Abstract
Anaerobic fermentation has been recognized as an effective approach to harness livestock manure resources. In the present study, cow dung and pig manure were employed as fermentation substrates. These were subjected to co-fermentation experiments with sodium hydroxide-pretreated corn straw. Additionally, biochar derived from artificial wetland substrate was introduced as a conditioner to investigate the impact of varying addition quantities on the pretreated anaerobic co-fermentation process. Our findings indicate that for the anaerobic co-fermentation of cow dung, an optimal addition of 4% biochar reduced the gas production cycle by 35–45%, although the total methane yield remained relatively unchanged. Conversely, in the anaerobic co-fermentation of pig manure, a 6% biochar addition proved most effective. This adjustment, while not significantly altering the gas production cycle, led to a marked increase in the total methane content, ranging from 18.53% to 150.18%. The PCA analysis results of the cow manure experimental group showed a significant positive correlation between the addition of biochar and ammonia nitrogen. For the pig manure fermentation system, the addition of biochar can increase the final methane production potential, from 47.43 mL/g VS to 122.24 mL/g VS in the P2C experimental group. Biochar mainly regulates the activity of anaerobic bacteria through changes in pH and conductivity, thereby affecting methane production.
1. Introduction
In recent years, there has been a significant increase in global greenhouse gas emissions, leading to considerable instability in the global climate. The excessive utilization of fossil fuels is the main cause of global warming and climate change. Bioenergy, a type of renewable energy, can be derived from animal manure, a byproduct of the livestock industry [1]. Notably, the livestock industry is also a substantial contributor to greenhouse gas production, emitting gases such as CO2, CH4, and N2O. Despite its economic benefits, it accounts for 27%, 29%, and 44% of global greenhouse gas emissions, respectively [2]. The management of livestock manure, an agricultural waste product, is a global challenge due to its large volume and the difficulty of treatment [3]. China, a significant agricultural and livestock producer, generates about 3.8 billion tons of livestock and poultry manure annually. However, the comprehensive utilization rate of this manure is less than 60%, indicating a severe underutilization [4]. The excessive accumulation of manure can lead to soil pollution, the release of unpleasant odors into the atmosphere, the spread of diseases, and potential contamination of groundwater through rainwater runoff, resulting in serious environmental pollution [5,6].
Anaerobic digestion is an efficacious biochemical process that converts organic solid waste and wastewater into energy and valuable by-products. This process plays a crucial role in resource recovery technologies that support the circular economy [7]. Over recent decades, anaerobic digestion has achieved widespread commercialization globally [8]. The biogas generated during this process serves as a viable source of heat and power [8], while the post-digestion residue, enriched with nutrients, can be safely utilized as a soil conditioner. This application significantly enhances soil fertility along with its physical and chemical properties [9]. However, the utilization of this process for manure fermentation alone is not feasible due to ammonia inhibition. This inhibition is a significant challenge in the anaerobic digestion of nitrogen-rich substrates, such as animal manures. The proteins and nitrogenous compounds present in these substrates generate ammonia. Elevated concentrations of ammonia, particularly in the form of NH3, tend to disrupt cellular functions, thereby impeding methanogenic activity [10]. To regulate the carbon-to-nitrogen ratio in the co-anaerobic digestion (Co-AD) of animal manure, agricultural straws, such as corn straw, rice straw, and wheat straw, are often incorporated as co-substrates. However, the lignin content in straw resists degradation into soluble substances, negatively impacting methane production efficiency. Therefore, the substrate may require appropriate pretreatment, such as acid addition, to boost the biodegradability of complex materials [11].
Carbon-based additives have been empirically demonstrated to enhance the efficiency of anaerobic fermentation, thereby increasing biogas production. This method boasts the advantages of widespread availability and cost-effective implementation [12]. A substantial number of -OH and -COOH functional groups on the biochar surface render it alkaline. When integrated into an anaerobic fermentation system, biochar elevates the system’s pH, mitigating acid accumulation during the acid production phase and subsequently bolstering methane production efficiency [13]. Furthermore, biochar possesses nitrogen-fixing capabilities, facilitating organic matter conversion and reducing greenhouse gas emissions during composting [14]. In co-composting barley straw and chicken manure, greenhouse gas emissions have been observed to decrease by 27–32% [15,16]. Shen et al. employed corn straw biochar in the anaerobic fermentation of sludge, achieving a CO2 removal rate of 86.3% and average increases of 42.4% and 7.0% in methane content and generation rate, respectively [17]. Biochar can influence shifts in microbial communities [18], and Luz et al. posited that biochar with a high specific surface area can more effectively interact with adjacent species, enhancing anaerobic fermentation [19].
Interspecies electron transfer between co-digesting bacteria and methanogenic bacteria is crucial for enhancing anaerobic fermentation performance. Methane generation is notably influenced by this interspecies electron transfer, facilitated by hydrogen and formate. However, recent research has demonstrated that a direct interspecies electron transfer, achieved via conductive materials, nanowires, and shuttle molecules, is more energy-efficient [20]. This is primarily because it operates independently of various enzymatic reactions, employing hydrogen and formate directly as electron carriers. Biochar is postulated to foster direct interspecies electron transfer across a range of materials, including kitchen waste, activated sludge, ethanol, and cow dung [20]. However, the precise mechanism through which biochar enhances anaerobic fermentation remains unelucidated, and the existence of additional related processes beyond interspecies electron transfer is uncertain. Furthermore, most existing studies concentrate solely on a singular method of improvement, despite the potential for multiple, non-conflicting approaches to enhance anaerobic fermentation. Therefore, investigating the effects of biochar in conjunction with pretreatment technologies on anaerobic fermentation is of paramount importance.
This study employs cow dung and pig manure as raw materials for anaerobic fermentation, constructing a co-fermentation system utilizing pretreated corn straw. The main purpose of this study is to investigate the promoting effect of adding biochar on the anaerobic fermentation process of cow manure and pig manure. Specifically, the objectives include the following: to (1) investigate how biochar can improve the gas production performance of anaerobic fermentation of livestock and poultry manure; (2) evaluate the impact of different amounts of biochar on the improvement of anaerobic fermentation gas production efficiency of livestock and poultry manure; and (3) elucidate the underlying mechanisms by which biochar enhances livestock manure anaerobic fermentation throughout the entire fermentation cycle.
2. Materials and Methods
2.1. Test Materials and Properties
The cow dung and pig manure utilized in this study were sourced from a farm in Jilin Province to ensure uniformity in the experimental raw materials. Similarly, the straw was procured from yellow storage straw grown in a field within the same province, maintaining homogeneity in the experimental conditions (the basic properties of the material are shown in Table 1). Biochar was manufactured by combusting corn straw under anaerobic conditions at 450 °C; the properties of biochar are shown in Table 2 [21]. Subsequently, it underwent water treatment via an artificial wetland system, after which it was recovered, dried, and ground, thus guaranteeing its quality and stability. The diatomite employed in the experiment was powdered secondary diatomite, sourced from a reputable diatomite functional material company, ensuring its provenance’s reliability. Furthermore, the biogas slurry used was obtained from cow manure biogas slurry generated through long-term anaerobic fermentation in the laboratory. It exhibited a stable pH of 7.03 and a total solid content of 1.01% [22], offering a dependable foundation for the experiment.

Table 1.
Basic properties of digestion raw materials.

Table 2.
Properties apparent density.
2.2. Experimental Design
In this experiment, both cattle and pig manure were utilized as raw materials for anaerobic fermentation, alongside a co-fermentation process with corn straw. The fermentation procedure was optimized by adding a suitable quantity of corn straw, determined by the C/N ratio of cattle manure, pig manure, and corn straw, to establish an initial C/N ratio of 35 for the cattle manure reactor and 28 for the pig manure reactor [23]. Subsequently, biochar amounting to 0%, 2%, 4%, and 8% of the total solids content (based on TS) was incorporated into each reactor, with the specific groupings detailed in Table 3 and Table 4. Three replicates were established for each experimental group. In order to treat corn straw, we employed a sodium hydroxide solution with a concentration of 0.5 mol/L and a pH exceeding 13, applying it for a duration of 24 h. Subsequent to this treatment, the corn straw was cleansed with tap water until the pH value reached 7. Thereafter, the treated straw was placed into a reactor and subjected to ultrasound for an additional 5 min to further stimulate the reaction.

Table 3.
Experimental material usage.

Table 4.
The preparation of materials in each group.
All materials prepared for anaerobic fermentation were subsequently placed into the reactors depicted in Figure 1, with tap water added to ensure the total solid content of the system remained at 8%. The initial C/N ratios for the cow dung and pig manure groups were 35 and 28, respectively. The reactors were then sealed and placed in a thermostatic water bath pot, with a starting temperature of 35 ± 1 °C. Three days post-sealing, approximately 10% of the total volume of biogas slurry was introduced into the reactor as an inoculum to facilitate the anaerobic fermentation process. The time when the biogas slurry is added is set as the start time of the experiment, and the entire experimental period is the production period of the experimental device.
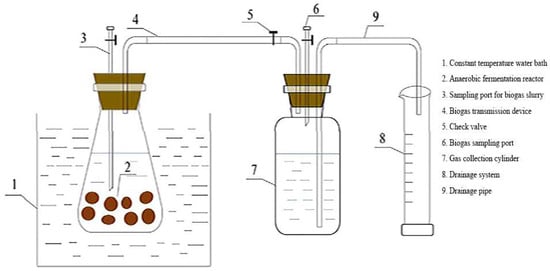
Figure 1.
Anaerobic digestion reactor.
Throughout the experiment, gas production was gauged daily at noon, with slurry samples collected triannually for the detection of NH3-N, volatile fatty acids (VFAs), pH, electric conductivity (EC), and other parameters. Concurrently, biogas samples were harvested every three days to ascertain the methane content, thereby facilitating the calculation of methane production. A modified Gompertz model was employed to fit the methane production data, enabling a deeper exploration into the gas production performance during anaerobic fermentation. Subsequently, a principal component analysis (PCA) of physicochemical indices and methane yield was executed to elucidate the impact and potential mechanisms through which additives influence the gas production performance in anaerobic fermentation.
2.3. Analytical Methods
Before the initiation of the experiment, a comprehensive measurement of the total organic carbon, total nitrogen, total solids content, and volatile solids content was conducted for each reactor to provide baseline data for future analysis. Throughout the experimental process, meticulous monitoring was performed on several parameters, including biogas yield, methane content, pH value, electrical conductivity, and variations in ammonia nitrogen and VFAs. Subsequent to the experiment, additional measurements were taken to determine the total phosphorus content as well as the various forms of heavy metals, specifically copper and zinc, present in the biofertilizer.
In the conducted experiments, the pH, EC, TOC, TN, and TP contents were all ascertained utilizing the national standard method of Organic Fertilizer (NY525-2012) [24]. The specific methodologies for determining VFAs, ammonia nitrogen, and TP were adopted from Wang et al. [25].
Every day at noon, the liquid volume in the measuring cylinder and the ambient temperature should be recorded precisely to ensure data accuracy. Concurrently, the reactor should be shaken for one minute in the morning, afternoon, and evening to ensure thorough mixture of the reactants. Gas samples should be collected using an air collection bag, with the methane content immediately determined using a portable methane detector. All relevant data should be promptly recorded.
Throughout the anaerobic digestion process, samples were collected at three-day intervals. On each occasion, a precise 10 mL of biogas slurry was extracted using a three-way valve. The pH value and conductivity of the sample were then measured, following the addition of an appropriate quantity of distilled water, in accordance with the total solid content of the extracted biogas slurry. Subsequent to centrifugation, the supernatant was analyzed for TP, NH3-N, and VFAs [25]. The completion of the anaerobic fermentation process was determined when gas production fell below 10% of its peak value.
2.4. Statistical Analysis
In the anaerobic fermentation system, three parallel samples were collected during sampling. The final measurement results were averaged, and data statistics were conducted using Microsoft Excel 2016. To simulate the trend of total methane production over time, the modified Gompertz model [26] was employed, with simulations carried out in Origin 9.0 software. The modified Gompertz model is represented in Equation (1). Furthermore, principal component analysis was executed using Canoco 5 software to delve into the impact of various factors on the gas production performance of anaerobic fermentation and its underlying mechanisms.
In the equation provided, the following symbols and units are used:
- -
- (P) represents the cumulative methane yield and has the unit of mL/g (in terms of VS);
- -
- (P0) denotes the final methane potential with the unit of mL/g (in terms of VS);
- -
- (Rm) signifies the maximum methane production rate, measured in mL/(g·d) (in terms of VS);
- -
- () refers to the retention time and is expressed in days (d);
- -
- (e) is the mathematical constant 2.718282.
3. Results
3.1. The Influence of Biochar Supplementation on Gas Production
As illustrated in Figure 2, significant fluctuations were observed in the daily gas production of cow manure fermentation groups upon the addition of biochar. Notably, the maximum daily gas production was recorded on the fifth day in the C8C group, registering at 17.48 mL/g VS. When examining the duration of the gas production cycle, it was found that the anaerobic fermentation cycle was notably shortened post-biochar addition. The control group, which did not receive biochar, completed its anaerobic fermentation by the 40th day post-initiation. However, for the experimental groups receiving biochar, the fermentation process concluded on the 22nd day for both the C6C and C8C groups, while the C2C group completed its cycle on the 26th day. This underscores that biochar supplementation considerably reduces the fermentation duration. Furthermore, in terms of daily gas production, biochar supplementation appears to amplify the peak daily gas output. Specifically, the C2C group reached its peak on the third day, the C6C group (with 6% biochar) achieved its apex on the fourth day, and the C8C group peaked on the fifth day. In contrast, the control CCK group only attained its maximum gas output on the seventh day.
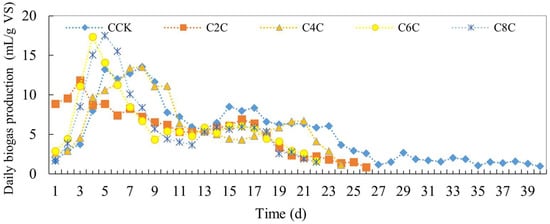
Figure 2.
Daily biogas production of anaerobic co-digestion of cow dung by adding biochar.
In examining the total biogas production curve for the cow dung experimental group (Figure 3), it is evident that during the initial 9 days, all experimental groups exhibited higher production than the CCK control group. This can be attributed to the inclusion of biochar, which enhanced the efficiency of anaerobic fermentation, subsequently leading to a minor decrease in overall gas production. Notably, post the 23rd day, the total gas production for all experimental groups was observed to be lower than that of the CCK control group. Nevertheless, it is worth noting that the gas production in this study’s experimental groups surpassed the 112.5 mL/g VS recorded for cow dung alone digestion as documented by Xu et al. [27].
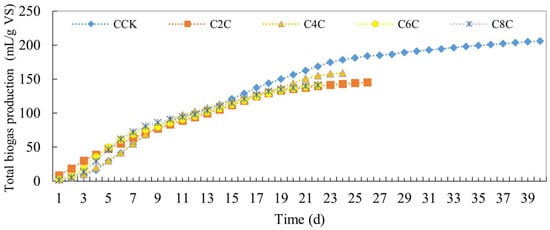
Figure 3.
Total biogas production of anaerobic co-digestion of cow dung by adding biochar.
The daily gas production from pig manure fermentation augmented with biochar is illustrated in Figure 4. Notably, the P6C experimental group exhibited the highest daily gas production, reaching up to 21.91 mL/g VS, and this group also reached its peak gas production the most rapidly. This suggests that the addition of biochar can enhance gas production and stabilize the process. However, the impact of biochar on the duration of the gas production cycle was not significant. The reaction in the control group concluded on the 35th day, while the fermentation cycle for the groups with added biochar ranged from 30 to 39 days. Among these, the reaction speed was fastest with 6% biochar added, concluding on the 30th day.
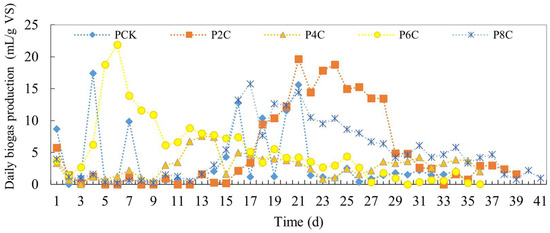
Figure 4.
Daily biogas production of anaerobic co-digestion of pig manure by adding biochar.
Figure 5 illustrates the total biogas yield derived from pig manure fermentation when supplemented with biochar. Notably, the P4C experimental group demonstrated a marginally lower total gas production compared to the control group, achieving a biogas yield of 112.33 mL/g VS. In contrast, the other experimental groups that incorporated biochar exhibited an upsurge in gas production. This was particularly pronounced in the group where 6% biochar was added, achieving a gas yield of 188.74 mL/g VS. This yield was notably superior to the 119.01 mL/g VS recorded by the control group.
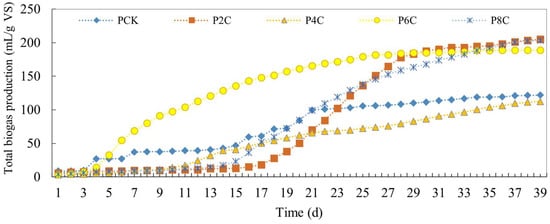
Figure 5.
Total biogas production of anaerobic co-digestion of pig manure by adding biochar.
3.2. The Effect of Biochar Addition on Biogas Composition
In the co-fermentation experiment of cattle manure and corn straw under anaerobic conditions, significant differences were observed in the gas production results of each experimental group with biochar addition (refer to Table 5 for specifics). Remarkably, biochar not only enhanced the methane content in biogas but also significantly reduced the fermentation reaction time, thereby boosting fermentation efficiency. Notably, the C4C experimental group achieved the highest gas production rate among all additive groups, peaking in both initial biogas volume and methane content. However, as fermentation progressed, this group exhibited a slight decline in final biogas content and methane yield. This trend indicates that while using biochar to optimize anaerobic fermentation, it is imperative to consider its effects on fermentation kinetics and end product distribution for a holistic enhancement in fermentation performance.

Table 5.
Methane production of anaerobic co-digestion of cow manure.
The daily methane yield following the addition of cow manure is illustrated in Figure 6. Upon introducing biochar into the anaerobic fermentation system of cow manure, there is a rapid surge to the peak of gas production. Notably, the methane yield surpasses that of the control group significantly. This suggests that biochar addition effectively enhances the growth and reproduction of methanogenic bacteria during the anaerobic co-fermentation of pretreated cow manure.
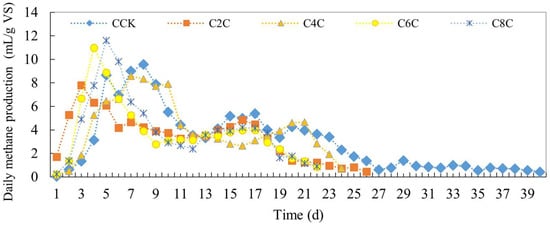
Figure 6.
Daily methane production of anaerobic co-digestion of cow manure by adding biochar.
The impact of added cow manure on total methane production is illustrated in Figure 7. The anaerobic fermentation system for cow manure rapidly peaked in gas production upon the addition of biochar, with the yield of methane surpassing that of the control group—a trend that mirrors the total biogas production. In the initial phase, total methane production from the experimental group with biochar was consistently higher than the control group. However, from day 15 onwards, the total methane production from all experimental groups fell below that of the control group. While biochar can enhance methane production by anaerobic bacteria, it does not improve total methane production, instead causing a slight decrease.
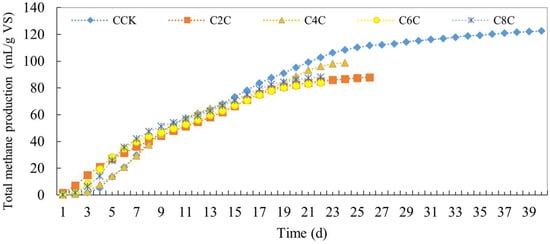
Figure 7.
Total methane production of anaerobic co-digestion of cow manure by adding biochar.
The data from the anaerobic co-fermentation experiment of pig manure and corn straw, with the addition of biochar, are presented in Table 6. It is evident that the inclusion of biochar substantially enhanced the production of methane, resulting in an increase ranging from 18.25% to 150.15%, depending on the specific conditions. Importantly, the fermentation cycle remained largely unaffected by the biochar addition. Notably, the experimental group that received a 6% biochar supplementation yielded the highest methane content, constituting 60% of the total biogas volume.

Table 6.
Effect of co-digestion of pig manure addition biochar.
The alterations in methane percentage within the pig manure experimental group are depicted in Figure 8. During the initial 9 days, the group with added biochar exhibited a slightly elevated methane percentage compared to the control group. Specifically, the addition of 6% biochar led to the quickest onset of methane production, while 8% biochar caused a slight delay in reaching the peak gas production. Overall, the methane percentage increased in each group that received biochar.
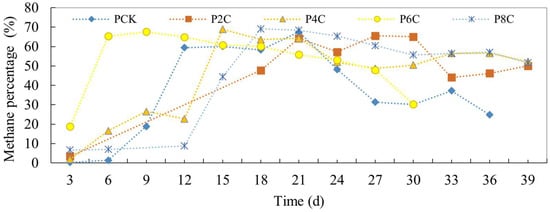
Figure 8.
Daily methane percentage of anaerobic co-digestion of pig manure by adding biochar.
After the addition of biochar, the daily methane production from pig manure anaerobic co-fermentation is shown in Figure 9. As can be seen from the figure, the group with 6% biochar addition rapidly produced methane, reaching its first gas production peak on the sixth day, with a methane yield of 14.31 mL/g VS. The control group produced less methane, and the yield dropped sharply after the balance was disrupted. However, the biochar addition group had stable gas production and continued to produce methane for a long time after reaching the peak.
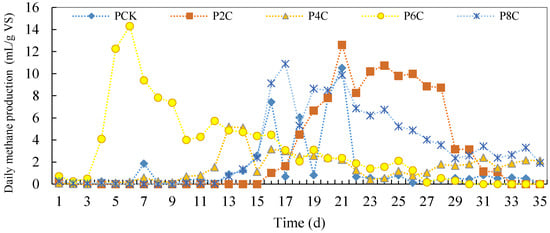
Figure 9.
Daily methane production of anaerobic co-digestion of pig manure by adding biochar.
The daily methane production of the pig manure biochar group is shown in Figure 10. The figure indicates that, after adding biochar, the total methane production of each experimental group exceeded that of the control group. The P6C experimental group quickly produced methane and its total output exceeding that of the control group. The P8C experimental group, which produced the most methane, reached 119.74 mL/g VS, while the fastest gas-producing P6C experimental group was 111.25 mL/g VS, far exceeding the 47.86 mL/g VS of the control group.
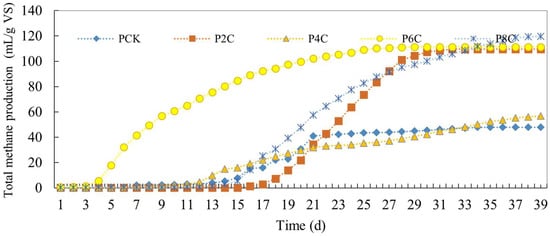
Figure 10.
Total methane production of anaerobic co-digestion of pig manure by adding biochar.
3.3. The Effect of Biochar Addition on Physicochemical Indicators
The impact of biochar addition on the anaerobic co-digestion of pig and cow manure with corn straw is illustrated in Figure 11 and Figure 12, respectively.
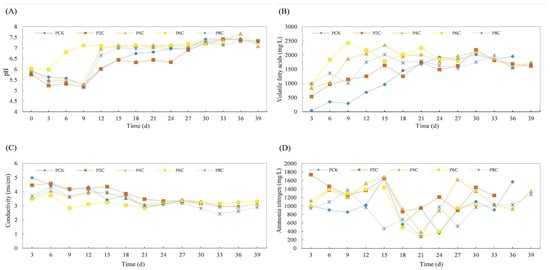
Figure 11.
Effect of physicochemical properties of anaerobic co-digestion of pig manure by adding biochar ((A): pH, (B): VFAs, (C): EC, and (D): ammonia nitrogen).
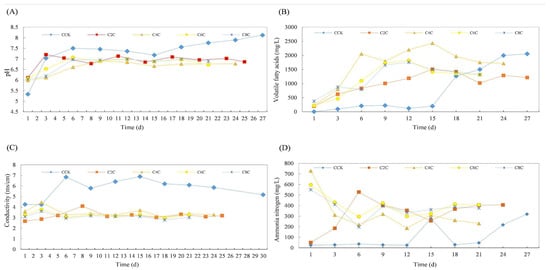
Figure 12.
Effect of physicochemical properties of anaerobic co-digestion of cow dung by adding biochar ((A): pH, (B): VFAs, (C): EC, and (D): ammonia nitrogen).
In the case of the cow manure experimental group, the pH data demonstrates that the introduction of biochar enables a rapid return to neutrality, stabilizing after the 6th day, with the ending pH nearing approximately 6.80. This can be attributed to the fact that the biochar experimental group was augmented with alkaline digestate containing methanogenic bacteria, which aids in achieving a smoother system startup post-integration. In the experimental group utilizing pig manure, the pH level of the group supplemented with 6% biochar rapidly reverted to neutral, subsequently initiating gas production. Conversely, in the remaining experimental groups, the pH initially declined before escalating, suggesting that the acidification process was not entirely conclusive. Only after the ninth day did the pH gradually stabilize at the neutral conditions optimal for methane-producing bacteria.
Regarding NH3-N levels, there was a notably high concentration on the initial day of the experiment when the biogas slurry was first introduced. For instance, in the 4% experimental group, which exhibited the highest gas production, the addition of 4% biochar led to the peak NH3-N content, reaching 728.58 mg/L. This concentration consistently decreased until the 6th day, when it started to fluctuate, eventually stabilizing at 230.42 mg/L by the experiment’s conclusion. This observed increase in NH3-N can potentially be attributed to the biochar’s pre-existing adsorption of a significant amount of NH3-N during water quality purification in the artificial wetland. Upon its addition to the anaerobic co-fermentation system with cow manure, a portion of this adsorbed NH3-N is likely released, resulting in a temporary rise in its concentration. In contrast, for the pig manure experimental group, the inherent high nitrogen content of the pig manure may lead to an initial surge in NH3-N levels, potentially causing an inhibitory effect on NH3-N.
The EC exhibits a decreasing trend in both cow dung and pig manure. Upon the addition of biochar, the EC in the anaerobic co-fermentation group of cow dung diminishes to approximately 3.2 ms/cm, while in pig manure, it stabilizes around 3.0 ms/cm. Notably, the EC peaks on the fifth day. In the biochar group, the conductivity undergoes fluctuations due to its saturation in the wetland system and the ongoing dynamic process of adsorption and release, rendering these variations relatively insignificant [28].
The anaerobic fermentation process is stimulated by biochar, resulting in the decomposition of VFAs by methanogenic bacteria once they accumulate to a significant degree. During the methane production process, these VFAs are utilized, leading to a reduction in their concentration after 12 days of reaction. The introduction of biochar decreases the VFAs concentration during the middle and final stages of the reaction, thereby mitigating the issue of system disruption caused by the accumulation of VFAs in anaerobic fermentation.
4. Discussion
4.1. The Effect of Biochar on Gas Production Efficiency
The performance of gas production in anaerobic fermentation can be evaluated using specific models. In particular, the results of methane production, as fitted by the modified Gompertz model, are presented in Table 7 and Table 8. Notably, the experimental groups involving cow manure anaerobic fermentation with added biochar demonstrated a reduced final methane production potential in comparison to groups without biochar. This potential decreased from a minimum of 122.43 mL/g VS to 87.74 mL/g VS in the C6C experimental group. While numerous studies have indicated that biochar enhances methane production efficiency, this study found a decrease in the final methane production potential following the alkali treatment of straw. This is attributed to the fact that the co-fermentation system, post-pretreatment, already provides optimal living conditions for methane-producing bacteria. Consequently, the addition of biochar does not significantly enhance the living space of these bacteria. Features of biochar, such as its large specific surface area and its neutral, non-toxic nature, do not markedly promote the activity of anaerobic bacteria.

Table 7.
Fitting results of modified Gompertz model for anaerobic co-digestion of cow dung.

Table 8.
Fitting results of modified Gompertz model for anaerobic co-digestion of pig manure.
The solid content significantly influences the gas production in anaerobic fermentation. When other variables are controlled, the direct incorporation of biochar alters the system’s solid content, thereby affecting the anaerobic fermentation process. For instance, in the C8C experimental group, where the solid content rose to 8.64%, there was a noticeable impact on the fermentation system. This may account for the observed decrease in total methane production. Furthermore, the introduction of biochar can modify the system’s carbon-to-nitrogen ratio. Even minor shifts in this ratio can profoundly affect methane-producing bacteria.
The experimental results from the pig manure group indicated an increase in the final methane potential following the addition of biochar. The introduction of 2% and 8% biochar marginally extended the gas production retention time, whereas 4% and 6% biochar markedly reduced the retention duration. Notably, the inclusion of biochar in pig manure has the potential to enhance the ultimate methane production, elevating it from a baseline of 47.43 mL/g VS to a peak of 122.24 mL/g VS, as observed in the P2C experimental group.
The retention time for the C4C experimental group in the cow dung cohort was slightly lower than that of the control group, which did not contain biochar—a difference of less than 3%. This minor discrepancy might be attributed to experimental error. However, the retention times for all other experimental groups were notably shorter than that of the control group. Notably, the retention time for the C2C experimental group was a mere 0.36 days, representing a reduction to 12.67% of the control group’s duration. Following the addition of biochar, there was a rapid enhancement in the activity of methanogenic bacteria. Overall, the introduction of biochar resulted in a slight extension of the retention time.
In the pig manure biochar group, the addition of 2% and 8% biochar extends the gas production retention time, while the introduction of 4% or 6% biochar significantly reduces it. Hydrolysis is a key rate-limiting step in anaerobic fermentation. The observed reduction in retention time suggests that biochar enhances the hydrolysis and acidification processes during anaerobic fermentation. This enables methanogenic bacteria to proliferate rapidly post-inoculum introduction, leading to a swift stabilization of the anaerobic fermentation system. Moreover, biochar facilitates interspecies direct electron transfer, further expediting the system’s transition to the methane production phase and consequently shortening the retention time.
From the perspective of peak methane production rate, the impact of biochar addition in the cow dung experimental groups is not significant. C2C and C6C exhibit lower rates than the control group, whereas C4C and C8C display higher rates. This variation might be attributed to differences in the rates of hydrolysis, acidification, and acetogenesis, and the utilization rate of methanogenic bacteria. Alternatively, it could be due to an unchanged abundance or dominance of methanogenic bacteria. Further research is required to ascertain the exact reasons for these observations. Overall, biochar supplementation does not enhance the peak methane production rate. Notably, the peak methane production rate for the P4C group in the pig dung experimental set is lower than that of its control group, while other experimental groups show a significant increase in their peak methane production rates.
From a goodness-of-fit standpoint, all experimental groups exhibited values above 0.9781, indicating a strong fit. However, in the cow manure experimental group, there was a noticeable decrease in the goodness of fit as the biochar quantity increased. This suggests that the addition of biochar may cause deviations from the modified Gompertz model in methane production. Notably, other studies have not reported this observation, which implies that the impact of additives on methane production from anaerobic fermentation might follow another pattern that warrants further investigation based on specific indicators. Future research should consider integrating these observations into the Gompertz model to enhance its predictive accuracy. For the pig manure experimental group, the goodness of fit was consistently high, with values above 0.9851. The use of digestate as inoculum, coupled with the presence of minimal VFAs in the digestate, facilitated system startup. Nevertheless, elevated VFA levels can impede biogas production [29]. Biochar, being a promoter of anaerobic fermentation, ensures the rapid utilization of the produced VFAs, thus expediting system startup. Li Dani et al. discovered that biochar addition during anaerobic dry fermentation of pig manure reduced the gas retention period by 31.23–83.90% [30]. In our study, the retention time for P6C was notably shorter, being 80.66% less than that of the control group.
4.2. Mechanism Analysis of Biochar’s Influence on Gas Production Performance
Through the PCA of the anaerobic fermentation data derived from cow manure, it was observed that PC1 and PC2 accounted for 76.74% and 21.32% of the variation in methane production, respectively. As illustrated in the PCA analysis results of the cow manure experimental group (Figure 13), the introduction of biochar was positively correlated with increased levels of ammonia nitrogen and negatively correlated with conductivity. This suggests that the presence of biochar significantly impacts methane production during the anaerobic fermentation process of cow manure. Within the anaerobic co-fermentation system of cow manure, biochar primarily influences overall gas production performance by modulating the content of ammonia nitrogen and conductivity.
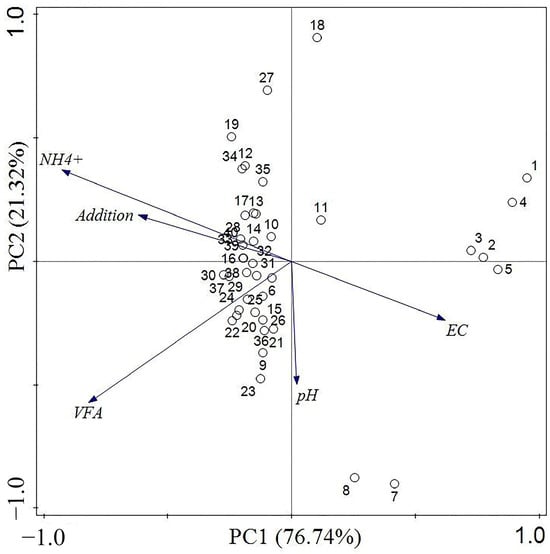
Figure 13.
PCA of anaerobic co-digestion of cow dung adding biochar.
The results of the PCA on the swine manure experimental group are presented in Figure 14. In the experiment concerning swine manure fermentation with biochar addition, the PC1 and PC2 accounted for 78.88% and 19.46% of the variability in methane production, respectively. The analysis revealed a significant positive correlation between biochar addition and pH, while it demonstrated a substantial negative correlation with alterations in EC. This suggests that biochar potentially influences the activity of anaerobic bacteria by impacting changes in pH and EC, thereby affecting methane production. Furthermore, elevated VFAs concentrations were identified as one of the key factors contributing to reduced methane yields. Concurrently, higher NH3-N levels were also identified as a factor leading to decreased methane production.
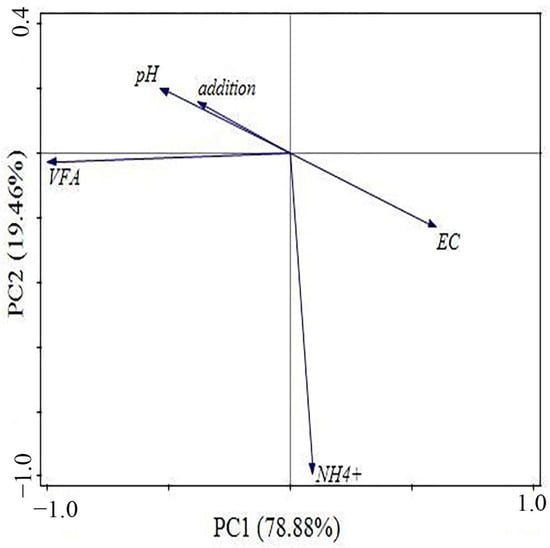
Figure 14.
PCA of anaerobic co-digestion of pig manure adding biochar.
In the context of gas production outcomes, the inclusion of biochar markedly enhances the efficiency of gas production in anaerobic fermentation and reduces the duration of the gas production cycle. Existing research indicates that hydrolysis is a pivotal rate-limiting step in anaerobic fermentation [31], with hydrolysis and methane production processes occurring concurrently in anaerobic systems. Thus, expediting the hydrolysis phase is key to augmenting the gas production rate. Presently, many researchers employ a two-phase anaerobic fermentation technique to segregate the stages of hydrolysis and methane production, thereby aiming to hasten the fermentation process, an approach that has yielded substantial successes. The implementation of suitable pretreatment methods can further accelerate the rate of hydrolysis [32]. This paper introduces a novel method wherein corn straw, following NaOH alkali pretreatment, is incorporated into a livestock manure anaerobic fermentation system to establish a co-fermentation system. Studies reveal that alkali pretreatment thins the cell wall, rendering the treated straw more susceptible to utilization by methane-producing bacteria and hydrolytic bacteria [33]. Subsequently, biochar is introduced as an additive, providing a growth medium for methane-producing bacteria, which further abbreviates the lag phase of methane production. The pH value declined from 7 prior to treatment to approximately 6 at the commencement of the reaction, suggesting that the stages of hydrolysis and acidification had been underway for a period.
Biochar, an adsorbent derived from agricultural residues, has garnered interest owing to its cost-effectiveness and expanding applications [34]. Despite its theoretical capacity for pH buffering, experimental findings, congruent with those of Luo et al. [35], indicate that biochar does not significantly mitigate pH fluctuations. This could be attributed to biochar’s enhancement of efficiency, which may expedite pH alterations during buffering. Wu Si Ya et al. ascertained that the gas production per unit dry matter and the average methane volume fraction for wheat straw anaerobic fermentation were 148.21–183.08 mL/g and 47.53–52.37%, respectively [36]. They noted an acidification phenomenon during the anaerobic fermentation of straw, necessitating artificial pH regulation to optimize gas production.
The significant increase in total phosphorus content in the digestate after biochar addition can be attributed to the phosphorus content inherent in the straw biochar and the initial release of some phosphorus into the slurry during the early phases of anaerobic fermentation. However, as fermentation progresses, the majority of the phosphorus in the system becomes enriched in the digestate. This observation aligns with those made by other researchers. For instance, Fu Guangqing and colleagues noted that 91.96% of the phosphorus added to feed cattle manure was enriched in the solids, suggesting a propensity for phosphorus to transition from the liquid phase to the solid phase [37]. The degradation of organic matter by anaerobic fermentation, particularly large particles in the digester, results in a reduction of the organic phosphorus content and coarse content in livestock slurry. The tendency for soluble phosphorus to form precipitates with ions such as Ca2+ and Mg2+ leads to the formation of birds’ nest stone and other precipitates during the anaerobic digestion process. These precipitates then settle into the digestate, thereby increasing the phosphorus content in the solid component [38]. The adsorption properties of biochar facilitate the conversion of phosphorus to solids. Following this reaction, the phosphorus content in the digestate ranged from 16.00–59.66 mg/g, surpassing the total phosphorus requirement of wheat (11.30 g/kg) [27].
Research has demonstrated that ammonia nitrogen is toxic to anaerobic fermentation due to its ability to penetrate the cell membrane and adversely affect anaerobic fermentation bacteria [39]. It has been shown that free ammonia is more toxic than NH3-N, as it is capable of penetrating the cell membrane. Zeshan et al. suggest that increasing the carbon-to-nitrogen ratio in the raw material can mitigate the potential impact of high-protein raw materials. This is because adding carbon reduces the concentration of nitrogen-rich substances and provides alternative metabolic pathways, thereby reducing the production of NH3-N [40]. When the carbon-to-nitrogen ratio of the raw material was increased to 32, a 30% decrease in the NH3-N content of the digestate and a 50–73% decrease in residual energy were observed. YanginGomec and Ozturk noted a 1.2-fold increase in methane production, reaching 771, when corn silage was co-digested with chicken and cow manure to mitigate ammonia toxicity. Other studies have indicated that the high NH3-N content of biogas slurry significantly enhances fertilization effects and crop yield in pot experiments. However, field experiments have shown that nitrogen loss may occur due to the slow and uneven release of biogas slurry [41]. This suggests that while biogas slurry with high NH3-N content is valuable, an optimal method of utilization has yet to be determined. Inhibition due to ammonia is commonly observed in continuous feeding anaerobic fermentation and high-solid-content anaerobic dry fermentation. However, instances of NH3-N inhibition are seldom reported in sequential batch anaerobic wet fermentation. This can be attributed to two primary reasons: firstly, the low solid content results in the generated ammonia nitrogen being diluted into the slurry through shaking, which mitigates its impact on anaerobic fermentation; secondly, the fermentation raw materials are introduced into the reactor before the initiation of the reaction, with no additional feeding during the process. This ensures that the total nitrogen content remains relatively low. Notably, a minimal amount of ammonia nitrogen can exert a stimulating effect on anaerobic microorganisms [42], thereby enhancing anaerobic fermentation. Such insights aid in a comprehensive understanding of the anaerobic fermentation process, particularly through the observation of ammonia nitrogen variations during fermentation [43].
In this study, the concentration of NH3-N in pig manure is notably high; however, it remains significantly below the 3390 mg/L threshold that inhibits anaerobic fermentation of NH3-N in comparable experiments. In contrast, the concentration of NH3-N in cow manure is considerably lower than that in pig manure during anaerobic fermentation—a distinction attributed to the inherent nature of the respective raw materials. The incorporation of biochar into pretreated pig manure markedly enhances methane production, a finding corroborated by other researchers. For instance, Wang et al. investigated the impact of biochar on the anaerobic fermentation of kitchen waste using three distinct types of temperature-pyrolyzed biochar, revealing that all three variants significantly enhanced anaerobic fermentation [44]. Luo et al. suggest that powdered biochar is more effective than its granular counterpart due to its greater specific surface area and shorter diffusion path for powder particles, enabling quicker adsorbate penetration of adsorbates into particle pores [35]. In our experiment, P8C and P6C were the two groups with the highest daily methane production. Furthermore, all four groups treated with biochar exhibited a substantial increase in daily methane proportions. This can be attributed to the ability of biochar to facilitate interspecific electron transfer, thereby elevating the activity of methanogenic bacteria and enhancing methane yield [45].
The electrical conductivity of biochar exhibits temporal fluctuations even after reaching adsorption saturation, both in pure water and formulated urban tailwater [28]. Straw biochar particles are lightweight and form suspended solids, which elevate the electrical conductivity (EC) in the liquid. Notably, biochar possesses robust adsorption capabilities. Furthermore, the ash concentration discharged into wastewater by straw biochar demonstrates significant variation. This becomes more pronounced with the introduction of pollutants, leading to an increase in suspended matter and subsequently altering the EC. Comprised mainly of C, H, N, and O, the C element is the most abundant in straw biochar [46]. Incorporating it into anaerobic fermentation systems offers a habitat for anaerobic bacteria while simultaneously causing fluctuations in conductivity. However, introducing biochar to cattle manure did not enhance methane production through anaerobic fermentation. Cimon et al. observed a similar phenomenon; the incorporation of a minor quantity of biochar did not significantly augment biogas yield compared to the control group [47]. Specifically, 1.8 g/g VS of powdered biochar reduced methane output by 12% relative to the control, and similar findings were observed when integrating powdered biochar into the anaerobic fermentation system of wastewater sludge. With the addition of 0.9 g and 3.7 g/g VS of powdered biochar, the production lag phase was reduced, and the initial methane rate was enhanced. Although methane output exceeded that of the control group in the first 19 days, the cumulative methane production ultimately remained lower than the control group [47]. Thus, while the inclusion of biochar in the pretreatment of anaerobic co-fermentation of cattle manure does not increase the total methane yield, it significantly reduces the gas production lag period.
5. Conclusions
- The incorporation of biochar into pretreated cattle manure during anaerobic fermentation significantly accelerates the gas production cycle and reduces gas retention time. However, it also leads to a decrease in both the total gas output and methane yield. Specifically, the total gas production decreased by 22.85–31.70%, while the total methane yield experienced a reduction ranging from 19.40–31.44%. Despite these reductions, the duration of the gas production cycle was shortened by 35–45%.
- The optimal amount of biochar to be added to the experimental group in cow manure anaerobic co-fermentation is 4%. The primary effect of biochar integration into the cow manure anaerobic co-fermentation system is on gas production performance, which is influenced by changes in ammonia nitrogen and conductivity.
- The addition of biochar to pig manure in the experimental group significantly increased total gas production and total methane yield, while also reducing gas retention time. The total methane content increased by 18.53% to 150.18%, although the alteration in the gas production cycle was not markedly evident.
- The optimal amount of biochar to be added for the anaerobic co-fermentation experiment of pig manure is 6%. The inclusion of biochar in the anaerobic co-fermentation system of pig manure has an impact on the activity of anaerobic bacteria, which subsequently influences methane production by altering pH and conductivity levels.
Author Contributions
Formal analysis, J.X. and J.S.; investigation, X.W. and J.S.; writing—original draft preparation, J.X. and J.S.; software, J.S. and Y.C.; data curation, J.S., W.Z. and R.H.; writing—review and editing, X.W., W.Z., R.H. and Y.C.; supervision, J.X.; project administration, J.X.; funding acquisition, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 42461013), Scientific Research Foundation of Hainan Tropical Ocean University (No. RHDRC202333), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA28020102), the Project of Changchun City Technology Bureau (No. 16SS06) and Strategic Research and Consulting Project of the Chinese Academy of Engineering (JL2024-18). The authors thank the editor for his efforts in this paper.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this article are not available because the data are part of an ongoing study. Requests to access the datasets should be directed to the corresponding author.
Conflicts of Interest
Author Xinyu Wang was employed by the Jilin Province Water Resources and Hydropower Consultative Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yılmaz, A.; Başkan, G.; Açıkel, Ü. Effect of Thermal, Acid and Alkaline Pretreatments on Biogas Production from Cattle Manure. Waste Biomass Valorization 2025, 16, 2301–2314. [Google Scholar] [CrossRef]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical Options for the Mitigation of Direct Methane and Nitrous Oxide Emissions from Livestock: A Review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K.; et al. Biological Nutrient Removal and Recovery from Solid and Liquid Livestock Manure: Recent Advance and Perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; He, P.; Dong, J.; Zhang, L. Thoughts on the Comprehensive Utilization of Agricultural Organic Waste. Chin. Consult. Eng. Mag. 2021, 12, 89–92. [Google Scholar] [CrossRef]
- Su, F. A Preliminary Analysis of Environmental Pollution Caused by Traditional Aquaculture Techniques in China. South China Agric. 2015, 15, 210–211. [Google Scholar]
- Zhang, H. Analysis of the Current Situation of Environmental Pollution in Rural Livestock and Poultry Breeding and Exploration of Governance Measures. Livest. Poult. Ind. 2021, 32, 82+84. [Google Scholar]
- Stahel, W.R. The Circular Economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, J.; Chulu, B.; Yang, M.; Nopens, I.; Wei, Y. Ammonia Stress Decreased Biomarker Genes of Acetoclastic Methanogenesis and Second Peak of Production Rates during Anaerobic Digestion of Swine Manure. Bioresour. Technol. 2020, 317, 124012. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C—J. Carbon Res. 2019, 5, 27. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Wang, J.; Li, Y.; Ma, L.; Chen, W.; Duan, Y.; Zhai, Y.; Li, D. High Value-Added Chemical Production through Anaerobic Codigestion of Corn Straw with a Microbial Consortium, Cow Manure and Cow Digestion Solution. Anaerobe 2024, 89, 102900. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment Methods of Lignocellulosic Biomass for Anaerobic Digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Loh, K.C. Activated Carbon Enhanced Anaerobic Digestion of Food Waste—Laboratory-Scale and Pilot-Scale Operation. Waste Manag. 2018, 75, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zang, S.; Xu, J.; Sheng, L. Dynamic Simulation Analysis of City Tail Water Treatment by Constructed Wetland with Biochar Substrate. Environ. Sci. Pollut. Res. 2023, 30, 108582–108595. [Google Scholar] [CrossRef]
- Qu, Y.; Qu, J.; Yan, W.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Influence of Biochar on Physico-Chemical, Microbial Community and Maturity during Biogas Residue Aerobic Composting Process. Fermentation 2022, 8, 623. [Google Scholar] [CrossRef]
- Feng, J.; Sun, J.; Xu, J.; Wang, H. Degradation of Acetochlor in Soil by Adding Organic Fertilizers with Different Conditioners. Soil Tillage Res. 2023, 228, 105651. [Google Scholar] [CrossRef]
- Wang, Z.; Han, L.; Sun, K.; Jin, J.; Ro, K.S.; Libra, J.A.; Liu, X.; Xing, B. Sorption of Four Hydrophobic Organic Contaminants by Biochars Derived from Maize Straw, Wood Dust and Swine Manure at Different Pyrolytic Temperatures. Chemosphere 2016, 144, 285–291. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Reubens, B.; Willekens, K.; De Neve, S. Composting for Increasing the Fertilizer Value of Chicken Manure: Effects of Feedstock on P Availability. Waste Biomass Valorization 2014, 5, 491–503. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Potential of Aeration Flow Rate and Bio-Char Addition to Reduce Greenhouse Gas and Ammonia Emissions during Manure Composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing Pipeline-Quality Biomethane via Anaerobic Digestion of Sludge Amended with Corn Stover Biochar with in-Situ CO2 Removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Haider, M.A.; Xu, J.; Sun, J.; Chen, Y. Study on the Effect of Conditioners on the Degradation of Tetracycline Antibiotics in Deer Manure Composting. Fermentation 2024, 10, 575. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar Characteristics and Early Applications in Anaerobic Digestion-a Review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.-K.; Kan, E. Effects of Dairy Manure-Derived Biochar on Psychrophilic, Mesophilic and Thermophilic Anaerobic Digestions of Dairy Manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Wang, H.; Liu, X.; Zhang, D. Study of the Performance of Biogas Production by Mixed Fermentation of Cow Dung, Deer Manure, and Mushroom Fungus. Energy Sci. Eng. 2020, 8, 466–475. [Google Scholar] [CrossRef]
- NY525-2012; Oganic Fertilizer. Standardization Administration of China: Beijing, China, 2012.
- Wang, H.; Xu, J.; Sheng, L.; Liu, X.; Zong, M.; Yao, D. Anaerobic Digestion Technology for Methane Production Using Deer Manure Under Different Experimental Conditions. Energies 2019, 12, 1819. [Google Scholar] [CrossRef]
- Xiong, R.; Meng, Y.; Li, Y.; Chen, L.; Du, Z.; Zhong, Q.; Han, R. Biogas Production Characteristics of Mixed Anaerobic Fermentation of Rapeseed Cake with High Solid Content and Cow Dung-Sheep Manure. Chin. J. Environ. Eng. 2021, 15, 2427–2435. [Google Scholar] [CrossRef]
- Xu, H.; Yun, S.; Wang, C.; Wang, Z.; Han, F.; Jia, B.; Chen, J.; Li, B. Improving Performance and Phosphorus Content of Anaerobic Co-Digestion of Dairy Manure with Aloe Peel Waste Using Vermiculite. Bioresour. Technol. 2020, 301, 122753. [Google Scholar] [CrossRef]
- Wang, H.; Sun, J.; Xu, J.; Sheng, L. Study on Clogging Mechanisms of Constructed Wetlands from the Perspective of Wastewater Electrical Conductivity Change under Different Substrate Conditions. J. Environ. Manag. 2021, 292, 112813. [Google Scholar] [CrossRef]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved Biogas Production from Whole Stillage by Co-Digestion with Cattle Manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef]
- Li, D.; Zhang, K.; Liang, J.; Gao, W.; Kong, D.; Du, L. Solid-State Anaerobic Digestion of Pig Manure with Three Kinds of Additives. J. Agro-Environ. Sci. 2019, 38, 1777–1785. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Microwave Pretreatment of Lignocellulosic Agroindustrial Waste for Methane Production. J. Environ. Chem. Eng. 2017, 5, 352–365. [Google Scholar] [CrossRef]
- Liu, X.; Du, M.; Yang, J.; Wu, Y.; Xu, Q.; Wang, D.; Yang, Q.; Yang, G.; Li, X. Sulfite Serving as a Pretreatment Method for Alkaline Fermentation to Enhance Short-Chain Fatty Acid Production from Waste Activated Sludge. Chem. Eng. J. 2020, 385, 123991. [Google Scholar] [CrossRef]
- Zhao, Q. Effect of Temperature on the Structure and Anaerobic Fermentation Performance of Alkali-Pretreated Corn Stover. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2021. [Google Scholar]
- Meng, X.; Sui, Q.; Liu, J.; Yu, D.; Wang, Y.; Wei, Y. Relieving Ammonia Inhibition by Zero-Valent Iron (ZVI) Dosing to Enhance Methanogenesis in the High Solid Anaerobic Digestion of Swine Manure. Waste Manag. 2020, 118, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of Eco-Compatible Biochar in Anaerobic Digestion to Relieve Acid Stress and Promote the Selective Colonization of Functional Microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, S.; Chen, Y.; Wang, X.; Li, J.; Chen, G. Effect of Extrinsic Extruding Pressure on Biogas Production of Agricultural Straw. China Biogas 2019, 37, 25–30. [Google Scholar]
- Fu, G.; Ye, X.; Jin, H.; Chang, Z.; Chen, G.; Du, J. Effect of Anaerobic Digestion on Phosphorus Transformation of Both Pig and Dairy Manure. J. Agro-Environ. Sci. 2013, 32, 179–184. [Google Scholar] [CrossRef]
- Li, Y.; Jones, D.L.; Chen, Q.; Ge, T.; Chadwick, D.R. Acidification and Anaerobic Digestion Change the Phosphorus Forms and Distribution in Particle Fractions of Cattle Slurry and Phosphorus Dynamics in Soil after Application. Biosyst. Eng. 2020, 200, 101–111. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The Challenges of Anaerobic Digestion and the Role of Biochar in Optimizing Anaerobic Digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N Ratio and Ammonia-N Accumulation in a Pilot-Scale Thermophilic Dry Anaerobic Digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Ge, H.; Luo, J.; Zhi, X.; Le, Y. A Review on Hormesis and Antibiotics in the Environment. Chin. J. Trop. Crops 2015, 36, 1719–1725. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The Effects of C/N (10–25) on the Relationship of Substrates, Metabolites, and Microorganisms in “Inhibited Steady-State” of Anaerobic Digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Z.; Zhang, Y. Enhancing Anaerobic Digestion of Kitchen Wastes with Biochar: Link between Different Properties and Critical Mechanisms of Promoting Interspecies Electron Transfer. Renew. Energy 2021, 167, 791–799. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding Granular Activated Carbon into Anaerobic Sludge Digestion to Promote Methane Production and Sludge Decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L. Preparation of Straw Biochar and Application of Constructed Wetland in China: A Review. J. Clean. Prod. 2020, 273, 123131. [Google Scholar] [CrossRef]
- Cimon, C.; Kadota, P.; Eskicioglu, C. Effect of Biochar and Wood Ash Amendment on Biochemical Methane Production of Wastewater Sludge from a Temperature Phase Anaerobic Digestion Process. Bioresour. Technol. 2020, 297, 122440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).