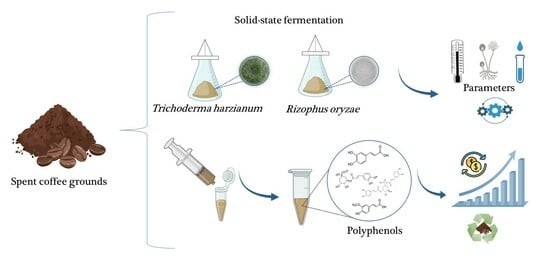
Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Raw Material
2.2.1. Characterization of the Raw Material
2.2.2. Determination of Cellulose, Hemicellulose, and Lignin Content
2.3. Solid-State Fermentation (SSF) Process
2.3.1. Microorganisms
2.3.2. Reactivation of Fungal Strains
2.3.3. Solid-State Fermentation (SSF) Strategy
2.3.4. Preliminary Selection of Variables Using the Hunter & Hunter Method
2.4. Optimization of Solid-State Fermentation Strategy
2.5. Total Polyphenols
2.5.1. Condensed Tannin Content
2.5.2. Total Polyonehol Content
2.6. Antioxidant Activity
2.6.1. Antioxidant Activity by DPPH
2.6.2. Antioxidant Activity Using the ABTS Method
2.6.3. Antioxidant Activity by FRAP
2.7. Fermentation Extract Identification Using HPLC-MS
2.8. Statistical Analysis
3. Results
3.1. Characterization of the Raw Material
3.2. Hunter & Hunter Preliminary Method for Obtaining Polyphenols
3.3. Pareto Analysis of Significant Factors Affecting Polyphenol Release Based on Hunter and Hunter Exploratory Design
3.4. Box-Behnken Design for the Production of Polyphenols
3.5. Response Surface Analysis for Polyphenol Release in Solid-State Fermentation
3.6. Antioxidant Activity of the Selected Treatments
3.7. Identification of Phenolic Compounds in the Fermented Extract Using HPLC-MS
4. Discussion
4.1. Material Characterization
4.2. Exploratory Design: Hunter & Hunter Method
Pareto Analysis—Hunter & Hunter Method
4.3. Evaluation Using the Box–Behnken Design
4.3.1. Antioxidant Activity
4.3.2. HPLC-MS Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montenegro, J.; Freitas-Silva, O.; Teodoro, A.J. Molecular mechanisms of coffee on prostate cancer prevention. BioMed Res. 2022, 2022, 3254420. [Google Scholar] [CrossRef] [PubMed]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Environ. Sci. Pollut. Res. 2020, 27, 34715–34733. [Google Scholar] [CrossRef] [PubMed]
- Setter, C.; Borges, F.A.; Cardoso, C.R.; Mendes, R.F.; Oliveira, T.J.P. Energy quality of pellets produced from coffee residue: Characterization of the products obtained via slow pyrolysis. Ind. Crops Prod. 2020, 154, 112731. [Google Scholar] [CrossRef]
- Colantoni, A.; Paris, E.; Bianchini, F.; Ferri, S.; Marcantonio, V.; Carnevale, M.; Gallucci, F. Spent coffee ground characterization, pelletization test and emissions assessment in the combustion process. Sci. Rep. 2021, 11, 5119. [Google Scholar] [CrossRef]
- Arias, A.; Ioannidou, S.M.; Giannakis, N.; Feijoo, G.; Moreira, M.T.; Koutinas, A. Review of potential and prospective strategies for the valorization of coffee grounds within the framework of a sustainable and circular bioeconomy. Ind. Crops Prod. 2023, 205, 117504. [Google Scholar] [CrossRef]
- Carboué, Q.; Rébufa, C.; Hamrouni, R.; Roussos, S.; Bombarda, I. Statistical approach to evaluate effect of temperature and moisture content on the production of antioxidant naphtho-gamma-pyrones and hydroxycinnamic acids by Aspergillus tubingensis in solid-state fermentation. Bioprocess Biosyst. Eng. 2020, 43, 2283–2294. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward circular economy: Potentials of spent coffee grounds in bioproducts and chemical production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Devi, M.H.; Munjam, S. Bioethanol production from rice straw and cellulose degradation using Aspergillus terreus and Trichoderma harzanium. Biosci. Biotechnol. Res. Asia 2022, 19, 699–711. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Volpe, V.; Zanini, S.; Vallino, M.; Abbà, S.; Bonfante, P. A rice GRAS gene has an impact on the success of arbuscular mycorrhizal colonization. Am. J. Plant Sci. 2015, 6, 1905–1915. [Google Scholar] [CrossRef]
- Lima, P.C.; Karimian, P.; Johnston, E.; Hartley, C.J. The Use of Trichoderma spp. for the Bioconversion of Agro-Industrial Waste Biomass via Fermentation: A Review. Fermentation 2024, 10, 442. [Google Scholar] [CrossRef]
- De la Rosa-Hernandez, M.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Inungaray, M.L.C.; Wong-Paz, J.E. Biotechnological cost-effective processes for the extraction of bioactive compounds. In Food Byproducts Management and Their Utilization; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 169–199. [Google Scholar]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A. Advances in bioprocess engineering for optimising Chlorella vulgaris fermentation: Biotechnological innovations and applications. Foods 2024, 13, 4154. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Gil-Ramírez, A.; Benítez, V.; Cañas, S.; Braojos, C.; Martin-Cabrejas, M.A. Biorefinery and stepwise strategies for valorizing coffee by-products as bioactive food ingredients and nutraceuticals. Appl. Sci. 2023, 13, 8326. [Google Scholar] [CrossRef]
- Vitale, E.; Motta, C.M.; Avallone, B.; Amoresano, A.; Fontanarosa, C.; Battaglia, G.; Arena, C. Sustainable reuse of espresso coffee by-products as a natural fertilizer to improve growth and photosynthesis in cucumber (Cucumis sativus L.) plants. Waste Biomass Valorization 2024, 15, 543–559. [Google Scholar] [CrossRef]
- Bevilacqua, E.; Cruzat, V.; Singh, I.; Rose’Meyer, R.B.; Panchal, S.K.; Brown, L. The potential of spent coffee grounds in functional food development. Nutrients 2023, 15, 994. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Shiva; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; López-Sandin, I.; Morais, A.R.C.; Ruiz, H.A. Enzymatic hydrolysis, kinetic modeling of hemicellulose fraction, and energy efficiency of autohydrolysis pretreatment using agave bagasse. BioEnergy Res. 2023, 16, 75–87. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Siller-Sánchez, A.; Aguilar, C.N.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Kumar Verma, D.; Aguilar-González, M. Solid-state fermentation-assisted extraction of flavonoids from grape pomace using co-cultures. Processes 2024, 12, 2027. [Google Scholar] [CrossRef]
- Van den Berg, R.; Haenen, G.R.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.; Ilina, A.; Torres-León, C.; Verma, D.K.; Chávez-González, M.L. Phenolic compounds and antioxidant activity of Lippia graveolens Kunth residual leaves fermented by two filamentous fungal strains in solid-state process. Food Bioprod. Process 2022, 136, 24–35. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Campos, D.A.; Gómez-García, R.; Pintado, M.; Oliveira, M.C.; Santos, D.I.; Alves, V.D. Optimization of natural antioxidants extraction from pineapple peel and their stabilization by spray drying. Foods 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Jomnonkhaow, U.; Plangklang, P.; Reungsang, A.; Peng, C.Y.; Chu, C.Y. Valorization of spent coffee grounds through integrated bioprocess of fermentable sugars, volatile fatty acids, yeast-based single-cell protein and biofuels production. Bioresour. Technol. 2024, 393, 130107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, L.; Zhang, Y.; Gu, Y.; Jiang, L.; Qin, B. Solid state fermentation of aquatic macrophytes for crude protein extraction. Ecol. Eng. 2009, 35, 1668–1676. [Google Scholar] [CrossRef]
- Rodrigues, M.A.M.; Pinto, P.; Bezerra, R.M.F.; Dias, A.A.; Guedes, C.V.M.; Cardoso, V.M.G.; Cone, J.W.; Ferreira, L.M.M.; Colaco, J.; Sequeira, C.A. Effect of enzyme extracts isolated from white-rot fungi on chemical composition and in vitro digestibility of wheat straw. Anim. Feed. Sci. Technol. 2008, 141, 326–338. [Google Scholar] [CrossRef]
- Vellozo-Echevarría, T.; Barrett, K.; Vuillemin, M.; Meyer, A.S. Mini-Review: The distinct carbohydrate active enzyme secretome of Rhizopus spp. represents fitness for mycelium remodeling and solid-state plant food fermentation. ACS Omega 2024, 9, 34185–34195. [Google Scholar] [CrossRef]
- De Villa, R.; Roasa, J.; Mine, Y.; Tsao, R. Impact of solid-state fermentation on the factors and mechanisms that influence the bioactive compounds of grains and processing byproducts. Crit. Rev. Food Sci. Nutr. 2023, 63, 5388–5413. [Google Scholar] [CrossRef]
- Jo, C.; Zhang, J.; Tam, J.M.; Church, G.M.; Khalil, A.S.; Segrè, D.; Tang, T.C. Unlocking the magic in mycelium: Using synthetic biology to optimize filamentous fungi for biomanufacturing and sustainability. Mater. Today Bio 2023, 19, 100560. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Wan, X.; Zhang, H.; Tian, J. Fabrication of biodegradable films with UV-blocking and high-strength properties from spent coffee grounds. Carbohydr. Polym. 2023, 321, 121290. [Google Scholar] [CrossRef]
- Mahmoud, L.S. A summary of secondary metabolites and nanoparticles generated by filamentous fungi and their application in medicine. Bull. Fac. Sci. Zagazig Univ. 2024, 4, 19–36. [Google Scholar] [CrossRef]
- Morilla, E.A.; Taddia, A.; Sortino, M.; Tubio, G. Mixed cultures of Aspergillus niger and Rhizopus oryzae using lignocellulosic substrates to improve hydrolytic enzyme production. Bioenergy Res. 2023, 16, 2285–2296. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Benabda, O.; M’hir, S.; Kasmi, M.; Mnif, W.; Hamdi, M. Optimization of protease and amylase production by Rhizopus oryzae cultivated on bread waste using solid-state fermentation. J. Chem. 2019, 2019, 3738181. [Google Scholar] [CrossRef]
- Abdullah, J.J.; Greetham, D.; Pensupa, N.; Tucker, G.A.; Du, C. Optimizing cellulase production from municipal solid waste (MSW) using solid state fermentation (SSF). J. Fundam. Renew. Energy Appl. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Chinnasamy Muthulakshmi, C.M.; Duraisamy Gomathi, D.G.; Kumar, D.G.; Ganesan Ravikumar, G.R.; Manokaran Kalaiselvi, M.K.; Chandrasekar Uma, C.U. Production, purification and characterization of protease by Aspergillus flavus under solid state fermentation. Jordan J. Biol. Sci. 2011, 4, 137–148. [Google Scholar]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine microbiome: A dynamic world of microbial interactions. Crit. Rev. Food Sci. 2017, 57, 856–873. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Q.; Nie, Y.; Wu, J.; Xu, Y. Construction of synthetic microbiota for reproducible flavor metabolism in Chinese light aroma type liquor produced by solid-state fermentation. Appl. Microbiol. Biotechnol. 2019, 10, e03090-18. [Google Scholar] [CrossRef]
- Tan, Y.W.; Zhong, H.P.; Zhao, D.; Du, H.; Xu, Y. Succession rate of microbial community causes flavor difference in strong-aroma Baijiu making process. Int. J. Food Microbiol. 2019, 311, 108350. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Wahab, R.A.; Huyop, F.; David, E.E.; Tin, L.C. Solid-state valorization of raw oil palm leaves by novel fungi Trichoderma asperellum UC1 and Rhizopus oryzae UC2 for sustainable production of cellulase and xylanase. J. Chem. Technol. Biotechnol. 2022, 97, 520–533. [Google Scholar] [CrossRef]
- Dos Santos, J.R.; de Souza Moreira, L.R.; Filho, E.X.F. Cellulose-degrading enzymes: Key players in biorefinery development. Biologia 2023, 78, 1759–1772. [Google Scholar] [CrossRef]
- Rasool, G.; Irfan, M. The role of microbial diversity in lignocellulosic biomass degradation: A biotechnological perspective. ChemBioEng Rev. 2024, 11, 613–635. [Google Scholar] [CrossRef]
- Biswa Sarma, J.; Mahanta, S.; Tanti, B. Maximizing microbial activity and synergistic interaction to boost biofuel production from lignocellulosic biomass. Arch. Microbiol. 2024, 206, 448. [Google Scholar] [CrossRef] [PubMed]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-state fermentation from organic wastes: A new generation of bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Arancibia-Díaz, A.; Astudillo-Castro, C.; Altamirano, C.; Soto-Maldonado, C.; Vergara-Castro, M.; Córdova, A.; Zúñiga-Hansen, M.E. Development of solid-state fermentation process of spent coffee grounds for the differentiated obtaining of chlorogenic, quinic, and caffeic acids. J. Sci. Food Agric. 2023, 103, 420–427. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Sharifi-Rad, J. Lifestyle, oxidative stress, and antioxidants: Bidirectional interactions in the pathophysiology of chronic diseases. Front. Physiol. 2020, 111, 694. [Google Scholar]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimecheva, I.P. Flaxseed oil stabilization using natural and synthetic antioxidants. Eur. J. Lipid Sci. Technol. 2017, 119, 1700079. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Ho, K.V.; Schreiber, K.L.; Park, J.; Vo, P.H.; Lei, Z.; Sumner, L.W.; Lin, C.H. Identification and quantification of bioactive molecules inhibiting pro-inflammatory cytokine production in spent coffee grounds using metabolomics analyses. Front. Pharmacol. 2020, 11, 229. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Chlorogenic acid isomers ameliorate oxidative stress in Caco-2 cells treated with pro-inflammatory proteins by activating the Nrf2/Keap1-ARE signaling pathway. J. Agric. Food Chem. 2018, 42, 11008–11017. [Google Scholar] [CrossRef]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Angeloni, C. Antioxidant and anti-inflammatory profiles of spent coffee ground extracts for the treatment of neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6620913. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva, L.F.S.; Corrêa, A.P.F.; Bach, E.; Brandelli, A. Total polyphenols, antioxidant, antimicrobial and allelopathic activities of spent coffee ground aqueous extract. Waste Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.; Fernandes, C.; Gonçalves, T. Antifungal activity of spent coffee ground extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef]





| Treatment | Temperature (°C) | Humidity (%) | Inoculum (Spores/mL) |
|---|---|---|---|
| 1 | 25 | 70 | 1 × 107 |
| 2 | 30 | 70 | 1 × 107 |
| 3 | 25 | 80 | 1 × 107 |
| 4 | 30 | 80 | 1 × 107 |
| 5 | 25 | 70 | 1 × 108 |
| 6 | 30 | 70 | 1 × 108 |
| 7 | 25 | 80 | 1 × 108 |
| 8 | 30 | 80 | 1 × 108 |
| Treatment | Temperature (°C) | Humidity (%) | Inoculum (Spores/mL) |
|---|---|---|---|
| T1 | 20 | 70 | 1 × 107 |
| T2 | 30 | 70 | 1 × 107 |
| T3 | 20 | 80 | 1 × 107 |
| T4 | 30 | 80 | 1 × 107 |
| T5 | 20 | 75 | 1 × 108 |
| T6 | 30 | 75 | 1 × 108 |
| T7 | 20 | 75 | 1 × 108 |
| T8 | 30 | 75 | 1 × 108 |
| T9 | 25 | 70 | 1 × 106 |
| T10 | 25 | 75 | 1 × 106 |
| T11 | 25 | 70 | 1 × 108 |
| T12 | 25 | 80 | 1 × 108 |
| T13 | 25 | 75 | 1 × 107 |
| T14 | 25 | 75 | 1 × 107 |
| T15 | 25 | 75 | 1 × 107 |
| Content (% w/w) | Unfermented SCGs | SCGs Fermented with R. oryzae | SCGs Fermented with T. harzianum |
|---|---|---|---|
| Protein | 24.06 ± 0.67 | 26.14 ± 0.09 | 25.85 ± 0.09 |
| Carbohydrates | 39.66 | 34.72 | 41.31 |
| Lipids | 16.94 ± 0.26 | 25.31 ± 0.97 | 19.17 ± 0.19 |
| Fiber | 16.62 ± 0.54 | 13.67 ± 0.29 | 13.11 ± 0.03 |
| Ash | 2.72 ± 0.71 | 0.16 ± 0.71 | 0.54 ± 0.19 |
| Cellulose | 10.95 ± 0.06 | N.D. | N.D. |
| Hemicellulose | 27.13 ± 0.01 | N.D. | N.D. |
| Lignin | 14.85 ± 0. 17 | N.D. | N.D. |
| Factor | Observed Minimum | Critical Values | Observed Maximum |
|---|---|---|---|
| T. harzianum | |||
| Temperature, °C | 20 | 25 | 30 |
| Inoculum, spores/mL | 1 × 106 | 1 × 107 | 1 × 108 |
| Humidity, % | 70 | 77 | 80 |
| R. oryzae | |||
| Temperature, °C | 20 | 28.6 | 30 |
| Inoculum, spores/mL | 1 × 106 | 1 × 107 | 1 × 108 |
| Humidity, % | 70 | 76 | 80 |
| Retention Time (min) | Mass (m/z −1) | Compound | Family | |
|---|---|---|---|---|
| Unfermented SCG Extract | 13.40 | 353 | Caffeoylquinic acid | Hydroxycinnamic acids |
| 15.58 | 179 | Caffeic acid | Hydroxycinnamic acids | |
| Extract from T. harzianum Fermentation | 13.01 | 353 | Caffeoylquinic acid | Hydroxycinnamic acids |
| 15.09 | 179 | Caffeic acid | Hydroxycinnamic acids | |
| 19.41 | 367 | Cynarin | Hydroxycinnamic acids | |
| 20.75 | 367 | Ferulic acid | Hydroxycinnamic acids | |
| R. oryzae Fermentation Extract | 12.83 | 353 | Caffeoylquinic acid | Hydroxycinnamic acids |
| 19.22 | 179 | Caffeic acid | Hydroxycinnamic acids | |
| 29.49 | 193 | Feruloylquinic | Hydroxycinnamic acids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, K.A.; Aguilar, C.N.; Ramírez-Guzmán, N.; Ruiz, H.A.; Martínez, J.L.; Chávez-González, M.L. Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds. Fermentation 2025, 11, 366. https://doi.org/10.3390/fermentation11070366
Luna KA, Aguilar CN, Ramírez-Guzmán N, Ruiz HA, Martínez JL, Chávez-González ML. Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds. Fermentation. 2025; 11(7):366. https://doi.org/10.3390/fermentation11070366
Chicago/Turabian StyleLuna, Karla A., Cristóbal N. Aguilar, Nathiely Ramírez-Guzmán, Héctor A. Ruiz, José Luis Martínez, and Mónica L. Chávez-González. 2025. "Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds" Fermentation 11, no. 7: 366. https://doi.org/10.3390/fermentation11070366
APA StyleLuna, K. A., Aguilar, C. N., Ramírez-Guzmán, N., Ruiz, H. A., Martínez, J. L., & Chávez-González, M. L. (2025). Bioprocessing of Spent Coffee Grounds as a Sustainable Alternative for the Production of Bioactive Compounds. Fermentation, 11(7), 366. https://doi.org/10.3390/fermentation11070366