Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives
Abstract
1. Introduction
2. Characteristics of Mannoproteins
3. Functional Properties of Mannoproteins and Mannoprotein-Rich Preparations
3.1. Anti-Biofilm Properties
3.2. Antimicrobial Properties
3.3. Prebiotic Activity
3.4. Immunostymulating Effect
3.5. Antioxidant Activity
3.6. Emulsifying Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell Wall Polysaccharides: Before and after Autolysis of Brewer’s Yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of Cell Wall Structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Schiavone, M.; Déjean, S.; Sieczkowski, N.; Castex, M.; Dague, E.; François, J.M. Integration of Biochemical, Biophysical and Transcriptomics Data for Investigating the Structural and Nanomechanical Properties of the Yeast Cell Wall. Front. Microbiol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Du, C.G.; Guo, Y.Q.; Zhao, Y.F.; Aorigele, C.; Wang, C.J.; Simujide, H.; Aqima, W.; Zhang, X.Y. Antibacterial Spectrum of Four Compounds from Yeasts in Koumiss. Pol. J. Vet. Sci. 2021, 24, 167–173. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.Y.; et al. Structure, Preparation, Modification, and Bioactivities of β-Glucan and Mannan from Yeast Cell Wall: A Review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Valáriková, J.; Čížová, A.; Račková, L.; Bystrický, S. Anti-Staphylococcal Activity of Quaternized Mannan from the Yeast Candida albicans. Carbohydr. Polym. 2020, 240, 116288. [Google Scholar] [CrossRef]
- Borovikova, D.; Teparić, R.; Mrša, V.; Rapoport, A. Anhydrobiosis in Yeast: Cell Wall Mannoproteins Are Important for Yeast Saccharomyces cerevisiae Resistance to Dehydration. Yeast 2016, 33, 347–353. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-Glucans and Cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Ovsyukova, M.V.; Shintyapina, A.; Vetvicka, V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules 2020, 25, 1819. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Vetvickova Jana Anti-Infectious and Anti-Tumor Activities of β-Glucans. Anticancer. Res. 2020, 40, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, S.; James, J.; Ellison, A. The Supplementation of a Prebiotic Improves the Microbial Community in the Gut and the Skin of Atlantic Salmon (Salmo salar). Aquac. Rep. 2022, 25, 101204. [Google Scholar] [CrossRef]
- Tang, N.; Wang, X.; Yang, R.; Liu, Z.; Liu, Y.; Tian, J.; Xiao, L.; Li, W. Extraction, Isolation, Structural Characterization and Prebiotic Activity of Cell Wall Polysaccharide from Kluyveromyces marxianus. Carbohydr. Polym. 2022, 289, 119457. [Google Scholar] [CrossRef]
- Abbott, D.W.; Martens, E.C.; Gilbert, H.J.; Cuskin, F.; Lowe, E.C. Coevolution of Yeast Mannan Digestion: Convergence of the Civilized Human Diet, Distal Gut Microbiome, and Host Immunity. Gut Microbes 2015, 6, 334–339. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kieliszek, M.; Pobiega, K.; Falana, K.; Janowicz, M. Modification of the Cell Wall Structure of Saccharomyces cerevisiae Strains during Cultivation on Waste Potato Juice Water and Glycerol towards Biosynthesis of Functional Polysaccharides. J. Biotechnol. 2018, 281, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thorwall, S.; Schwartz, C.; Chartron, J.W.; Wheeldon, I. Stress-Tolerant Non-Conventional Microbes Enable next-Generation Chemical Biosynthesis. Nat. Chem. Biol. 2020, 16, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Kosel, J.; Čadež, N.; Raspor, P. Factors Affecting Volatile Phenol Production During Fermentations with Pure and Mixed Cultures of Dekkera bruxellensis and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2014, 52, 35–45. [Google Scholar]
- Kosel, J.; Raspor, P.; Cadež, N. Maximum Residue Limit of Fungicides Inhibits the Viability and Growth of Desirable Non-Saccharomyces Wine Yeasts. Aust. J. Grape Wine Res. 2019, 25, 43–52. [Google Scholar] [CrossRef]
- Kosel, J.; Cadež, N.; Schuller, D.; Carreto, L.; Franco-Duarte, R.; Raspor, P. The Influence of Dekkera bruxellensis on the Transcriptome of Saccharomyces cerevisiae and on the Aromatic Profile of Synthetic Wine Must. FEMS Yeast Res. 2017, 17, fox018. [Google Scholar] [CrossRef]
- Zupan, J.; Avbelj, M.; Butinar, B.; Kosel, J.; Šergan, M.; Raspor, P. Monitoring of Quorum-Sensing Molecules during Minifermentation Studies in Wine Yeast. J. Agric. Food Chem. 2013, 61, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Kręgiel, D.; Pawlikowska, E.; Antolak, H. Non-Conventional Yeasts in Fermentation Processes: Potentialities and Limitations. Old. Yeasts New Quest. 2017, 87. [Google Scholar] [CrossRef]
- Binati, R.L.; Salvetti, E.; Bzducha-Wróbel, A.; Bašinskienė, L.; Cizeikienė, D.; Bolzonella, D.; Felis, G.E. Non-Conventional Yeasts for Food and Additives Production in a Circular Economy Perspective. FEMS Yeast Res. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Gao, H.; Qian, X.; Jiang, Y.; Zhou, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Biotechnological Applications of the Non-Conventional Yeast Meyerozyma guilliermondii. Biotechnol. Adv. 2021, 46, 107674. [Google Scholar] [CrossRef]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent Advances in Systems and Synthetic Biology Approaches for Developing Novel Cell-Factories in Non-Conventional Yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 13: Suitability of Taxonomic Units Notified to EFSA until September 2020. EFSA J. 2021, 19, e06377. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the Characterisation of Microorganisms Used as Feed Additives or as Production Organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Herman, L.; Lindqvist, R.; Nørrung, B.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 5: Suitability of Taxonomic Units Notified to EFSA until September 2016. EFSA J. 2017, 15, e04663. [Google Scholar] [CrossRef]
- Deckers, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef]
- Zhang, W.; Ballou, C.E. Saccharomyces kluyveri Cell Wall Mannoprotein. Structures of the O- and N-Linked Carbohydrate Components. J. Biol. Chem. 1981, 256, 10073–10079. [Google Scholar] [CrossRef]
- Van Rinsum, J.; Klis, F.M.; Ende, H. Van Den Cell Wall Glucomannoproteins of Saccharomyces cerevisiae Mnn9. Yeast 1991, 7, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Bussey, H. Yeast Kre1p Is a Cell Surface O-Glycoprotein. Mol. Gen. Genet. MGG 1995, 249, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Davies, M.J.; Bailey, D.; Gradwell, M.J.; Smestad-Paulsen, B.; Wold, J.K.; Barnes, R.M.R.; Hounsell, E.F. Characterization of Oligosaccharides from an Antigenic Mannan of Saccharomyces cerevisiae. Glycoconj. J. 1998, 15, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Jigami, Y.; Odani, T. Mannosylphosphate Transfer to Yeast Mannan. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1426, 335–345. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces pastorianus Cell Wall Polysaccharides with Brewing Process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell Wall Construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Teparić, R.; Mrša, V. Proteins Involved in Building, Maintaining and Remodeling of Yeast Cell Walls. Curr. Genet. 2013, 59, 171–185. [Google Scholar] [CrossRef]
- Klis, F.M.; De Groot, P.; Hellingwerf, K. Molecular Organization of the Cell Wall of Candida albicans. Med. Mycol. Suppl. 2001, 39, 1–8. [Google Scholar] [CrossRef]
- Hamada, K.; Fukuchi, S.; Arisawa, M.; Baba, M.; Kitada, K. Screening for Glycosylphosphatidylinositol (GPI)-Dependent Cell Wall Proteins in Saccharomyces cerevisiae. Mol. General. Genet. 1998, 258, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.Y.; De Groot, P.W.J.; Dekker, H.L.; De Jong, L.; Klis, F.M.; De Koster, C.G. Comprehensive Proteomic Analysis of Saccharomyces cerevisiae Cell Walls: Identification of Proteins Covalently Attached via Glycosylphosphatidylinositol Remnants or Mild Alkali-Sensitive Linkages. J. Biol. Chem. 2005, 280, 20894–20901. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell Wall Polysaccharides Released during the Alcoholic Fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and Characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Sauer, M.; Resina, D.; Vai, M.; Porro, D.; Valero, F.; Ferrer, P.; Mattanovich, D. Cloning, Disruption and Protein Secretory Phenotype of the GAS1 Homologue of Pichia pastoris. FEMS Microbiol. Lett. 2006, 264, 40–47. [Google Scholar] [CrossRef]
- Walencka, E.; Wieckowska-Szakiel, M.; Rozalska, S.; Sadowska, B.; Rozalska, B. A Surface-Active Agent from Saccharomyces cerevisiae Influences Staphylococcal Adhesion and Biofilm Development. Z. Naturforschung Sect. C J. Biosci. 2007, 62, 433–438. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yu, H.H.; Song, Y.J.; Park, Y.J.; Lee, N.K.; Paik, H.D. Anti-Biofilm Effect of the Cell-Free Supernatant of Probiotic Saccharomyces cerevisiae against Listeria monocytogenes. Food Control 2021, 121, 107667. [Google Scholar] [CrossRef]
- Rasheed, H.G.; Haydar, N.H. Purification, characterization and evaluation of biological activity of mannoprotein produced from Saccharomyces cerevisiae by. Iraqi J. Agric. Sci. 2023, 54, 347–359. [Google Scholar] [CrossRef]
- Bzducha Wróbel, A.; Farkaš, P.; Chraniuk, P.; Popielarz, D.; Synowiec, A.; Pobiega, K.; Janowicz, M. Antimicrobial and Prebiotic Activity of Mannoproteins Isolated from Conventional and Nonconventional Yeast Species-the Study on Selected Microorganisms. World J. Microbiol. Biotechnol. 2022, 38, 256, Erratum in World J. Microbiol. Biotechnol. 2023, 39, 92. https://doi.org/10.1007/S11274-023-03528-0. [Google Scholar] [CrossRef]
- Kuntsova, M.; Meledina, T.; Davydenko, S.; Manshin, D.; Andreeva, A. Obtaining Yeast Mannoproteins with Antimicrobial Properties. Funct. Foods Health Dis. 2023, 13, 437–447. [Google Scholar] [CrossRef]
- Ali, L.H.; Ali, W.S. Production and Antibacterial Activity of Biosurfactant from Saccharomyces cerevisiae. J. Phys. Conf. Ser. 2019, 1234, 012080. [Google Scholar] [CrossRef]
- Mahmood Nibras Nazar Effect of Biosurfactants Purified from Saccharomyces cerevisiae Against Corynebacterium Urealyticum. Available online: https://www.proquest.com/docview/2030154838?pq-origsite=gscholar&fromopenview=true (accessed on 27 January 2023).
- Trevisi, P.; Priori, D.; Gandolfi, G.; Colombo, M.; Coloretti, F.; Goossens, T.; Bosi, P. In Vitro Test on the Ability of a Yeast Cell Wall Based Product to Inhibit the Escherichia coli F4ac Adhesion on the Brush Border of Porcine Intestinal Villi. J. Anim. Sci. 2012, 90, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Greco, D.; Marquis, V.; Raspoet, R.; D’Ascanio, V.; Logrieco, A.F.; Avantaggiato, G. Antimicrobial Activity of Yeast Cell Wall Products Against Clostridium Perfringens. Foodborne Pathog. Dis. 2019, 16, 638–647. [Google Scholar] [CrossRef]
- Rahimi, S.; Kathariou, S.; Fletcher, O.; Grimes, J.L. Effect of a Direct-Fed Microbial and Prebiotic on Performance and Intestinal Histomorophology of Turkey Poults Challenged with Salmonella and Campylobacter. Poult. Sci. 2019, 98, 6572–6578. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Dawson, K.; Graugnard, D.; Dyck, M.; Willing, B.P. Dietary Supplementation of Weaned Piglets with a Yeast-Derived Mannan-Rich Fraction Modulates Cecal Microbial Profiles, Jejunal Morphology and Gene Expression. Animal 2019, 13, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Liu, Z.; Wang, Y.; Wang, J. Effect of Supplemental Yeast Cell Walls on Growth Performance, Gut Mucosal Glutathione Pathway, Proteolytic Enzymes and Transporters in Growing Broiler Chickens. J. Anim. Sci. 2018, 96, 1330–1337. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; De Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Effect of Mannoproteins on the Growth, Gastrointestinal Viability, and Adherence to Caco-2 Cells of Lactic Acid Bacteria. J. Food Sci. 2012, 77, M176–M180. [Google Scholar] [CrossRef]
- Xue, G.D.; Wu, S.B.; Choct, M.; Swick, R.A. Effects of Yeast Cell Wall on Growth Performance, Immune Responses and Intestinal Short Chain Fatty Acid Concentrations of Broilers in an Experimental Necrotic Enteritis Model. Anim. Nutr. 2017, 3, 399–405. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human Gut Bacteroidetes Can Utilize Yeast Mannan through a Selfish Mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Galinari, É.; Almeida-Lima, J.; Macedo, G.R.; Mantovani, H.C.; Rocha, H.A.O. Antioxidant, Antiproliferative, and Immunostimulatory Effects of Cell Wall α-d-Mannan Fractions from Kluyveromyces marxianus. Int. J. Biol. Macromol. 2018, 109, 837–846. [Google Scholar] [CrossRef]
- Liu, L.; Dang, Y. Antimicrobial Activity of Mannose Binding Lectin in Grass Carp (Ctenopharyngodon idella) In Vivo and In Vitro. Fish. Shellfish. Immunol. 2020, 98, 25–33. [Google Scholar] [CrossRef]
- Pontón, J.; Omaetxebarría, M.J.; Elguezabal, N.; Alvarez, M.; Moragues, M.D. Immunoreactivity of the Fungal Cell Wall. Med. Mycol. 2001, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kroll, F.S.A.; Putarov, T.C.; Zaine, L.; Venturini, K.S.; Aoki, C.G.; Santos, J.P.F.; Pedrinelli, V.; Vendramini, T.H.A.; Brunetto, M.A.; Carciofi, A.C. Active Fractions of Mannoproteins Derived from Yeast Cell Wall Stimulate Innate and Acquired Immunity of Adult and Elderly Dogs. Anim. Feed. Sci. Technol. 2020, 261, 114392. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Lee, S.M.; Chang, H.I.; Ha, C.H. Mannoproteins from Saccharomyces cerevisiae Stimulate Angiogenesis by Promoting the Akt-ENOS Signaling Pathway in Endothelial Cells. Biochem. Biophys. Res. Commun. 2019, 519, 767–772. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, M.; Cao, G.F.; Yang, Y.F. Saccharomyces cerevisiae Mannan Induces Sheep Beta-Defensin-1 Expression via Dectin-2-Syk-P38 Pathways in Ovine Ruminal Epithelial Cells. Vet. Res. 2019, 50, 8. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rodriguez-Lecompte, J.C.; Rogiewicz, A.; Patterson, R.; Slominski, B.A. Effect of Yeast-Derived Products and Distillers Dried Grains with Solubles (DDGS) on Growth Performance, Gut Morphology, and Gene Expression of Pattern Recognition Receptors and Cytokines in Broiler Chickens. Poult. Sci. 2016, 95, 507–517. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Sifri, M.; Selvaraj, R.K. Effect of Yeast Cell Product Supplementation on Broiler Cecal Microflora Species and Immune Responses during an Experimental Coccidial Infection. Poult. Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Haldar, S.; Bedford, M.R.; Muthusami, N.; Samanta, I. Assessment of Yeast Cell Wall as Replacements for Antibiotic Growth Promoters in Broiler Diets: Effects on Performance, Intestinal Histo-Morphology and Humoral Immune Responses. J. Anim. Physiol. Anim. Nutr. 2012, 96, 275–284. [Google Scholar] [CrossRef]
- Cruz, A.; Håkenåsen, I.M.; Skugor, A.; Mydland, L.T.; Åkesson, C.P.; Hellestveit, S.S.; Sørby, R.; Press, C.M.L.; Øverland, M. Candida Utilis Yeast as a Protein Source for Weaned Piglets: Effects on Growth Performance and Digestive Function. Livest. Sci. 2019, 226, 31–39. [Google Scholar] [CrossRef]
- Agboola, J.; Schiavone, M.; Øverland, M.; Morales-Lange, B.; Lagos, L.; Arntzen, M.; Lapeña, D.; Eijsink, V.; Horn, S.; Mydland, L.; et al. Impact of Down-Stream Processing on Functional Properties of Yeasts in Diets of Atlantic Salmon (Salmo salar): Implications for Gut Health. Sci Rep. 2021, 11, 4496. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The Antioxidant Activities of Six (1 → 3)-β-d-Glucan Derivatives Prepared from Yeast Cell Wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G. The Derivatization and Antioxidant Activities of Yeast Mannan. Int. J. Biol. Macromol. 2018, 107, 755–761. [Google Scholar] [CrossRef]
- Zhong, L.; Guo, X.; Xue, H.; Qiao, Y.; Mao, D.; Ye, X.; Cui, Z.; Li, Z.; Hu, G.; Huang, Y. Quality Characteristics of Reduced-Fat Emulsified Sausages Made with Yeast Mannoprotein Enzymatically Prepared with a β-1,6-Glucanase. Foods 2023, 12, 2486. [Google Scholar] [CrossRef]
- Elsaygh, Y.A.; Gouda, M.K.; Elbahloul, Y.; Hakim, M.A.; El Halfawy, N.M. Production and Structural Characterization of Eco-Friendly Bioemulsifier SC04 from Saccharomyces cerevisiae Strain MYN04 with Potential Applications. Microb. Cell Fact. 2023, 22, 176. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Fleischer, L.G.; Kurz, T. In Vitro Potential Antioxidant Activity of (1→3),(1→6)-β-d-Glucan and Protein Fractions from Saccharomyces cerevisiae Cell Walls. J. Agric. Food Chem. 2007, 55, 4710–4716. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.Q.; Jia, S.C.; Chen, Y.K.; Wang, J.P. Effect of Yeast Cell Wall on the Growth Performance and Gut Health of Broilers Challenged with Aflatoxin B1 and Necrotic Enteritis. Poult. Sci. 2018, 97, 477–484. [Google Scholar] [CrossRef]
- Da Silva Araújo, V.B.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed Extraction of β-Glucan and Mannoprotein from Spent Brewer’s Yeast (Saccharomyces uvarum) and Application of the Obtained Mannoprotein as a Stabilizer in Mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, Nutritional and Sensory Characteristics of French Salad Dressing Made with Mannoprotein from Spent Brewer’s Yeast. LWT—Food Sci. Technol. 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S. Characterization of the Composition and the Techno-Functional Properties of Mannoproteins from Saccharomyces cerevisiae Yeast Cell Walls. Food Chem. 2019, 297, 124867. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xia, C.; Liu, L.; Tang, L.; Wang, J.; Xu, C.; Wang, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Structural Characterization and Emulsifier Property of Yeast Mannoprotein Enzymatically Prepared with a β-1,6-Glucanase. LWT 2022, 168, 113898. [Google Scholar] [CrossRef]
- Hajhosseini, A.; Doroud, D.; Sharifan, A.; Eftekhari, Z. Stress Response and Characterization of Oil-in-Water Emulsions Stabilized with Kluyveromyces marxianus Mannoprotein. J. Food Sci. 2021, 86, 454–462. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Curioni, A.; Marangon, M.; Vincenzi, S.; Kantureeva, G.; Lomolino, G. Characterization and Emulsifying Properties of Extracts Obtained by Physical and Enzymatic Methods from an Oenological Yeast Strain. J. Sci. Food Agric. 2019, 99, 5702–5710. [Google Scholar] [CrossRef]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L. Evaluation of Kluyveromyces marxianus FII 510700 Grown on a Lactose-Based Medium as a Source of a Natural Bioemulsifier. J. Ind. Microbiol. Biotechnol. 2003, 30, 715–720. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
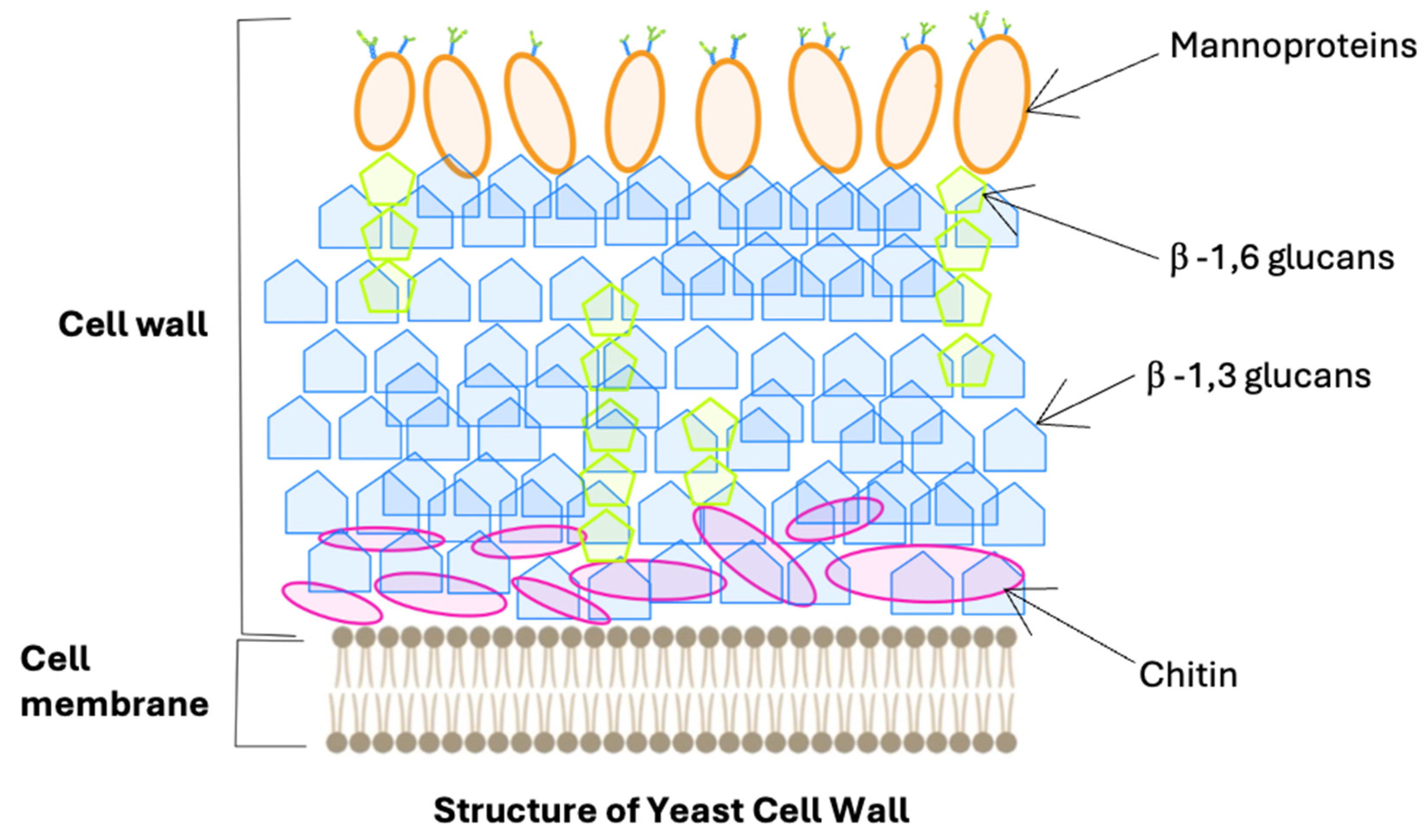
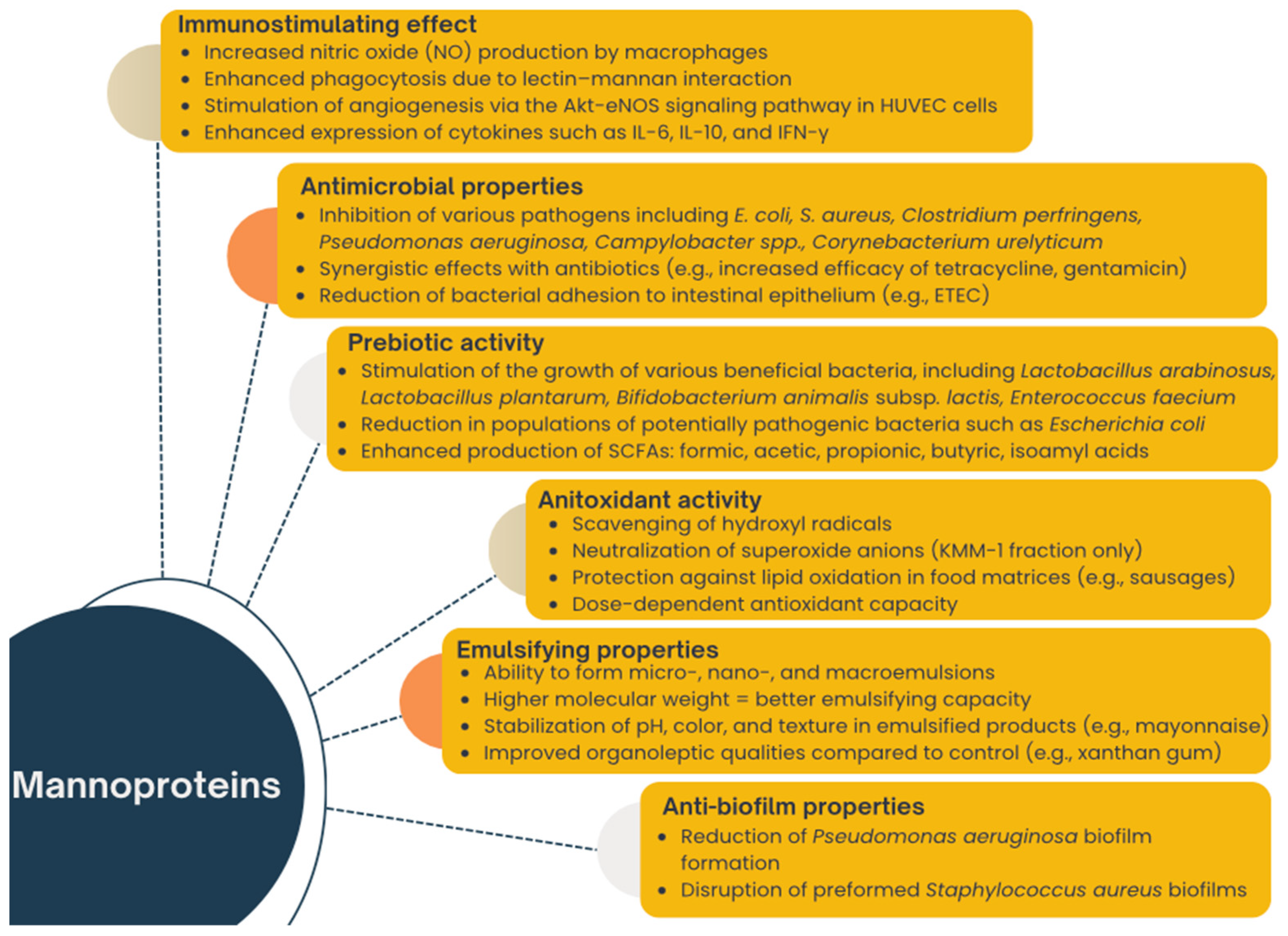
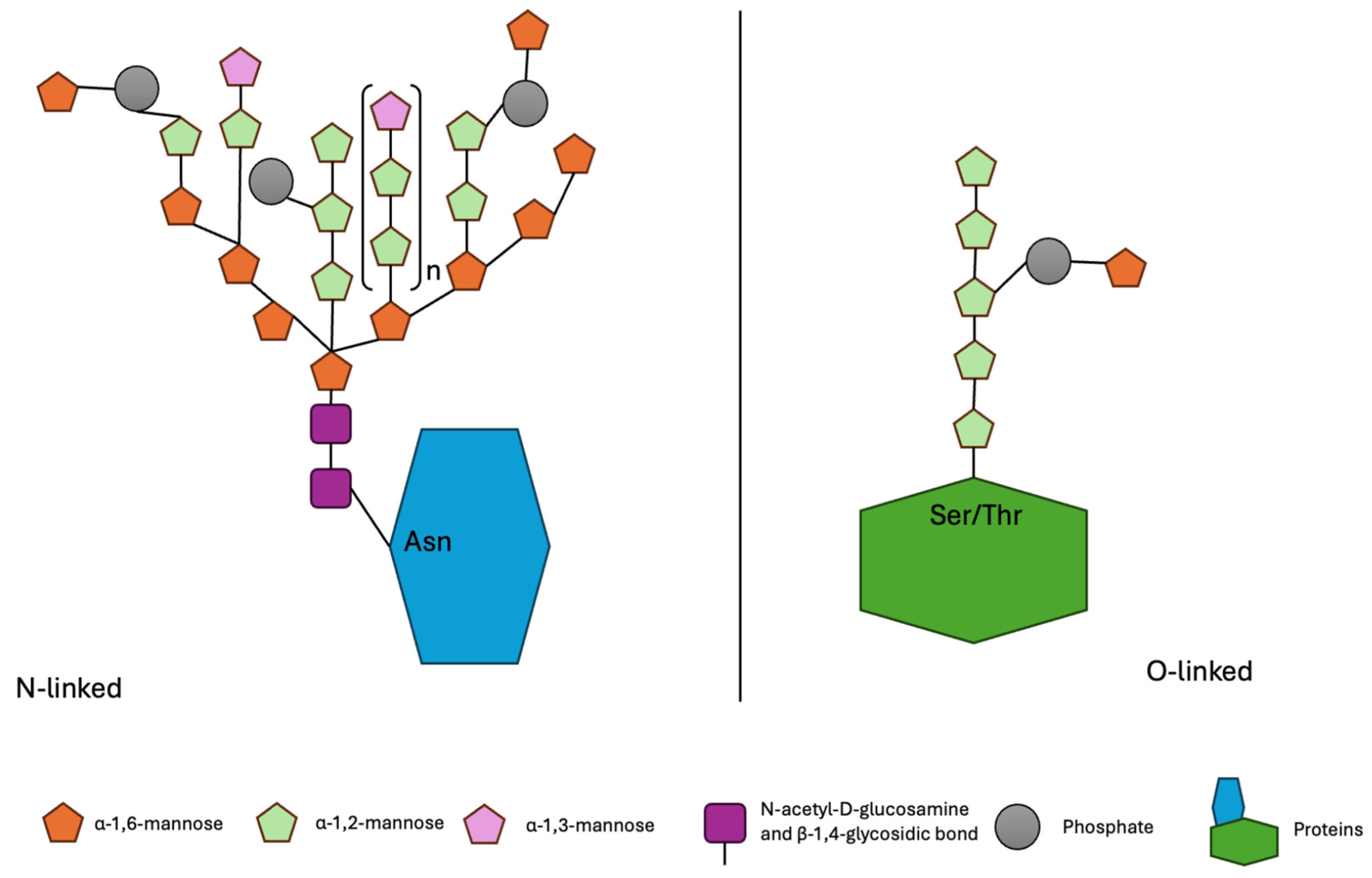
| Action | Preparation | Dosage of Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|
| Reduction in formation of biofilm Staphylococcus aureus ATCC 29213 (biofilm inhibition of 12–87%) | Yeast cell wall isolated from S. cerevisiae from Tokaj wine production | 10% | Protein content in the raw extract: 7.2%; 15–25 kDa, no information about the polysaccharide fraction | [45] |
| Reduction in formation of biofilm Listeria monocytogenes (biofilm inhibition of 52.6–79.5%) | CFS—cell free supernatant of S. cerevisiae | 2 mL (undiluted CFS) | No characteristics of the preparation, presumably the supernatant containing mannoprotein fractions | [46] |
| Reduction in formation of biofilm Pseudomonas aeruginosa and Staphylococcus aureus (biofilm inhibition: P. aeruginosa—51.8%, S. aureus—19.7%) | Mannoproteins isolated from the cell wall of the yeast S. cerevisiae BY | 200 mg/mL | Protein to mannan ratio 87.1%, Characterization of preparations using FT-IR | [47] |
| Reduction in formation of biofilm S. aureus ATCC 29213 (maximum biofilm inhibition 63.4% at 4%) | Mannoproteins isolated from the cell wall of the yeast S. cerevisiae 102 | 2–10% (max effect at 4%) | Protein concentration: 31.7%; carbohydrate concentration: 65% | [48] |
| Action | Preparation | Dosage of Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|
| Inhibition of the growth of Pseudomonas aeruginosa | Biosurfactant from the yeast S. cerevisiae | Not specified (100 μL applied in agar well diffusion assay) | No detailed characteristics | [50] |
| Growth inhibition of Corynebacterium urelyticum (inhibition zone 18 mm) | Biosurfactant from the yeast S. cerevisiae | Not specified (biosurfactant activity evaluated using oil spreading and E24 tests) | Protein concentration: 0.0535 mg/mL; carbohydrate concentration 0.08839 mg/mL; molecular mass 89, 100 kDa | [51] |
| Inhibition of the growth of Escherichia coli and Bacillus subtilis (37% inhibition of E. coli at 5% concentration, 80% inhibition of B. subtilis at 3% concentration) | Mannoproteins isolated from the cell wall of the yeast S. cerevisiae | 5% (E. coli) 3% (B. subtilis) | No detailed characteristics | [49] |
| Inhibition of the growth of Pseudomonas aeruginosa and Staphylococcus aureus (zone of inhibition: 16.2 mm (P. aeruginosa), 13.7 mm (S. aureus) at 200 mg/mL) | Mannoproteins isolated from the cell wall of the yeast S. cerevisiae BY | 50–200 mg/mL | Protein-to-mannose ratio 87.1% Characterization of preparations using FT-IR | [47] |
| Reduction in the growth of P. aeruginosa ATCC 27853, P. mirabilis ATCC 27853, and S. Enteritidis ATCC 13076 (reduction in bacterial growth ranged from 77% to 95%) | Mannoproteins isolated from Saccharomyces cerevisiae ATCC 7090 | 2–6% | Molecular weight: 65, 14, 1.9 (Mw/kDa), nitrogen: 6.96%, carbon: 31.9%, hydrogen: 4.42%, protein: 42.3%, total sugars: 55.7% Characterization of preparations using NMR and FT-IR | [48] |
| Reduction in the growth of S. aureus ATCC 25923 and E. coli ATCC 25922 (inhibition: 93.6% (S. aureus), 91% (E. coli) at 6% concentration) | Mannoproteins isolated from the cell biomass of Metschnikowia reukaufii WLP 4650 | 2–6% | Molecular weight: 150, 13, 1.9 (Mw/kDa), (nitrogen: 6.21%, carbon: 30.38%, hydrogen: 4.12%, protein: 37.8%, total sugars: 60.0% Characterization of preparations using NMR and FT-IR | [48] |
| Reduction in the growth of P. aeruginosa ATCC 29212 and E. coli ATCC 25922 (inhibition: 84.4% (P. aeruginosa at 6%), 45.5–70.5% (E. coli at 2–6%)) | Mannoproteins isolated from cell biomass of the yeast Wickerhamomyces anomalus CCY 38-1-13 | 2–6% | Molecular weight: 84, 20, 1.9 (Mw/kDa), nitrogen: 5.41%, carbon: 28.77%, hydrogen: 3.93%, protein: 32.9%, total sugars: 52.8% Characterization of preparations using NMR and FT-IR | [48] |
| Inhibition of the growth of E. coli F4ac (ETEC) in the intestinal lumen of pigs by binding and excretion with the components of SENTIGUARD | SENTIGUARD product—containing beta-glucans, mannoproteins, oligosaccharides and yeast bile salts—Nutriad, Turnhout, Belgium | 0.5%, 5%, 10% (w/v) | No detailed characteristics | [52] |
| Inhibition of the growth of Clostridium perfringens (reduction in growth rate and maximum growth level; effect was time- and dose-dependent) | The cell wall of the yeast S. cerevisiae | 0.5–2% (w/v), min 1.25 mg/mL | Mannan content: 13–26.8% DM; protein content: 17.5–23.8% DM | [53] |
| Inhibition of the growth of Salmonella cells in the feces of female large white turkey chicks (reduction from 3.99 log CFU/g (control) to 2.60 log CFU/g (treated group) after 21 days) | S. cerevisiae yeast cell wall—containing mannan–oligosaccharides composed by Quality Technology International, Inc., (QTI) Elgin, IL, USA | 0.5 g/kg feed | No detailed characteristics | [54] |
| Reduction in the number of Campylobacter cells in piglets | MRF (mannan-rich fraction from yeast cell wall)—Actigen™ | 800 mg/kg feed | Mannan 12%; raw fat 1.85%; protein 33.5%; total ash 6.78% | [55] |
| Reduction in the number of E. coli in vitro after 24 h of fermentation in intestinal juice | Three fractions of α-mannan (LZ-MPS; MC-MPS, G-MPS) isolated from Kluyveromyces marxianus | 1% (w/v) | LZ-MPS: yield 71.1 mg/g, sugars—mannose, sugar content: 97.1%, protein: –; MC-MPS: yield 84.7 mg/g, sugars—mannose, sugar content: 96.88%, protein: –; G-MPS: yield 77.3 mg/g, sugars—mannose, sugar content: 91.13%, protein: – | [13] |
| Action | Preparation | Dosage of the Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|
| Higher FROM 600 Lactobacillus paracasei ssp. ZY-1 tolerance towards the control group and inulin | Three fractions of α-mannan (LZ-MPS, MC-MPS, G-MPS) isolated from Kluyveromyces marxianus | 2% (20 mg/mL) in MRS for growth; 100 mg/9 mL in fecal fermentation; 80 mg/10 mL in digestion test | LZ-MPS: yield 71.1 mg/g, sugars—mannose, sugar content: 97.1%, protein: –; MC-MPS: yield 84.7 mg/g, sugars—mannose, sugar content: 96.88%, protein: –; G-MPS: yield 77.3 mg/g, sugars—mannose, sugar content: 91.13%, protein: – | [13] |
| Stimulation of Lactobacillus arabinosus ATCC 8014 growth by ~159% and Bifidobacterium animalis subsp. lactis B12 by ~135% compared to control group (at 1–2% concentration) | Mannoproteins isolated from cell biomass of the yeast Saccharomyces cerevisiae ATCC 7090 | 0.5–2% | Molecular weight: 65, 14, 1.9 (Mw/kDa), nitrogen: 6.96%, carbon: 31.9%, hydrogen: 4.42%, protein: 42.3%, total sugars: 55.7% Characterization of preparations using NMR and FT-IR | [48] |
| Stimulation of Lactobacillus arabinosus ATCC 8014 growth by ~214% at 2% concentration | Mannoproteins isolated from the cell biomass of the yeast Metschnikowia reukaufii WLP 4650 | 0.5–2% | Molecular weight: 150, 13, 1.9 (Mw/kDa), nitrogen: 6.21%, carbon: 30.38%, hydrogen: 4.12%, protein: 37.8%, total sugars: 60.0% Characterization of preparations using NMR and FT-IR | [48] |
| Stimulation of Lactobacillus arabinosus ATCC 8014 growth by ~140–160%, depending on the dose (0.5–2%) | Mannoproteins isolated from cell biomass of the yeast Wickerhamomyces anomalus CCY 38-1-13 | 0.5–2% | Molecular weight: 84, 20, 1.9 (Mw/kDa), nitrogen: 5.41%, carbon: 28.77%, hydrogen: 3.93%, protein: 32.9%, total sugars: 52.8% Characterization of preparations using NMR and FT-IR | [48] |
| Increased cecal counts of Lactobacillus (7.80 log CFU/g) and Bifidobacterium (8.60 log CFU/g) in Cobb broilers compared to control group (7.50 and 8.37 log CFU/g, respectively) | Wall cellular yeast from Luoyang Hongxiang Biological Feed Laboratory of Henan University of Science and Technology (Henan, China) | 0.5, 1.0, and 1.5 g/kg feed | Yeast cell wall (48.3% D-glucose; 32.3% D-mannose) | [59] |
| Improving adhesion to the intestinal wall: Enterococcus faecium, Lactobacillus plantarum, and Lactobacillus salivarius in an artificial intestine | Mannoprotein extracts isolated from the yeast S. cerevisiae (Laffort, Guipuzcoa, Spain) | 15 mg/mL | Protein: 31.3%, polysaccharides 66.1%, mannose 92.7% of individual sugars from polysaccharides | [60] |
| Increased concentrations of formic (1.05 vs. 0.73 mmol/g), acetic (76.92 vs. 57.81 mmol/g), propionic (30.08 vs. 21.66 mmol/g), isoamyl (1.02 vs. 0.86 mmol/g), and butyric (18.73 vs. 11.71 mmol/g) acids in cecal content of broilers vs. control group | S. cerevisiae yeast cell wall extract (Actigen, Alltech, Nicholasville, KY, USA) | 0.5 g/kg feed | No detailed characteristics | [61] |
| Action | Preparation | Dosage of the Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|
| Producing more H2O2 during bacterial lipopolysaccharide stimulation in Beagle dogs | Preparation of mannoproteins isolated from the cell wall of the yeast S. cerevisiae (Actigen, Alltech, Lexington, KY, USA) | 400 mg/kg feed | No detailed characteristics | [66] |
| Stimulation of nitric oxide-induced angiogenesis in umbilical vein endothelial cells ex vivo -eNOS signaling pathway in umbilical vein endothelial cells ex vivo | Mannoproteins isolated from the yeast cell wall of S. cerevisiae K48L3 (mutant) | 100, 500, and 1000 ng/mL | No detailed characteristics | [67] |
| Increased in vitro production of SBD-1 (increased 5.8-fold compared to control) protein in rumen epithelial cells | Mannan isolated from the cell wall of the yeast S. cerevisiae (Sigma, Munich, Germany) | 50 µg/mL | No detailed characteristics | [68] |
| Increased expression of cytokines in blood serum after challenge with E. coli bacterial lipopolysaccharide | Yeast cell wall (Canadian Bio-System Inc., Calgary, AB, Canada) | 0.25% | Polysaccharides 43.3%, including sugars: mannose 22.9%, glucose 20.0%; protein 17.2% | [69] |
| E. coli bacteria cells by 31% Salmonella cells by 20% Increased production of γ interferon (IFN-γ mRNA) in the intestine during coccidial infection Depletion of regulatory T cells (Tregs) | Yeast cell wall of Pichia guilliermondii; CitriStim, ADM, Quincy, IL, USA | 0.1 or 0.2% | No detailed characteristics | [70] |
| Reducing the number of E. coli and Salmonella cells in the intestinal content of broilers Increased humoral memory of the immune response of broilers to Newcastle disease | Cell wall of the yeast S. cerevisiae (AB Vista, Marlborough, Wiltshire, UK) | 1000 mg/kg feed | Weight: 975 g/kg DM; crude protein: 610.2 g/kg, including 97.6 g/kg total N; raw fat: 39 g/kg; total ash: 37 g/kg; glucans and mannan in total approximately 40% of the total sample | [71] |
| Increase in the height of intestinal villi in the jejunum of pigs Increase in the content of dry fecal matter in pigs Increasing the duration of intestinal transit of pig digestive contents | Biomass yeast Candida utilis LYCC 7549 (Lallemand Inc., Salutaguse, Estonia) | 40% of the protein content in the diet came from the yeast biomass C. utilis | Dry weight: 970 g/kg; protein: 470 g/kg; raw fat 16 g/kg; ash 78 g/kg | [72] |
| Action | Preparation | Dosage of the Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|
| Delay in the rate of lipid oxidation in pork sausages | Mannoproteins isolated from the cell wall of S. cerevisiae | 25, 50, 75, 100% | Ratio of mannan to protein: 14.5 | [76] |
| 51.8% DPPH free radical scavenging | S. cerevisiae yeast extract MYN04 | 0.5 mg/mL emulsifier addition | Carbohydrates: 27.1%, protein: 72.9% | [77] |
| Neutralization of free hydroxyl radicals Chelating effect on copper and iron The ability to scavenge peroxide radicals by the KMM-1 fraction | Mannan fractions isolated from Kluyveromyces marxianus yeast | 0.05, 0.1, 0.25, 0.5, 1.0 mg/mL | KMM-1 (glucose–mannose sugars: 85.9%, protein: 0.11%, molecular mass: 7.6 kDa) KMM-2 (glucose–mannose sugars: 95.2%, protein: 0.51%, molecular mass: 26.1 kDa) KMM-3 (mannose sugars: 96.1%, protein: –, molecular weight: 41.3 kDa) KMM-4 (mannose sugars: 93.4%, protein: 1.21%, molecular weight: 75.1 kDa) | [63] |
| Radical scavenging ability (PM and CMP-M) Antioxidant capacity towards lipids (all tested preparations) (PM and CMP-M: +15% hydroxyl radical scavenging vs. unmodified mannan) | Mannans extracted from the yeast cell wall and subjected to chemical modification: mannan phosphorylated (PM), sulfated mannan (SM), mannan carboxymethylated (CM-M), mannan carboxymethylated–phosphorylated (CMP-M) and mannan carboxymethylated–sulfated (CMS-M) | 0.1, 0.2, 0.4, 0.8, 1.6, and 3.2 mg/mL | Mannan: sugars 96.15%, PM: sugars 76.33%, SM: sugars 64.08%, CM-M: sugars 57.28%, CMP-M: sugars 38.03%, CMS-M: sugars 23.25% | [75] |
| Increase in the concentration of reduced glutathione, glutathione reductase, and glutathione S-transfer in the blood of broilers after 21 days of supplementation with a yeast cell wall preparation (increased the rate of r-GSH (13.8–16.6%), GR (9.7–24.6%) at 0.5–1.5 g/kg feed) | Wall cellular yeast from Luoyang Hongxiang Biological Feed Laboratory of Henan University of Science and Technology (Henan, China) | 0.5, 1.0, 1.5 g/kg feed | D-glucose: 46.2%, D-mannose: 29.8% | [59] |
| Action | Preparation | O/W Phase Ratio | Dosage of the Preparation | Structure and Chemical Composition | Literature |
|---|---|---|---|---|---|
| Maintaining emulsion stability at a dose of 6 and 8% mannoproteins after 7 days of storage (100% emulsion phase preserved at 8%) | Mannoproteins isolated from the cell wall of S. cerevisiae | 50%/5% | 2, 4, 6, 8% | Ratio of mannan to protein: 14.5 | [83] |
| Improved emulsion stability observed at 1.25% and 1.5% mannoprotein concentrations; associated with higher negative zeta potential, smaller oil droplets, and increased viscosity | Mannoproteins isolated from the cell wall of Kluyveromyces marxianus IBRC-M 30114 | 20%/80% and 80%/20% | 0.5, 0.75, 1, 1.25, and 1.5% | Molecular weight: 107.2 kDa, protein: 28.8%, carbohydrates: 68.22% | [84] |
| Improved textural properties of pork sausages with 25–100% replacement of animal fat by emulsions containing mannoproteins | Mannoproteins isolated from the cell wall of S. cerevisiae | 50% oil/5% sodium caseinate/6% mannoprotein preparation (w/v) | Oil replacement in doses: 25, 50, 75, 100% | Ratio of mannan to protein: 14.5 | [76] |
| Maintenance of emulsion phase after 48 h at pH 3 with 25 mg/7.5 mL extract addition; better stability at acidic pH values | Saccharomyces cerevisiae EC 1118 yeast extract (Lalle—mand Inc., Montreal, QC, Canada) | 2:1 | 25 mg of added extract, additionally: corn oil 5 mL, Mcllvine buffer 2.5 mL | Sugars: 564.4–980.5 mg/g s.s. (depending on the isolation method), including mannose: 69.4–93.5% (depending on the isolation method), protein: 10.6–48.3 mg/g DS (depending on the insulation method) | [85] |
| Maintaining a stable emulsion for 90 days at 4 °C (76% of the emulsion phase) | Mannoproteins isolated from the yeast K. marxianus FII 510700 (FRR 1586) | 0.12 g of mannoprotein, 4 mL of water, 6 mL of corn oil | Carbohydrates: 90% (mannan), protein: 4–6% | [86] | |
| Emulsification index of 80% for wheat germ oil, corn oil, and olive oil after 24 h with 0.5 mg/mL extract addition | S. cerevisiae yeast extract MYN04 | - | 0.5 mg/mL emulsifier addition | Carbohydrates: 27.1%, protein: 72.9% | [77] |
| Maintained mayonnaise stability after 28 days at refrigeration with 0.6–1.0 g/100 g mannoprotein addition; stable pH and improved color | Mannoproteins derived from the yeast cell wall after Saccharomyces beer production uvarum | - | 0.6, 0.8, 1.0 g/100 g | Fractions of 58 kDa and 64 kDa | [80] |
| Emulsion stability after 30 days at 4:6 oil-to-water ratio; emulsifying capacity at 72% with 1% mannoprotein addition (ultraturrax method) | Mannoproteins derived from Saccharomyces yeast pastorianus (BSY) Super Bock Group, S.A. (Leça do Balio, Portugal) | 4:6 | 1% | Alkaline insulation: yield: 3–17%; sugars: 16–79%, including glucose: 23–90 mol%, mannose: 10–77 mol%; protein: 32% Subcritical fluid insulation with high temperature: yield: 8–24%; sugars: 15–79%, including glucose: 50–71 mol%, mannose: 29–50 mol%; protein: 32% | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chraniuk, P.; Bzducha-Wróbel, A. Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives. Fermentation 2025, 11, 374. https://doi.org/10.3390/fermentation11070374
Chraniuk P, Bzducha-Wróbel A. Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives. Fermentation. 2025; 11(7):374. https://doi.org/10.3390/fermentation11070374
Chicago/Turabian StyleChraniuk, Paulina, and Anna Bzducha-Wróbel. 2025. "Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives" Fermentation 11, no. 7: 374. https://doi.org/10.3390/fermentation11070374
APA StyleChraniuk, P., & Bzducha-Wróbel, A. (2025). Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives. Fermentation, 11(7), 374. https://doi.org/10.3390/fermentation11070374






