Microalgal Valorization of CO2: A Sustainable Pathway to Biofuels and High-Value Chemicals
Abstract
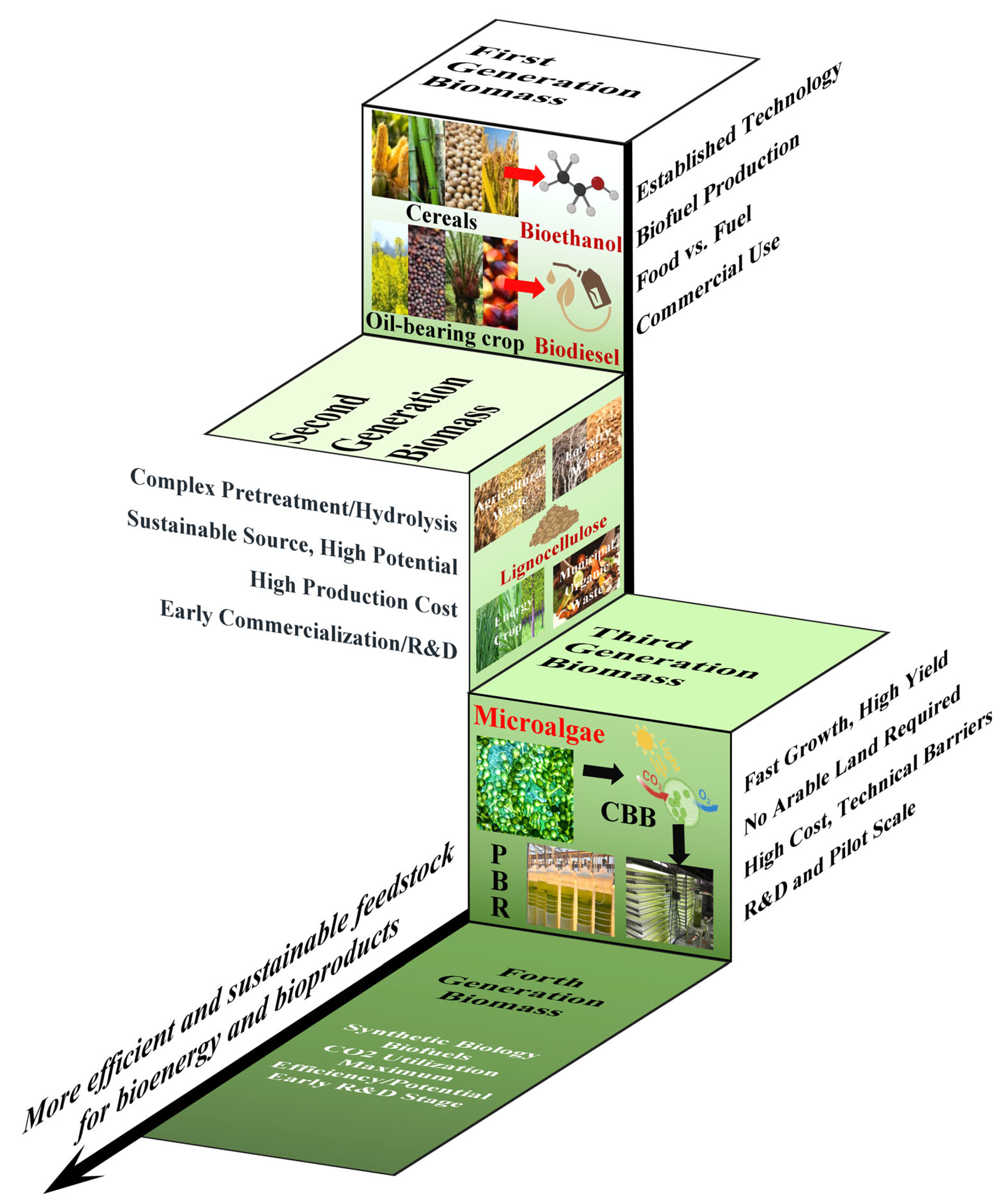
1. Introduction
2. Photoautotrophic Microalgae-Driven CO2 Fixation
2.1. Microalgal Species
| Phyla | Species | Carbon Source | Type of Reactor | CO2 Fixation Rate | Products/Efficiency | References |
|---|---|---|---|---|---|---|
| yanophyta (Cyanobacteria) | Synechococcus elongatus PCC 7942 | CO2-enriched air | PBR | / | 8.9 g L−1 succinic acid | [35] |
| Synechococcus elongatus UTEX 2973 | Flue gas (3–6% CO2) | PBR | / | 75.2 mg L−1 d−1 PHB | [36] | |
| Synechococcus elongatus UAM-C/S03 | Pure CO2 | PBR | 674 mg L−1 d−1 | 58.1 mg L−1 d−1 PHB | [37] | |
| Synechococcus 2973-phaCAB | 5% CO2 | PBR | / | 6.9 g m−2 d−1 PHB | [38] | |
| Thermosynechococcus sp. CL-1 | Inorganic carbon | Flat panel PBR | 21.98 mg L−1 h−1 | 98.1 mg g−1 phycocyanin | [39] | |
| Thermosynechococcus elongatus E542 | 5–15% CO2 | PBR | / | Biomass | [40] | |
| Thermosynechococcus sp. CL-1 | Inorganic carbon | PBR | 11.41 mg L−1 h−1 | 0.043 mg L−1 h−1 carotenoid | [41] | |
| Spirulina platensis | Flue gas (99% CO2) | Incubator | / | 38.3 g m−2 d−1 biomass | [42] | |
| Spirulina. sp. | Flue gas (99% CO2) | Raceway ponds | 51.3 g m−2 d−1 | Biomass | [43] | |
| Phormidium alkaliphilum | Capture ambient CO2 | Tubular PBR | / | 5.8 g m−2 d−1 dry weight | [44] | |
| Chlorophyta | Chlorella vulgaris | 5% CO2 | Flask | / | 989.4 mg L−1 lipid | [45] |
| Chlorella vulgaris FACHB-31 | 15% CO2 | PBR | / | 2.1 g L−1 biomass | [46] | |
| Chlorella vulgaris FACHB 24 | 10% CO2 | B-PAMFC | 605.3 mg L−1 d−1 | 105.9 mg L−1 d−1 lipid | [47] | |
| Chlorella sorokiniana TH01 | 2% CO2 | Flat-panel PBR | / | 3.0 mg L−1 d−1 lutein | [48] | |
| Chlorella fusca LEB 111 | 15% CO2 | PBR | 89.2 mg L−1 d−1 | 26.8 mg L−1 carbohydrate | [49] | |
| Chlorella mutant PY-ZU1 | 15% CO2 | Tubular PBR | / | 5.5 g L−1 biomass | [50] | |
| Immobilized Scenedesmus obliquus | 5% CO2 | Incubator | / | 628 mg g−1 protein | [51] | |
| Scenedesmus obliquus CPCC05 | 1–25% CO2 | PBR | 0.44 kg m−3 d−1 | 0.36 kg m−3 d−1 biomass | [52] | |
| Haematococcus pluvialis | 15% CO2 | PBR | / | Astaxanthin | [53] | |
| Chlamydomonas reinhardtii | 5% CO2 | PBR | / | Lutein and lipid | [54] | |
| Chrysophyta | Emiliania huxleyi CCMP 371 | Pure CO2 | PBR | / | Biomass | [55] |
| Nannochloroposis oculata CCMP525 | Capture ambient CO2 | PBR | / | Lipid | [56] | |
| Rhodophyta | Colaconema formosanum | Inorganic carbon | Incubator | / | 6 mg g−1 phycobiliprotein | [57] |
2.2. Pathways of Photosynthetic Carbon Fixation in Microalgae
2.3. Microalgal Carbon Fixation Systems
2.3.1. Open Cultivation Systems
2.3.2. Closed Photobioreactors
2.3.3. Integrated Wastewater Treatment Systems
3. Factors Influencing Microalgal Carbon Fixation
3.1. Light and Photoperiod
3.2. Temperature
3.3. Gas–Liquid Mass Transfer Efficiency
4. Microalgal Resource Utilization Methods: The Core of Valorization
5. Future Outlook: Pioneering the Next Wave of Microalgal Biotechnology
5.1. Strain Improvement and Synthetic Biology Driven Innovation: Engineering ‘Super Algae’
5.2. Cultivation System and Engineering Process Collaborative Upgrades: Towards Intelligent and Scalable Bioproduction
5.3. Biorefinery and Full Industry Chain Synergy: Maximizing Value and Sustainability
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A.; et al. Global Carbon Budget 2024. Earth Syst. Sci. Data 2025, 17, 965–1039. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, C.; Wang, Y.-F.; Chang, Z.-S. Comparative Study on Discharge Characteristics of Low Pressure CO2 Driven by Sinusoidal AC Voltage: DBD and Bare Electrode Structure. Acta Phys. Sin. 2022, 71, 115204. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, P.; Hu, S.; Ye, J. Achieving Artificial Carbon Cycle via Integrated System of High-Emitting Industries and CCU Technology: Case of China. J. Environ. Manag. 2023, 340, 118010. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.Y.; Jung, I.L.; Park, D.H. Conversion of Carbon Dioxide to Metabolites by Clostridium acetobutylicum KCTC1037 Cultivated with Electrochemical Reducing Power. Adv. Microbiol. 2012, 2, 332–339. [Google Scholar] [CrossRef]
- Huang, F.; Wang, F.; Liu, Y.; Guo, L. Cu-ZnS Modulated Multi-Carbon Coupling Enables High Selectivity Photoreduction CO(2) to CH(3)CH(2)COOH. Adv. Mater. 2025, 37, e2416708. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal Biomass Valorization for Biofuel Production and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of Biofuels from Microalgae—A Review on Cultivation, Harvesting, Lipid Extraction, and Numerous Applications of Microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Udayan, A.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J. Mass Cultivation and Harvesting of Microalgal Biomass: Current Trends and Future Perspectives. Bioresour. Technol. 2022, 344, 126406. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; Pereira, A.S.A.d.P.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from Microalgae Biomass: Technology, Sustainability, Challenges and Opportunities. Chemosphere 2022, 305, 135508. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-Mediated Carbon Capture and Utilization by Microalgae Towards Sustainable CO2 Biofixation and Biomass Valorization—A Review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Padhi, D.; Kashyap, S.; Mohapatra, R.K.; Dineshkumar, R.; Nayak, M. Microalgae-Based Flue Gas CO2 Sequestration for Cleaner Environment and Biofuel Feedstock Production: A Review. Environ. Sci. Pollut. Res. Int. 2025, 30, 13539–13565. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Gupta, V.K.; Verma, P. Multifaceted Application of Microalgal Biomass Integrated with Carbon Dioxide Reduction and Wastewater Remediation: A Flexible Concept for Sustainable Environment. J. Clean. Prod. 2022, 339, 130654. [Google Scholar] [CrossRef]
- Scapini, T.; Woiciechowski, A.L.; Manzoki, M.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Fanka, L.S.; Duda, L.J.; Vale, A.d.S.; de Carvalho, J.C.; Soccol, C.R. Microalgae-Mediated Biofixation as an Innovative Technology for Flue Gases Towards Carbon Neutrality: A Comprehensive Review. J. Environ. Manag. 2024, 363, 121329. [Google Scholar] [CrossRef]
- Cheng, D.; Li, X.; Yuan, Y.; Yang, C.; Tang, T.; Zhao, Q.; Sun, Y. Adaptive Evolution And Carbon Dioxide Fixation of Chlorella sp. in Simulated Flue Gas. Sci. Total Environ. 2019, 650, 2931–2938. [Google Scholar] [CrossRef]
- Tanvir, R.U.; Zhang, J.; Canter, T.; Chen, D.; Lu, J.; Hu, Z. Harnessing Solar Energy Using Phototrophic Microorganisms: A Sustainable Pathway To Bioenergy, Biomaterials, and Environmental Solutions. Renew. Sustain. Energy Rev. 2021, 146, 111181. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.; Suely, A.; Boyce, A.; Faruq, G. Bioethanol Production From Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Rahul, S.; Valliammai, N.; Varshiny, S.; Lakshaya, M.; Prabakaran, S.; Sudalai, S.; Arumugam, A. Utilization of Lignocellulosic Biomass for Advanced Simultaneous Biofuel and Biomaterials Production. In Sustainable Development of Renewable Energy; Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Bouwer, E. Biogas Production from Algae and Cyanobacteria Through Anaerobic Digestion: A Review, Analysis, and Research Needs. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013. [Google Scholar]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2bio-Mitigation Using Microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Yang, Z.-Y.; Vadiveloo, A.; Li, C.; Chen, Q.-G.; Chen, D.-Z.; Gao, F. Enhancing Lipid Production and Sedimentation of Chlorella pyrenoidosa in Saline Wastewater Through the Addition of AGRICULTURAL phytohormones. J. Environ. Manag. 2024, 354, 120445. [Google Scholar] [CrossRef]
- Gehad, I.; Lemar Al, G. The Production of Biodiesel from Algae Using Flocculation and Nanoparticles. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 3. Bioengineering 2022, 9, 328. [Google Scholar] [CrossRef]
- Patil, A.D.; Kasabe, P.J.; Dandge, P.B. Pharmaceutical and Nutraceutical Potential of Natural Bioactive Pigment: Astaxanthin. Nat. Prod. Bioprospect. 2022, 12, 25. [Google Scholar] [CrossRef]
- Hu, J.; Wang, D.; Chen, H.; Wang, Q. Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity. Int. J. Mol. Sci. 2023, 24, 1898. [Google Scholar] [CrossRef]
- Goodchild-Michelman, I.M.; Church, G.M.; Schubert, M.G.; Tang, T.-C. Light and carbon: Synthetic biology toward new cyanobacteria-based living biomaterials. Mater. Today Bio 2023, 19, 100583. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, J.; Pakrasi, H.B. Cpf1 Is A Versatile Tool for CRISPR Genome Editing Across Diverse Species of Cyanobacteria. Sci. Rep. 2016, 6, 39681. [Google Scholar] [CrossRef]
- Lin, W.-R.; Ng, I.-S. Development of CRISPR/Cas9 System in Chlorella Vulgaris FSP-E to Enhance Lipid Accumulation. Enzym. Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef]
- Baek, K.; Yu, J.; Jeong, J.; Sim, S.J.; Bae, S.; Jin, E. Photoautotrophic Production of Macular Pigment in a Chlamydomonas reinhardtii Strain Generated by Using DNA-free CRISPR-Cas9 RNP-Mediated Mutagenesis. Biotechnol. Bioeng. 2017, 115, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, E.C.; Graf, L.; Yang, J.H.; Qiu, H.; Zelzion, U.; Chan, C.X.; Stephens, T.G.; Weber, A.P.M.; Boo, G.H.; et al. Analysis of the Draft Genome of the Red Seaweed Gracilariopsis chorda Provides Insights into Genome Size Evolution in Rhodophyta. Mol. Biol. Evol. 2018, 35, 1869–1886. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.J.; Tsai, J.C.; Lan, E.I. CRISPRi-Enhanced Direct Photosynthetic Conversion of Carbon Dioxide to Succinic Acid by Metabolically Engineered Cyanobacteria. Bioresour. Technol 2022, 366, 128131. [Google Scholar] [CrossRef]
- Roh, H.; Lee, J.S.; Choi, H.I.; Sung, Y.J.; Choi, S.Y.; Woo, H.M.; Sim, S.J. Improved CO(2)-Derived Polyhydroxybutyrate (Phb) Production by Engineering Fast-Growing Cyanobacterium Synechococcus elongatus UTEX 2973 for Potential Utilization of Flue Gas. Bioresour. Technol 2021, 327, 124789. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Resendiz, L.; Sanchez-Garcia, L.; Hernandez-Martinez, I.; Vigueras-Ramirez, G.; Jimenez-Garcia, L.F.; Lara-Martinez, R.; Morales-Ibarria, M. Photoautotrophic Poly(3-Hydroxybutyrate) Production by a Wild-Type Synechococcus elongatus Isolated from an Extreme Environment. Bioresour. Technol. 2021, 337, 125508. [Google Scholar] [CrossRef]
- Lee, J.S.; Sung, Y.J.; Kim, D.H.; Lee, J.Y.; Sim, S.J. Development of a Limitless Scale-Up Photobioreactor for Highly Efficient Photosynthesis-Based Polyhydroxybutyrate (Phb)-Producing Cyanobacteria. Bioresour. Technol. 2022, 364, 128121. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Hsueh, H.T.; Chu, H. CO(2) Fixation and Cultivation of Thermosynechococcus sp. CL-1 for the Production of Phycocyanin. Bioresour. Technol. 2022, 364, 128105. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, J.; Luo, Y.; Kaczmarek, M.B.; Li, X.; Daroch, M. Thermosynechococcus as a Thermophilic Photosynthetic Microbial Cell Factory for CO(2) Utilisation. Bioresour. Technol. 2019, 278, 255–265. [Google Scholar] [CrossRef]
- Winayu, B.N.R.; Chang, Y.-L.; Hsueh, H.-T.; Chu, H. Simultaneous 17beta-Estradiol Degradation, Carbon Dioxide Fixation, and Carotenoid Accumulation by Thermosynechococcus sp. CL-1. Bioresour. Technol. 2022, 354, 127197. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, W.; Liu, W.; Wang, H.; Zhang, D.; Qiao, Z.; Jin, G.; Liu, T. Field Study on Attached Cultivation of Arthrospira (Spirulina) with Carbon Dioxide as Carbon Source. Bioresour. Technol. 2019, 283, 270–276. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, W.; Ameer Ali, K.; Ye, Q.; Jin, G.; Qiao, Z. Promoting Helix Pitch and Trichome Length to Improve Biomass Harvesting Efficiency and Carbon Dioxide Fixation Rate by Spirulina sp. in 660 m2 Raceway Ponds Under Purified Carbon Dioxide from a Coal Chemical Flue Gas. Bioresour. Technol. 2018, 261, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.; Vadlamani, A.; Richardson, W.D.L.; Strous, M. Pilot-Scale Outdoor Trial of a Cyanobacterial Consortium at pH 11 in a Photobioreactor at High Latitude. Bioresour. Technol. 2022, 354, 127173. [Google Scholar] [CrossRef]
- You, S.K.; Ko, Y.J.; Shin, S.K.; Hwang, D.H.; Kang, D.H.; Park, H.M.; Han, S.O. Enhanced CO(2) Fixation and Lipid Production of Chlorella vulgaris Through the Carbonic Anhydrase Complex. Bioresour. Technol. 2020, 318, 124072. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Liao, Q.; Zhu, X.; Xia, A.; Zhu, X.; Chang, J.S. Boosting Photo-Biochemical Conversion and Carbon Dioxide Bio-Fixation of Chlorella vulgaris in an Optimized Photobioreactor with Airfoil-Shaped Deflectors. Bioresour. Technol. 2021, 337, 125355. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Luo, J.; Tan, C.; Tian, X.; Su, P.; Gu, T. Carbon Dioxide Sequestration Accompanied by Bioenergy Generation Using a Bubbling-Type Photosynthetic Algae Microbial Fuel Cell. Bioresour. Technol. 2019, 280, 95–103. [Google Scholar] [CrossRef]
- Do, C.V.T.; Dinh, C.T.; Dang, M.T.; Tran, T.D.; Le, T.G. A Novel Flat-Panel Photobioreactor for Simultaneous Production of Lutein and Carbon Sequestration by Chlorella sorokiniana TH01. Bioresour. Technol. 2022, 345, 126552. [Google Scholar] [CrossRef]
- Vaz, B.D.S.; Mastrantonio, D.; Costa, J.A.V.; Morais, M.G. Green Alga Cultivation with Nanofibers as Physical Adsorbents of Carbon Dioxide: Evaluation of Gas Biofixation and Macromolecule Production. Bioresour. Technol. 2019, 287, 121406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, J.; Ye, Q.; Lai, X.; Zhang, X.; Zhou, J. Strengthening Mass Transfer of Carbon Dioxide Microbubbles Dissolver in a Horizontal Tubular Photo-Bioreactor for Improving Microalgae Growth. Bioresour. Technol. 2019, 277, 11–17. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Wang, J.; Zuo, J.; Peng, F.; Wu, J.; San, E. Carbon Dioxide Fixation Coupled with Ammonium Uptake by Immobilized Scenedesmus obliquus and Its Potential for Protein Production. Bioresour. Technol. 2019, 289, 121685. [Google Scholar] [CrossRef]
- Deprá, M.C.; Dias, R.R.; Severo, I.A.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. Carbon Dioxide Capture and Use in Photobioreactors: The Role of the Carbon Dioxide Loads in the Carbon Footprint. Bioresour. Technol. 2020, 314, 123745. [Google Scholar] [CrossRef]
- Sung, Y.J.; Sim, S.J. Multifaceted Strategies for Economic Production of Microalgae Haematococcus pluvialis-Derived Astaxanthin via Direct Conversion of CO2. Bioresour. Technol. 2022, 344, 126255. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Effendi, S.S.W.; Ng, I.-S. Enhanced Carbon Capture and Utilization (Ccu) using Heterologous Carbonic Anhydrase in Chlamydomonas reinhardtii for Lutein and Lipid Production. Bioresour. Technol. 2022, 351, 127009. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Eustance, E.; Shesh, T.; Frias, Z.; Rittmann, B.E. Achieving Superior Carbon Transfer Efficiency and pH Control Using Membrane Carbonation with a Wide Range of CO(2) Contents for the Coccolithophore Emiliania huxleyi. Sci. Total. Environ. 2022, 822, 153592. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Lo, E.; Philippidis, G.P. A Two-Prong Mutagenesis and Adaptive Evolution Strategy to Enhance the Temperature Tolerance and Productivity of Nannochloropsis oculata. Bioresour. Technol. 2022, 364, 128101. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.Y.; Wang, W.L.; Nan, F.H.; Lee, M.C. Enhanced Colaconema formosanum Biomass and Phycoerythrin Yield After Manipulating Inorganic Carbon, Irradiance, and Photoperiod. Bioresour. Technol. 2022, 352, 127073. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-Generation Biorefineries as the Means to Produce Fuels and Chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Singh, S.K.; Sundaram, S.; Sinha, S.; Rahman, M.A.; Kapur, S. Recent Advances in CO2 uptake and Fixation Mechanism of Cyanobacteria and Microalgae. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1297–1323. [Google Scholar] [CrossRef]
- Guo, Z. Evolutionary Escape from the Rubisco Activase Requirement. Ph.D Thesis, Nanyang Technological University, Singapore, 2019. [Google Scholar] [CrossRef]
- Taylor-Kearney, L.J.; Wang, R.Z.; Shih, P.M. Evolution and Origins of Rubisco. Curr. Biol. 2024, 34, R764–R767. [Google Scholar] [CrossRef]
- Lin, M.T.; Occhialini, A.; Andralojc, P.J.; Parry, M.A.; Hanson, M.R. A Faster Rubisco with Potential to Increase Photosynthesis in Crops. Nature 2014, 513, 547–550. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Song, W.; Du, X.; Yang, W. CO2 Gradient Domestication Produces Gene Mutation Centered on Cellular Light Response for Efficient Growth of Microalgae in 15% CO2 from Flue Gas. Chem. Eng. J. 2022, 429, 131968. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 Concentrating Mechanisms in Algae: Mechanisms, Environmental Modulation, and Evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ho, S.H. Microalgae as a Solution of Third World Energy Crisis for Biofuels Production from Wastewater Toward Carbon Neutrality: An Updated Review. Chemosphere 2022, 291, 132863. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Toyokawa, C.; Shimamura, D.; Matsuoka, T.; Fukuzawa, H. CO2-Dependent Migration and Relocation of LCIB, a Pyrenoid-Peripheral Protein in Chlamydomonas reinhardtii. Plant Physiol. 2021, 188, 1081–1094. [Google Scholar] [CrossRef]
- Keys, M.; Hopkinson, B.; Highfield, A.; Chrachri, A.; Brownlee, C.; Wheeler, G.L. The Requirement for External Carbonic Anhydrase in Diatoms Is Influenced by the Supply and Demand for Dissolved Inorganic Carbon. J. Phycol. 2023, 60, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Mackey, K.; Morris, J.J.; Morel, F.; Kranz, S. Response of Photosynthesis to Ocean Acidification. Oceanography 2015, 25, 74–91. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Ng, I.-S. Enhanced Carbon Capture, Lipid and Lutein Production in Chlamydomonas reinhardtii Under Meso-thermophilic Conditions Using Chaperone and CRISPRi System. Bioresour. Technol. 2023, 384, 129340. [Google Scholar] [CrossRef]
- Südfeld, C.; Kiyani, A.; Wefelmeier, K.; Wijffels, R.H.; Barbosa, M.J.; D’aDamo, S. Expression of Glycerol-3-Phosphate Acyltransferase Increases Non-Polar Lipid Accumulation in Nannochloropsis oceanica. Microb. Cell Factories 2023, 22, 12. [Google Scholar] [CrossRef]
- Mikalayeva, V.; Ceslevičienė, I.; Sarapinienė, I.; Žvikas, V.; Skeberdis, V.A.; Jakštas, V.; Bordel, S. Fatty Acid Synthesis and Degradation Interplay to Regulate the Oxidative Stress in Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1348. [Google Scholar] [CrossRef]
- Gomez, J.A.; Höffner, K.; Barton, P.I. Mathematical Modeling of a Raceway Pond System for Biofuels Production. Comput.-Aided Chem. Eng. 2016, 38, 2355–2360. [Google Scholar] [CrossRef]
- Testa, R.L.; Delpino, C.; Estrada, V.; Diaz, M.S. Metabolic Network Design of Synechocystis sp. Pcc 6803 to Obtain Bioethanol Under Autotrophic Conditions. Comput.-Aided Chem. Eng. 2017, 40, 2857–2862. [Google Scholar] [CrossRef]
- Wen, X.; Tao, H.; Peng, X.; Wang, Z.; Ding, Y.; Xu, Y.; Liang, L.; Du, K.; Zhang, A.; Liu, C.; et al. Sequential Phototrophic–Mixotrophic Cultivation of Oleaginous Microalga Graesiella sp. Wbg-1 in a 1000 m2 Open Raceway Pond. Biotechnol. Biofuels 2019, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Khazi, M.I.; Shi, L.; Liaqat, F.; Yang, Y.; Li, X.; Yang, D.; Li, J. Sequential Continuous Mixotrophic and Phototrophic Cultivation Might Be a Cost-Effective Strategy for Astaxanthin Production from the Microalga Haematococcus lacustris. Front. Bioeng. Biotechnol. 2021, 9, 740533. [Google Scholar] [CrossRef]
- de Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of Four Outdoor Pilot-Scale Photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Hydrothermal Kinetic Modeling for Microalgae Biomass Under Subcritical Condition Cultivated in a Close Bubble Tubular Photobioreactor. Fuel 2023, 334, 126585. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Cuaresma, M.; de Vree, J.; Ruiz-Domínguez, M.C.; García, M.C.M.; Uronen, P.; Barbosa, M.J.; Wijffels, R. Neochloris oleoabundans Oil Production in an Outdoor Tubular Photobioreactor at Pilot Scale. J. Appl. Phycol. 2021, 33, 1327–1339. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Z.; Wu, X.; Wen, S.; Yu, J.; Cong, W. Outdoor Cultivation of Chlorella sp. in an Improved Thin-Film Flat-Plate Photobioreactor in Desertification Areas. J. Biosci. Bioeng. 2020, 129, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Yaqoubnejad, P.; Rad, H.A.; Taghavijeloudar, M. Development a Novel Hexagonal Airlift Flat Plate Photobioreactor for the Improvement of Microalgae Growth That Simultaneously Enhance CO2 Bio-Fixation and Wastewater Treatment. J. Environ. Manag. 2021, 298, 113482. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Hsueh, H.T.; Li, T.Y.; Huang, L.C.; Chu, Y.L.; Tseng, C.M.; Chu, H. Effects of Light Availability on the Biomass Production, CO2 Fixation, and Bioethanol Production Potential of Thermosynechococcus CL-1. Bioresour. Technol. 2013, 145, 162–165. [Google Scholar] [CrossRef]
- Mahata, C.; Mishra, S.; Dhar, S.; Ray, S.; Mohanty, K.; Das, D. Utilization of Dark Fermentation Effluent for Algal Cultivation in a Modified Airlift Photobioreactor for Biomass and Biocrude Production. J. Environ. Manag. 2023, 330, 117121. [Google Scholar] [CrossRef]
- Tao, Q.; Gao, F.; Qian, C.-Y.; Guo, X.-Z.; Zheng, Z.; Yang, Z.-H. Enhanced Biomass/Biofuel Production and Nutrient Removal in an Algal Biofilm Airlift Photobioreactor. Algal Res. 2017, 21, 9–15. [Google Scholar] [CrossRef]
- Fan, F.; Wan, M.; Huang, J.; Wang, W.; Bai, W.; He, M.; Li, Y. Modeling of Astaxanthin Production in the Two-Stage Cultivation of Haematococcus pluvialis and Its Application on the Optimization of Vertical Multi-Column Airlift Photobioreactor. Algal Res. 2021, 58, 102301. [Google Scholar] [CrossRef]
- Rayen, F.; Behnam, T.; Dominique, P. Optimization of a Raceway Pond System for Wastewater Treatment: A Review. Crit. Rev. Biotechnol. 2019, 39, 422–435. [Google Scholar] [CrossRef]
- Barboza-Rodríguez, R.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Aguado, M.L.R.; Ruiz, H.A. Photobioreactor Configurations in Cultivating Microalgae Biomass for Biorefinery. Bioresour. Technol. 2024, 394, 130208. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Panagakis, P.; Chionidis, M.; Tzenos, D.; Aris, S.L.; Christos, G.T.; Kostas, B.; Anita, J. An Experimental Helical-Tubular Photobioreactor for Continuous Production of Nannochloropsis sp. Bioresour. Technol. 2010, 101, 6768–6777. [Google Scholar] [CrossRef]
- Abbas, A.; Marta, C.; Damian, A.; David, G.W.; Siegfried, E.V. Control Tools to Selectively Produce Purple Bacteria for Microbial Protein in Raceway Reactors. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Zhang, H.; Zhao, J.; Liu, H.; Wang, J.; Li, G.; Liang, H. Membrane-Based Technology in Water and Resources Recovery from the Perspective of Water Social Circulation: A Review. Sci. Total Environ. 2024, 908, 168277. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Katam, K.; Show, P.L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A Comprehensive Review on the Use of Algal-Bacterial Systems for Wastewater Treatment with Emphasis on Nutrient and Micropollutant Removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Yoon, Y.; Ngo, H.H.; Jang, A. The Application of Microalgae in Removing Organic Micropollutants in Wastewater. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1187–1220. [Google Scholar] [CrossRef]
- de Morais, E.G.; Sampaio, I.C.F.; Gonzalez-Flo, E.; Ferrer, I.; Uggetti, E.; García, J. Microalgae Harvesting for Wastewater Treatment and Resources Recovery: A Review. New Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, W.; Chen, W.; Wang, N. A Conceptual Structure for Heat Transfer Imitating the Transporting Principle of Plant Leaf. Int. J. Heat Mass Transf. 2014, 71, 79–90. [Google Scholar] [CrossRef]
- Huang, P.; Dong, G.; Zhong, X.; Pan, M. Numerical Investigation of the Fluid Flow and Heat Transfer Characteristics of Tree-Shaped Microchannel Heat Sink with Variable Cross-Section. Chem. Eng. Process. Process Intensif. 2020, 147, 107769. [Google Scholar] [CrossRef]
- Mink, A.; Schediwy, K.; Posten, C.; Nirschl, H.; Simonis, S.; Krause, M.J. Comprehensive Computational Model for Coupled Fluid Flow, Mass Transfer, and Light Supply in Tubular Photobioreactors Equipped with Glass Sponges. Energies 2022, 15, 7671. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Lim, J.S.M.; Chong, C.C.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Foo, H.C.Y.; Show, P.L.; Lim, S. Unravelling CO2 Capture Performance of Microalgae Cultivation and Other Technologies via Comparative Carbon Balance Analysis. J. Environ. Chem. Eng. 2021, 9, 106519. [Google Scholar] [CrossRef]
- Madiha, A.; Ani, I.; Attaullah, B.; Suzana, W. Intensity of Blue LED Light: A Potential Stimulus for Biomass and Lipid Content in Fresh Water Microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.-Y.; Lu, T. A Model for Autotrophic Growth of Chlorella vulgaris Under Photolimitation and Photoinhibition in Cylindrical Photobioreactor. Biochem. Eng. J. 2015, 99, 55–60. [Google Scholar] [CrossRef]
- Khoeyi, Z.A.; Seyfabadi, J.; Ramezanpour, Z. Effect of Light Intensity and Photoperiod on Biomass and Fatty Acid Composition of the Microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Markina, Z.V.; Zinov, A.A. Population Growth of the Microalga Tisochrysis lutea (Haptophyta) and the Contents of Carotenoids and Neutral Lipids at Different Illumination Levels in a Panel Bioreactor. Russ. J. Mar. Biol. 2025, 51, 33–40. [Google Scholar] [CrossRef]
- Vanags, J.; Kunga, L.; Dubencovs, K.; Galvanauskas, V.; Grīgs, O. Influence of Light Intensity and Temperature on Cultivation of Microalgae Desmodesmus Communis in Flasks and Laboratory-Scale Stirred Tank Photobioreactor. Latv. J. Phys. Tech. Sci. 2015, 52, 59–70. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, Y.; Zhao, Y. Biocapture of CO2 by Different Microalgal-Based Technologies for Biogas Upgrading and Simultaneous Biogas Slurry Purification under Various Light Intensities and Photoperiods. Int. J. Environ. Res. Public Health 2018, 15, 528. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, H.; Zhang, Y.; Yan, C.; Zhao, Y. Purifying Synthetic High-Strength Wastewater by Microalgae Chlorella vulgaris under Various Light Emitting Diode Wavelengths and Intensities. J. Environ. Health Sci. Eng. 2013, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Flügel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The Photorespiratory Metabolite 2-Phosphoglycolate Regulates Photosynthesis and Starch Accumulation in Arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef]
- Levey, M.; Timm, S.; Mettler-Altmann, T.; Borghi, G.L.; Koczor, M.; Arrivault, S.; Weber, A.P.; Bauwe, H.; Gowik, U.; Westhoff, P. Efficient 2-Phosphoglycolate Degradation Is Required to Maintain Carbon Assimilation and Allocation in the C4 plant Flaveria bidentis. J. Exp. Bot. 2018, 70, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Usman, P. Effect of Light Intensity and Photoperiod on Growth of Chlorella pyrenoidosa and CO2 Biofixation. Zenodo 2018, 31, 03003. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light Requirements in Microalgal Photobioreactors: An Overview of Biophotonic Aspects. Appl. Microbiol. Biotechnol. 2010, 89, 1275–1288. [Google Scholar] [CrossRef]
- Vejrazka, C.; Janssen, M.; Streefland, M.; Wijffels, R.H. Photosynthetic Efficiency of Chlamydomonas reinhardtii in Flashing Light. Biotechnol. Bioeng. 2011, 108, 2905–2913. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Politaeva, N.; Ilin, I.; Velmozhina, K.; Shinkevich, P. Carbon Dioxide Utilization Using Chlorella Microalgae. Environments 2023, 10, 109. [Google Scholar] [CrossRef]
- Kim, B.; Praveenkumar, R.; Choi, E.; Lee, K.; Jeon, S.G.; Oh, Y.-K. Prospecting for Oleaginous and Robust Chlorella spp. for Coal-Fired Flue-Gas-Mediated Biodiesel Production. Energies 2018, 11, 2026. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vakulenko, G.; Smith, D.R.; Zhang, X.; Hüner, N.P.A. Temperature Stress in Psychrophilic Green Microalgae: Minireview. Physiol. Plant. 2022, 174, e13811. [Google Scholar] [CrossRef]
- Meeks, J.C.; Castenholz, R.W. Growth and Photosynthesis in an Extreme Thermophile, Synechococcus lividus (Cyanophyta). Arch. Microbiol. 1971, 78, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Yan, J.; Ming, C.; Bai, X.; Shi, H.-m. Scale Deposition Mechanism and Low-Damage Underbalanced Drilling Fluids for High CO2 Content Gas Reservoirs. Pet. Explor. Dev. 2011, 38, 750–755. [Google Scholar] [CrossRef]
- Li, M.; Young, J.N. Temperature Sensitivity of Carbon Concentrating Mechanisms in the Diatom Phaeodactylum tricornutum. Photosynth. Res. 2023, 156, 205–215. [Google Scholar] [CrossRef]
- Yue, L.; Chen, W. Isolation and Determination of Cultural Characteristics of a New Highly CO2 Tolerant Fresh Water Microalgae. Energy Convers. Manag. 2005, 46, 1868–1876. [Google Scholar] [CrossRef]
- Mehlitz, T. Temperature Influence and Heat Management Requirements of Microalgae Cultivation in Photobioreactors. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2016. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Zhao, G.; Xiong, P. Optimization Strategies for CO2 Biological Fixation. Biotechnol. Adv. 2024, 73, 108364. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, I.; Solanki, M.K.; Roychowdhury, R.; Kumar, A.; Gupta, R.K. Advancement of Abiotic Stresses for Microalgal Lipid Production and Its Bioprospecting into Sustainable Biofuels. Sustainability 2023, 15, 13678. [Google Scholar] [CrossRef]
- Qiu, R.; Gao, S.; Lopez, P.A.; Ogden, K.L. Effects of pH on Cell Growth, Lipid Production and CO2 Addition of Microalgae Chlorella sorokiniana. Algal Res. 2017, 28, 192–199. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y.; Zeng, R.J. Enhancement of Acetate Productivity in a Thermophilic (55 °C) Hollow-Fiber Membrane Biofilm Reactor with Mixed Culture Syngas (H2/CO2) Fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627. [Google Scholar] [CrossRef]
- Ma, Z.; Cheah, W.Y.; Ng, I.S.; Chang, J.S.; Zhao, M.; Show, P.L. Microalgae-Based Biotechnological Sequestration of Carbon Dioxide for Net Zero Emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef]
- Chang, M.; Liu, K. Arthrospira platensis as Future Food: A review on Functional Ingredients, Bioactivities and Application in the Food Industry. Int. J. Food Sci. Technol. 2023, 59, 1197–1212. [Google Scholar] [CrossRef]
- Sharma, P.K.; Lakhawat, S.S.; Malik, N.; Kumar, V.; Kumar, S. Implications of Crispr-Cas9 in Developing Next Generation Biofuel: A Mini-Review. Curr. Protein Pept. Sci. 2022, 23, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-R.; Jang, J.; Jin, E. Genome Engineering via Gene Editing Technologies in Microalgae. Bioresour. Technol. 2023, 373, 128701. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.A.; Onyeaka, H.; Miri, T.; Soltani, F. Chlorella vulgaris as a Food Substitute: Applications and Benefits in the Food Industry. J. Food Sci. 2024, 89, 8231–8247. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total. Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-Based Biorefineries for Sustainable Resource Recovery from Wastewater. J. Water Process. Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.J.; Moon, M.; Lee, J.S.; Min, K. Recent Progress and Challenges in Microbial Polyhydroxybutyrate (PHB) Production from CO2 as a Sustainable Feedstock: A State-of-the-Art Review. Bioresour. Technol. 2021, 339, 125616. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-Based Wastewater Treatment for Nutrients Recovery: A Review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-Bacteria Consortium for Wastewater Treatment and Biomass Production. Sci. Total. Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
- You, X.; Yang, L.; Zhou, X.; Zhang, Y. Sustainability and Carbon Neutrality Trends for Microalgae-Based Wastewater Treatment: A Review. Environ. Res. 2022, 209, 112860. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; Junior, R.G.d.O.; Fuentes-Grünewald, C.; Picot, L. Analysis of Scientific Research Driving Microalgae Market Opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wen, J.; Wang, C.; Gomari, S.R.; Xu, X.; Zheng, S.; Su, Y.; Li, L.; Hao, Y.; Li, D. Current Status and Development Trends of CO2 Storage with Enhanced Natural Gas Recovery (Cs-Egr). Fuel 2023, 349, 128555. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Kumar, S.P.J.; Kumar, N.S.S. Metabolic Engineering and Genome Editing Strategies for Enhanced Lipid Production in Microalgae. Biocell 2024, 48, 1181–1195. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.R.; Huang, C.-H.; Sung, L.-Y.; Wu, M.-Y.; Hu, Y.-C. Crispr-Cas9 for the Genome Engineering of Cyanobacteria and Succinate Production. Metab. Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Hu, J.; Meng, W.; Su, Y.; Qian, C.; Fu, W. Emerging Technologies for Advancing Microalgal Photosynthesis and Metabolism Toward Sustainable Production. Front. Mar. Sci. 2023, 10, 1260709. [Google Scholar] [CrossRef]
- Liang, W.; Wei, L.; Wang, Q.; You, W.; Poetsch, A.; Du, X.; Lv, N.; Xu, J. Knocking out Chloroplastic Aldolases/Rubisco Lysine Methyltransferase Enhances Biomass Accumulation in Nannochloropsis oceanica under High-Light Stress. Int. J. Mol. Sci. 2024, 25, 3756. [Google Scholar] [CrossRef]
- Badr, M.M.; Amany, A.M. Design and Evaluation of a Helical-Tubular Photobioreactor for Microalgae Production. Misr J. Agric. Eng. 2019, 36, 1265–1284. [Google Scholar] [CrossRef]
- Helleckes, L.M.; Hemmerich, J.; Wiechert, W.; von Lieres, E.; Grünberger, A. Machine Learning in Bioprocess Development: From Promise to Practice. Trends Biotechnol. 2023, 41, 817–835. [Google Scholar] [CrossRef]
- Cheng, F.; Xie, W.; Zheng, H. Digital Twin Calibration for Biological System-of-Systems: Cell Culture. arXiv 2024, arXiv:2405.03913. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Gabel, B.V.; Sinetova, M.A.; Gabrielian, A.K.; Markelova, A.G.; Shcherbakova, N.V.; Los, D.A. Optimization of CO2 Supply for the Intensive Cultivation of Chlorella sorokiniana IPPAS C-1 in the Laboratory and Pilot-Scale Flat-Panel Photobioreactors. Life 2022, 12, 1469. [Google Scholar] [CrossRef] [PubMed]
- del Rio-Chanona, E.A.; Wagner, J.L.; Ali, H.; Fiorelli, F.; Zhang, D.; Hellgardt, K. Deep Learning-Based Surrogate Modeling and Optimization for Microalgal Biofuel Production and Photobioreactor Design. AIChE J. 2018, 65, 915–923. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of Microalgal Cultures: A Major Bottleneck to Algae-Based Fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Okoro, V.; Azimov, U.; Munoz, J.; Hernandez, H.H.; Phan, A.N. Microalgae Cultivation and Harvesting: Growth Performance and Use of Flocculants-A Review. Renew. Sustain. Energy Rev. 2019, 115, 109364. [Google Scholar] [CrossRef]
- Zhu, J.; Wakisaka, M.; Omura, T.; Yang, Z.; Yin, Y.; Fang, W. Advances in Industrial Harvesting Techniques for Edible Microalgae: Recent Insights into Sustainable, Efficient Methods and Future Directions. J. Clean. Prod. 2024, 436, 140626. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Shah, S.M.U.; Shanab, S.M.M.; Ali, H.E.A. Integrated Algal Bioprocess Engineering for Enhanced Productivityof Lipid, Carbohydrate and High-Value Bioactive Compounds. Res. Rev. J. Microbiol. Biotechnol. 2017, 6, 61–92. [Google Scholar]
- Liu, S.; Hajar, H.A.A.; Riefler, G.; Stuart, B.J. Investigation of Electrolytic Flocculation for Microalga Scenedesmus sp. Using Aluminum and Graphite Electrodes. RSC Adv. 2018, 8, 38808–38817. [Google Scholar] [CrossRef]
- bin Azmi, A.A.; Sankaran, R.; Show, P.L.; Ling, T.C.; Tao, Y.; Munawaroh, H.S.H.; Kong, P.S.; Lee, D.-J.; Chang, J.-S. Current Application of Electrical Pre-Treatment for Enhanced Microalgal Biomolecules Extraction. Bioresour. Technol. 2020, 302, 122874. [Google Scholar] [CrossRef]
- Kim, D.; Kang, S.-G.; Chang, Y.K.; Kwak, M. Two-Step Macromolecule Separation Process with Acid Pretreatment and High-Shear-Assisted Extraction for Microalgae-Based Biorefinery. Sustainability 2024, 16, 7589. [Google Scholar] [CrossRef]

| Types of PBR | Microalgal Species | Production | Strengths | Limitations | References | |
|---|---|---|---|---|---|---|
| Open | Raceway pond | Graesiella sp. WBG-1 | 12.5 g/(m2∙d) | Simple technology, low investment, large-scale cultivation | Large footprint, unstable culture conditions, susceptible to contamination | [74] |
| Haematococcus lacustris | 2.2% DW | [75] | ||||
| Nannochloropsis salina | 24.5 g/(L∙d) | [76] | ||||
| Closed | Tubular | Spirulina platensis LEB-5 | 0.86 g/L | High light surface area to volume ratio, easy amplification | Dissolved oxygen accumulation, easy to stick to the wall | [77] |
| Neochloris oleoabundans | 7.4 g/(dw2∙d) | [78] | ||||
| Flat panel | Chlorella sp. | 49.79 g/(m2∙d) | Large illumination area, simple structure, easy to clean, easy to operate | Short optical range, difficult to amplify, some degree of wall attachment | [79] | |
| Chlorella sorokinina Pa.91 | 0.85 g/(L∙d) | [80] | ||||
| Thermosynechococcus CL–1 | 1.61 g/(L∙d) | [81] | ||||
| Airlift | Chlorella sorokiniana | 0.83 g/(L∙d) | Compact structure, high mass transfer efficiency, no bacterial contamination, algae growth is fast | High cost of construction, large operation and maintenance costs, complex operation | [82] | |
| Chlorella sorokiniana | 15.93 g/(L∙d) | [83] | ||||
| Haematococcus pluvialis | 0.56 g/(L∙d) | [84] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Ye, K.; Zheng, X.; Zhao, L. Microalgal Valorization of CO2: A Sustainable Pathway to Biofuels and High-Value Chemicals. Fermentation 2025, 11, 371. https://doi.org/10.3390/fermentation11070371
Wu S, Ye K, Zheng X, Zhao L. Microalgal Valorization of CO2: A Sustainable Pathway to Biofuels and High-Value Chemicals. Fermentation. 2025; 11(7):371. https://doi.org/10.3390/fermentation11070371
Chicago/Turabian StyleWu, Shutong, Kaiyin Ye, Xiaochuan Zheng, and Lei Zhao. 2025. "Microalgal Valorization of CO2: A Sustainable Pathway to Biofuels and High-Value Chemicals" Fermentation 11, no. 7: 371. https://doi.org/10.3390/fermentation11070371
APA StyleWu, S., Ye, K., Zheng, X., & Zhao, L. (2025). Microalgal Valorization of CO2: A Sustainable Pathway to Biofuels and High-Value Chemicals. Fermentation, 11(7), 371. https://doi.org/10.3390/fermentation11070371






