Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease
Abstract
1. Introduction
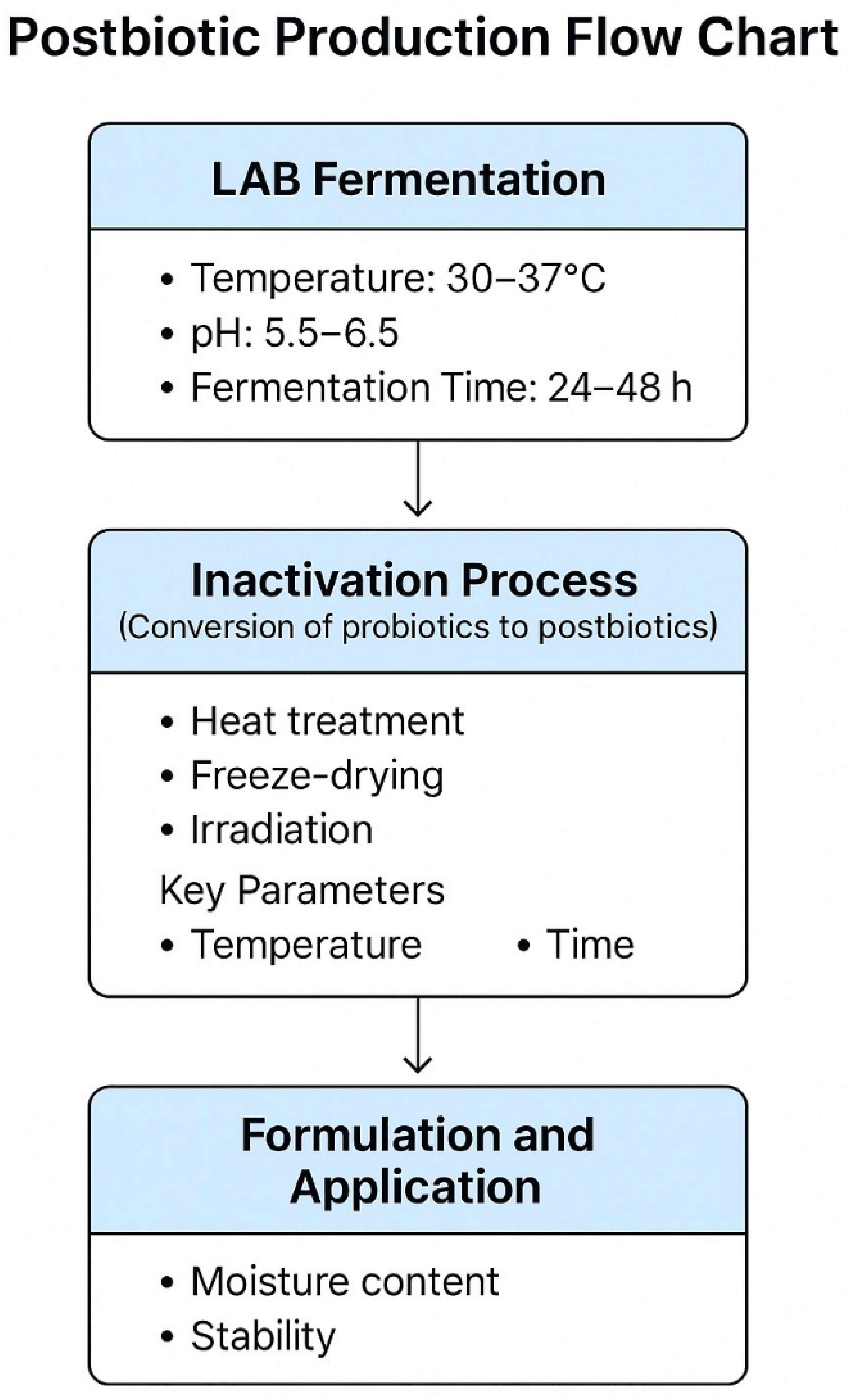
2. Postbiotic: Mechanisms of Action and Benefits
3. Pathophysiology of Muscular Complications in Inflammatory Bowel Disease
4. Application of Postbiotics Derived from Lactic Acid Bacteria in Muscular Complications
5. Postbiotics as Adjuvant Therapy in the Management of Muscular Complications of Inflammatory Bowel Disease
6. Challenges and Limitations in the Use of Postbiotics for Muscular Complications in Inflammatory Bowel Disease
7. Future Perspectives on the Use of Postbiotics Derived from Lactic Acid Bacteria
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| EPS | Exopolysaccharide |
| IBD | Inflammatory bowel disease |
| IL-1β | Interleukin 1 beta |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| LAB | Lactic acid bacteria |
| mTOR | Mammalian Target of Rapamycin |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| TNF-α | Tumor necrosis factor |
References
- Ashton, J.J.; Beattie, R.M. Inflammatory Bowel Disease: Recent Developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bernstein, C.N. Environmental Risk Factors for Inflammatory Bowel Disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakamura, S.; Miyazaki, T.; Kakimoto, K.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J. Clin. Med. 2021, 10, 4214. [Google Scholar] [CrossRef] [PubMed]
- Oli, A.K.; Maidur, R.N.; Hurkadli, P.S.; Javalgi, A.P.; Javaregowda, P.K.; Goni, M. Incidence of Inflammatory Bowel Disease: A Single Centre Retrospective Study. Arq. Gastroenterol. 2022, 59, 345–351. [Google Scholar] [CrossRef]
- Fatani, H.; Olaru, A.; Stevenson, R.; Alharazi, W.; Jafer, A.; Atherton, P.; Brook, M.; Moran, G. Systematic Review of Sarcopenia in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 1276–1291. [Google Scholar] [CrossRef]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and Malnutrition in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.; Simões, C.D.; Martins, J.O.; Sousa, A.S. Inflammatory Bowel Disease and Sarcopenia: A Focus on Muscle Strength—Narrative Review. Arq. Gastroenterol. 2023, 60, 373–382. [Google Scholar] [CrossRef]
- Tie, Y.; Huang, Y.; Chen, R.; Li, L.; Chen, M.; Zhang, S. Current Insights on the Roles of Gut Microbiota in Inflammatory Bowel Disease-Associated Extra-Intestinal Manifestations: Pathophysiology and Therapeutic Targets. Gut Microbes 2023, 15, 2265028. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]
- Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An Alternative and Innovative Intervention for the Therapy of Inflammatory Bowel Disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic Production: Harnessing the Power of Microbial Metabolites for Health Applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, L.F.; Gervasoni, K.; de Oliveira Silva, K.; Ferraz Mendes, M.E.; Maddela, N.R.; Prasad, R.; Winkelstroter, L.K. Postbiotics in Active Food Packaging: The Contribution of Cellulose Nanocomposites. Sustain. Chem. Pharm. 2023, 36, 101280. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-Inactivated Bifidobacterium Bifidum MIMBb75 (SYN-HI-001) in the Treatment of Irritable Bowel Syndrome: A Multicentre, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Favero, C.; Giordano, L.; Mihaila, S.M.; Masereeuw, R.; Ortiz, A.; Sanchez-Niño, M.D. Postbiotics and Kidney Disease. Toxins 2022, 14, 623. [Google Scholar] [CrossRef]
- Ying, Z.-H.; Mao, C.-L.; Xie, W.; Yu, C.-H. Postbiotics in Rheumatoid Arthritis: Emerging Mechanisms and Intervention Perspectives. Front. Microbiol. 2023, 14, 1290015. [Google Scholar] [CrossRef]
- Kvakova, M.; Kamlarova, A.; Stofilova, J.; Benetinova, V.; Bertkova, I. Probiotics and Postbiotics in Colorectal Cancer: Prevention and Complementary Therapy. World J. Gastroenterol. 2022, 28, 3370–3382. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2024, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16, 2244. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Rodriguez, J.; Wee, J. Dietary Postbiotics Reduce Cytotoxicity and Inflammation Induced by Crystalline Silica in an In Vitro RAW 264.7 Macrophage Model. Foods 2022, 11, 877. [Google Scholar] [CrossRef]
- Wang, M.; Ren, Y.; Guo, X.; Ye, Y.; Zhu, H.; Zhang, J.; Huang, Z.; Yu, K. Postbiotics from Lactobacillus Delbrueckii Alleviate Intestinal Inflammation by Promoting the Expansion of Intestinal Stem Cells in S. Typhimurium-Induced Mice. Foods 2024, 13, 874. [Google Scholar] [CrossRef]
- Di Chiano, M.; Rocchetti, M.T.; Spano, G.; Russo, P.; Allegretta, C.; Milior, G.; Gadaleta, R.M.; Sallustio, F.; Pontrelli, P.; Gesualdo, L.; et al. Lactobacilli Cell-Free Supernatants Modulate Inflammation and Oxidative Stress in Human Microglia via NRF2-SOD1 Signaling. Cell. Mol. Neurobiol. 2024, 44, 60. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Mishra, A.K.; Mohanta, Y.K.; Yadavalli, R.; Agrawal, D.C.; Reddy, H.P.; Gorrepati, R.; Reddy, C.N.; Mandal, S.K.; Shamim, M.Z.; et al. Postbiotics: The New Horizons of Microbial Functional Bioactive Compounds in Food Preservation and Security. Food Prod. Process. Nutr. 2024, 6, 28. [Google Scholar] [CrossRef]
- Alameri, F.; Tarique, M.; Osaili, T.; Obaid, R.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Fernandez-Cabezudo, M.; Liu, S.-Q.; Al-Ramadi, B.; et al. Lactic Acid Bacteria Isolated from Fresh Vegetable Products: Potential Probiotic and Postbiotic Characteristics Including Immunomodulatory Effects. Microorganisms 2022, 10, 389. [Google Scholar] [CrossRef]
- Allouche, R.; Hafeez, Z.; Dary-Mourot, A.; Genay, M.; Miclo, L. Streptococcus Thermophilus: A Source of Postbiotics Displaying Anti-Inflammatory Effects in THP 1 Macrophages. Molecules 2024, 29, 1552. [Google Scholar] [CrossRef]
- Tarique, M.; Ali, A.H.; Kizhakkayil, J.; Liu, S.-Q.; Oz, F.; Dertli, E.; Kamal-Eldin, A.; Ayyash, M. Exopolysaccharides from Enterococcus faecium and Streptococcus thermophilus: Bioactivities, Gut Microbiome Effects, and Fermented Milk Rheology. Food Chem. X 2023, 21, 101073. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Liu, S.; Han, Y.; Zhou, Z. Modelling Growth and Bacteriocin Production by Pediococcus Acidilactici PA003 as a Function of Temperature and pH Value. Appl. Biochem. Biotechnol. 2012, 166, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.d.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial Properties of Pediococcus Acidilactici and Pediococcus Pentosaceus Isolated from Silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G.-J. Fermentation of Plant-Based Dairy Alternatives by Lactic Acid Bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Huang, F.-C.; Huang, S.-C. The Combined Beneficial Effects of Postbiotic Butyrate on Active Vitamin D3-Orchestrated Innate Immunity to Salmonella Colitis. Biomedicines 2021, 9, 1296. [Google Scholar] [CrossRef]
- Pesce, M.; Seguella, L.; Del Re, A.; Lu, J.; Palenca, I.; Corpetti, C.; Rurgo, S.; Sanseverino, W.; Sarnelli, G.; Esposito, G. Next-Generation Probiotics for Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 5466. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus Plantarum NCU116 Regulate Intestinal Barrier Function via STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef]
- Sigala-Robles, R.; Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Peptides, Exopolysaccharides, and Short-Chain Fatty Acids from Fermented Milk and Perspectives on Inflammatory Bowel Diseases. Dig. Dis. Sci. 2022, 67, 4654–4665. [Google Scholar] [CrossRef]
- Yadav, R.S. Multifunctional Nanomaterials: Synthesis, Properties and Applications. Int. J. Mol. Sci. 2021, 22, 12073. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; and Wang, J. Oxidative Stress Tolerance and Antioxidant Capacity of Lactic Acid Bacteria as Probiotic: A Systematic Review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Łepecka, A.; Szymański, P.; Okoń, A.; Zielińska, D. Antioxidant Activity of Environmental Lactic Acid Bacteria Strains Isolated from Organic Raw Fermented Meat Products. LWT 2023, 174, 114440. [Google Scholar] [CrossRef]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-Free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Calvillo, Á.; Pellicer, T.; Carnicer, M.; Planas, A. Bioprocess Strategies for Vitamin B12 Production by Microbial Fermentation and Its Market Applications. Bioengineering 2022, 9, 365. [Google Scholar] [CrossRef]
- Pérez-Alvarado, O.; Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; Vinderola, G.; García-Cayuela, T. Role of Lactic Acid Bacteria and Yeasts in Sourdough Fermentation during Breadmaking: Evaluation of Postbiotic-like Components and Health Benefits. Front. Microbiol. 2022, 13, 969460. [Google Scholar] [CrossRef]
- D’ambrosio, S.; Dabous, A.; Sadiq, S.; Casillo, A.; Schiraldi, C.; Cassese, E.; Bedini, E.; Corsaro, M.M.; Cimini, D. Bifidobacterium Animalis Subsp. Lactis HN019 Live Probiotics and Postbiotics: Production Strategies and Bioactivity Evaluation for Potential Therapeutic Properties. Front. Bioeng. Biotechnol. 2024, 12, 1379574. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2025, 17, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, X.; Huang, K.; Liang, Z. Pre-Administration of Saccharomyces Boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice. Foods 2025, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Pizzoferrato, M.; de Sire, R.; Ingravalle, F.; Mentella, M.C.; Petito, V.; Martone, A.M.; Landi, F.; Miggiano, G.A.D.; Mele, M.C.; Lopetuso, L.R.; et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients 2019, 11, 2281. [Google Scholar] [CrossRef]
- Steell, L.; Gray, S.R.; Russell, R.K.; MacDonald, J.; Seenan, J.P.; Wong, S.C.; Gaya, D.R. Pathogenesis of Musculoskeletal Deficits in Children and Adults with Inflammatory Bowel Disease. Nutrients 2021, 13, 2899. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Ludwiczek, O.; Gabriel, M.; Obrist, P.; Wolf, A.M.; Tilg, H. The RANKL/OPG System Is Activated in Inflammatory Bowel Disease and Relates to the State of Bone Loss. Gut 2005, 54, 479–487. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. Effects of Vitamin D Supplementation on Blood Markers in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2022, 61, 23–35. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Xue, G.; Han, L.; Jia, H.; Wang, Z.; Zhang, J.; Liu, P.; Yang, C.; Zhou, Y. Role of Short-Chain Fatty Acids in Host Physiology. Anim. Models Exp. Med. 2024, 7, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; Ticinesi, A.; Sokol, H.; Barreto, P.D.S. Therapeutic Perspectives of Pre-, pro-, Post-Biotics in the Treatment of Sarcopenia. J. Nutr. Health Aging 2024, 28, 100298. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Kim, J.-H.; Jung, Y.-J.; Kwak, M.-S.; Sung, M.-H.; Imm, J.-Y. KL-Biome (Postbiotic Formulation of Lactiplantibacillus Plantarum KM2) Improves Dexamethasone-Induced Muscle Atrophy in Mice. Int. J. Mol. Sci. 2024, 25, 7499. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- Lee, M.-C.; Ho, C.-S.; Hsu, Y.-J.; Huang, C.-C. Live and Heat-Killed Probiotic Lactobacillus Paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Zeng, Z.; Fei, L.; Yang, J.; Zuo, J.; Huang, Z.; Li, H. MiR-27a-3p Targets GLP1R to Regulate Differentiation, Autophagy, and Release of Inflammatory Factors in Pre-Osteoblasts via the AMPK Signaling Pathway. Front. Genet. 2021, 12, 783352. [Google Scholar] [CrossRef]
- Bednarska, O.; Biskou, O.; Israelsen, H.; Winberg, M.E.; Walter, S.; Keita, Å.V. A Postbiotic Fermented Oat Gruel May Have a Beneficial Effect on the Colonic Mucosal Barrier in Patients with Irritable Bowel Syndrome. Front. Nutr. 2022, 9, 1004084. [Google Scholar] [CrossRef]
- Freitas, A.D.S.; Barroso, F.A.L.; Campos, G.M.; Américo, M.F.; Viegas, R.C.D.S.; Gomes, G.C.; Vital, K.D.; Fernandes, S.O.A.; Carvalho, R.D.d.O.; Jardin, J.; et al. Exploring the Anti-Inflammatory Effects of Postbiotic Proteins from Lactobacillus Delbrueckii CIDCA 133 on Inflammatory Bowel Disease Model. Int. J. Biol. Macromol. 2024, 277, 134216. [Google Scholar] [CrossRef]
- Han, S.; Seo, K.-H.; Gyu Lee, H.; Kim, H. Effect of Cucumis melo L. Peel Extract Supplemented Postbiotics on Reprograming Gut Microbiota and Sarcopenia in Hindlimb-Immobilized Mice. Food Res. Int. 2023, 173, 113476. [Google Scholar] [CrossRef]
- Lin, D.; Rezaei, M.J. Plant Polysaccharides and Antioxidant Benefits for Exercise Performance and Gut Health: From Molecular Pathways to Clinic. Mol. Cell. Biochem. 2025, 480, 2827–2846. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Kim, H.J.; Chae, J.; Ku, B.-H.; Lim, J.-M.; Kim, Y.-S.; Choi, J.-S. Preventing Effects of Exopolymers Purified from Aureobasidium Pullulans (EAP) Supplementation and Resistance Exercise on Muscle Aging and Loss in the Korean Elderly: A Randomized Controlled Trial. Toxicol. Environ. Health Sci. 2021, 13, 237–250. [Google Scholar] [CrossRef]
- Kang, C.-H.; Jung, E.-S.; Jung, S.-J.; Han, Y.-H.; Chae, S.-W.; Jeong, D.Y.; Kim, B.-C.; Lee, S.-O.; Yoon, S.-J. Pasteurized Akkermansia Muciniphila HB05 (HB05P) Improves Muscle Strength and Function: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2024, 16, 4037. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Nigro, F.; Porpora, M.; Bellomo, C.; Furone, F.; Budelli, A.L.; Nigro, R.; Barone, M.V.; Nanayakkara, M. Gliadin Peptide P31-43 Induces mTOR/NFkβ Activation and Reduces Autophagy: The Role of Lactobacillus Paracasei CBA L74 Postbiotc. Int. J. Mol. Sci. 2022, 23, 3655. [Google Scholar] [CrossRef]
- Rocha, R.; de J Santos, G.; Santana, G. Influence of Nutritional Status in the Postoperative Period of Patients with Inflammatory Bowel Disease. World J. Gastrointest. Pharmacol. Ther. 2021, 12, 90–99. [Google Scholar] [CrossRef]
- Avci, G.A.; Yilmaz, Ü.İ.; Avci, E. Efficacy of Probiotics, Paraprobiotics, and Postbiotics in Colorectal Cancer Cell Line and Their Role in Immune Response. Rev. Assoc. Med. Bras. 2024, 70, e20240226. [Google Scholar] [CrossRef]
- Li, C.; Dong, N.; Wu, B.; Mo, Z.; Xie, J.; Lu, Q. Dihydroberberine, an Isoquinoline Alkaloid, Exhibits Protective Effect against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Phytomedicine 2021, 90, 153631. [Google Scholar] [CrossRef]
- Olsen, W.; Liang, N.; Dallas, D.C. Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests. Nutrients 2023, 15, 4942. [Google Scholar] [CrossRef]
- Ferreiro, B.; Llopis-Salinero, S.; Lardies, B.; Granados-Colomina, C.; Milà-Villarroel, R. Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients 2021, 13, 3623. [Google Scholar] [CrossRef]
- Kim, M.; Eo, H.; Lim, J.G.; Lim, H.; Lim, Y. Can Low-Dose of Dietary Vitamin E Supplementation Reduce Exercise-Induced Muscle Damage and Oxidative Stress? A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 1599. [Google Scholar] [CrossRef]
- Maioli, T.U.; Trindade, L.M.; Souza, A.; Torres, L.; Andrade, M.E.R.; Cardoso, V.N.; Generoso, S.V. Non-Pharmacologic Strategies for the Management of Intestinal Inflammation. Biomed. Pharmacother. 2022, 145, 112414. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Pan, H.; Zhang, Y.; Zhang, Y.; Zhu, Q.; Hong, Y.; Huang, Z.; Li, Y.; Feng, X.; Fang, Y.; et al. GelNB Molecular Coating as a Biophysical Barrier to Isolate Intestinal Irritating Metabolites and Regulate Intestinal Microbial Homeostasis in the Treatment of Inflammatory Bowel Disease. Bioact. Mater. 2023, 19, 251–267. [Google Scholar] [CrossRef]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-Lactic Acid, a Metabolite of Tryptophan, Secreted by Bifidobacterium longum Subspecies Infantis Is Anti-Inflammatory in the Immature Intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Sun, L.; Liu, X.; Zhang, W.; Hao, J. Indole-3-Acetic Acid Alleviates DSS-Induced Colitis by Promoting the Production of R-Equol from Bifidobacterium pseudolongum. Gut Microbes 2024, 16, 2329147. [Google Scholar] [CrossRef]
- Del Chierico, F.; Masi, L.; Petito, V.; Baldelli, V.; Puca, P.; Benvenuto, R.; Fidaleo, M.; Palucci, I.; Lopetuso, L.R.; Caristo, M.E.; et al. Solute Transporter OCTN1/Slc22a4 Affects Disease Severity and Response to Infliximab in Experimental Colitis: Role of Gut Microbiota and Immune Modulation. Inflamm. Bowel Dis. 2024, 30, 2259–2270. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Lorente-Cebrián, S.; Aranaz, P.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef] [PubMed]
- Xingrong, L.; Gorish, B.M.T.; Qaria, M.A.; Hussain, A.; Abdelmula, W.I.Y.; Zhu, D. Unlocking Ectoine’s Postbiotic Therapeutic Promise: Mechanisms, Applications, and Future Directions. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Calik, A.; Blue, C.E.C.; Dalloul, R.A. Impact of Early Postbiotic Supplementation on Broilers’ Responses to Subclinical Necrotic Enteritis. Poult. Sci. 2024, 103, 104420. [Google Scholar] [CrossRef]
- Qureshi, W.; Dar, M.A.; Rather, M.Y. New Therapy for Metabolic Syndrome: Gut Microbiome Supplementation. World J. Diabetes 2024, 15, 1833–1836. [Google Scholar] [CrossRef]
- Rahimi, A.; Qaisar, S.A.; Janeh, T.; Karimpour, H.; Darbandi, M.; Moludi, J. Clinical Trial of the Effects of Postbiotic Supplementation on Inflammation, Oxidative Stress, and Clinical Outcomes in Patients with CVA. Sci. Rep. 2024, 14, 24021. [Google Scholar] [CrossRef]
- Ríus, A.G.; Kaufman, J.D.; Li, M.M.; Hanigan, M.D.; Ipharraguerre, I.R. Physiological Responses of Holstein Calves to Heat Stress and Dietary Supplementation with a Postbiotic from Aspergillus Oryzae. Sci. Rep. 2022, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.R.; Mahajan, J.; Teleki, A. Iron Oxide Nanoparticles for Treatment and Diagnosis of Chronic Inflammatory Diseases: A Systematic Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1963. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzyme Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Free, L.; Hinds, L.M.; Vijayaraghavan, R.K.; Daniels, S.; O’Donnell, C.P.; Tiwari, B.K. Effect of Non-Thermal Plasma Technology on Microbial Inactivation and Total Phenolic Content of a Model Liquid Food System and Black Pepper Grains. LWT 2020, 118, 108716. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.-J.; Jiao, Z.; Li, X. Probiotics, Prebiotics, and Postbiotics in Health and Disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Ma, Y.; Liu, N.-N.; Wang, H. Beneficial Insights into Postbiotics against Colorectal Cancer. Front. Nutr. 2023, 10, 1111872. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-Biotics, and Post-Biotics. Front. Nutr. 2021, 8, 634897. [Google Scholar] [CrossRef]
- Bacterial Lysates-Containing Medicinal Products Indicated for Respiratory Conditions—Referral|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/referrals/bacterial-lysates-containing-medicinal-products-indicated-respiratory-conditions (accessed on 30 May 2025).
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.-H.; Li, J.; Chow, S.K.-H.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the Gut Microbiota and Sarcopenia: A Systematic Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef] [PubMed]



| Category | Biometabolite | Mechanism | Proprieties | Functionality | References |
|---|---|---|---|---|---|
| Short chain fatty acids | Butyrate Acetate Propionate | Histone inhibition and activation of G-protein-coupled surface receptors | Anti-inflammatory, antioxidant, anti-microbial, anti-catabolic | Intestinal barrier maintenance, muscle restoration, protection against pathogens | [36,37,38,39]. |
| Carbohydrates | Exopolysaccharides | Stimulation of dendritic cells and macrophages, neutralization of reactive oxygen species and activation of STAT3 | Anti-inflammatory and antioxidant | Immune response modulation, oxidative stress reduction and barrier restoration | [17,26,40,41]. |
| Enzymes | Glutathione peroxidase Superoxide dismutase NADH peroxidase | Dismutation and removal of superoxide and hydrogen peroxide radicals | Antioxidant | Oxidative stress reduction | [14,42,47]. |
| Organic acids | Lactic acid Propionic acid 3-phenyllactic acid | pH reduction | Antimicrobial | Protection against pathogens | [15,45]. |
| Proteins | S-Layer Bacteriocins | Inhibition of adhesion and formation of pores in the membrane of microorganisms | Antimicrobial | Protection against pathogens | [15,26,46,48]. |
| Vitamins | Vitamin B Vitamin K | Enzymatic cofactors | Maintains homeostasis | Cellular maintenance | [15,49]. |
| Biosurfactants | - | Inhibition of adhesion of microorganisms | Antimicrobial | Protection against pathogens and inhibition of biofilm formation | [15]. |
| Therapeutic Component | Objective | Example | Synergistic Benefits | Potential Adverse Effects/Limitations | References | |
|---|---|---|---|---|---|---|
| Biotic therapy | Postbiotics | Reduction in inflammation and mitochondrial improvement | Short-chain fatty acids, exopolysaccharides, bacterial peptidoglycans | Reduced inflammation, improved cellular function | Individual responses vary, need for standardization | [70,80]. |
| Nutritional therapies | Protein diet | Stimulation of protein synthesis | Leucine, whey protein | Muscle mass recovery and maintenance | Risk of renal overload in patients with renal failure | [78,79]. |
| Omega-3 and antioxidants | Oxidative stress reduction and inflammation | Supplementation with omega-3, vitamin E | Reduced muscle damage and chronic inflammation | Possible interactions with anticoagulants | [80,81]. | |
| Biotic therapy | Anti-inflammatories | Control of intestinal inflammation | Mesalazine, corticosteroids | Reduced intestinal inflammatory activity | Adverse effects such as osteoporosis and insulin resistance | [82,83]. |
| Immunosuppressants | Modulation of the immune response | Azathioprine, infliximab | Prevention of inflammatory flare-ups and muscle protection | Increased risk of infections and systemic adverse effects | [77,84,85]. |
| Form of Administration | Example of Postbiotic | Advantages | Challenges | Bioavailability | References |
|---|---|---|---|---|---|
| Food supplements | Short-chain fatty acids, bioactive peptides | Precise dosage control, convenience | Need for standardization of the ideal dose | Medium, depends on formulation and intestinal absorption | [79,80]. |
| Functional foods | Postbiotics enriched in yogurts, fermented beverages | Good consumer acceptance, easy daily consumption | There may be degradation of bioactive compounds | Variable, influenced by digestive processes | [79,81,82]. |
| Nutritional formulas | Mixture of metabolites derived from Lactobacillus and Bifidobacterium | Indicated for patients with eating difficulties | High cost and lower adherence in healthy individuals | High, due to formulation optimized for absorption | [77,79]. |
| Nanotechnology | Nanoencapsulation of postbiotics | Increases bioavailability and protection of compounds | Technology still under development, high cost | Very high, protection against gastric degradation | [77,83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, E.B.T.; Silva, K.d.O.; Mendes, M.E.F.; de Oliveira, L.B.; Menezes, F.P.d.; Imperador, A.C.; Correia, L.F.; Winkelstroter, L.K. Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease. Fermentation 2025, 11, 362. https://doi.org/10.3390/fermentation11070362
Bueno EBT, Silva KdO, Mendes MEF, de Oliveira LB, Menezes FPd, Imperador AC, Correia LF, Winkelstroter LK. Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease. Fermentation. 2025; 11(7):362. https://doi.org/10.3390/fermentation11070362
Chicago/Turabian StyleBueno, Emili Bruna Toso, Kimberlly de Oliveira Silva, Maria Eduarda Ferraz Mendes, Lívia Batista de Oliveira, Felipe Prado de Menezes, Anna Cardoso Imperador, Lucimeire Fernandes Correia, and Lizziane Kretli Winkelstroter. 2025. "Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease" Fermentation 11, no. 7: 362. https://doi.org/10.3390/fermentation11070362
APA StyleBueno, E. B. T., Silva, K. d. O., Mendes, M. E. F., de Oliveira, L. B., Menezes, F. P. d., Imperador, A. C., Correia, L. F., & Winkelstroter, L. K. (2025). Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease. Fermentation, 11(7), 362. https://doi.org/10.3390/fermentation11070362








