Optimization of Pectinase Production from Silkworm Excrement Using Aspergillus niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Substrate
2.2. Medium and Fermentation Conditions
2.3. Experimental Design
2.3.1. Single-Factor Experiments
2.3.2. Box–Behnken Design
2.4. Pectinase Assay
3. Results
3.1. Single-Factor Analysis of Pectinase Production
3.2. Optimization of Medium Components for Pectinase Production by Box–Behnken Design
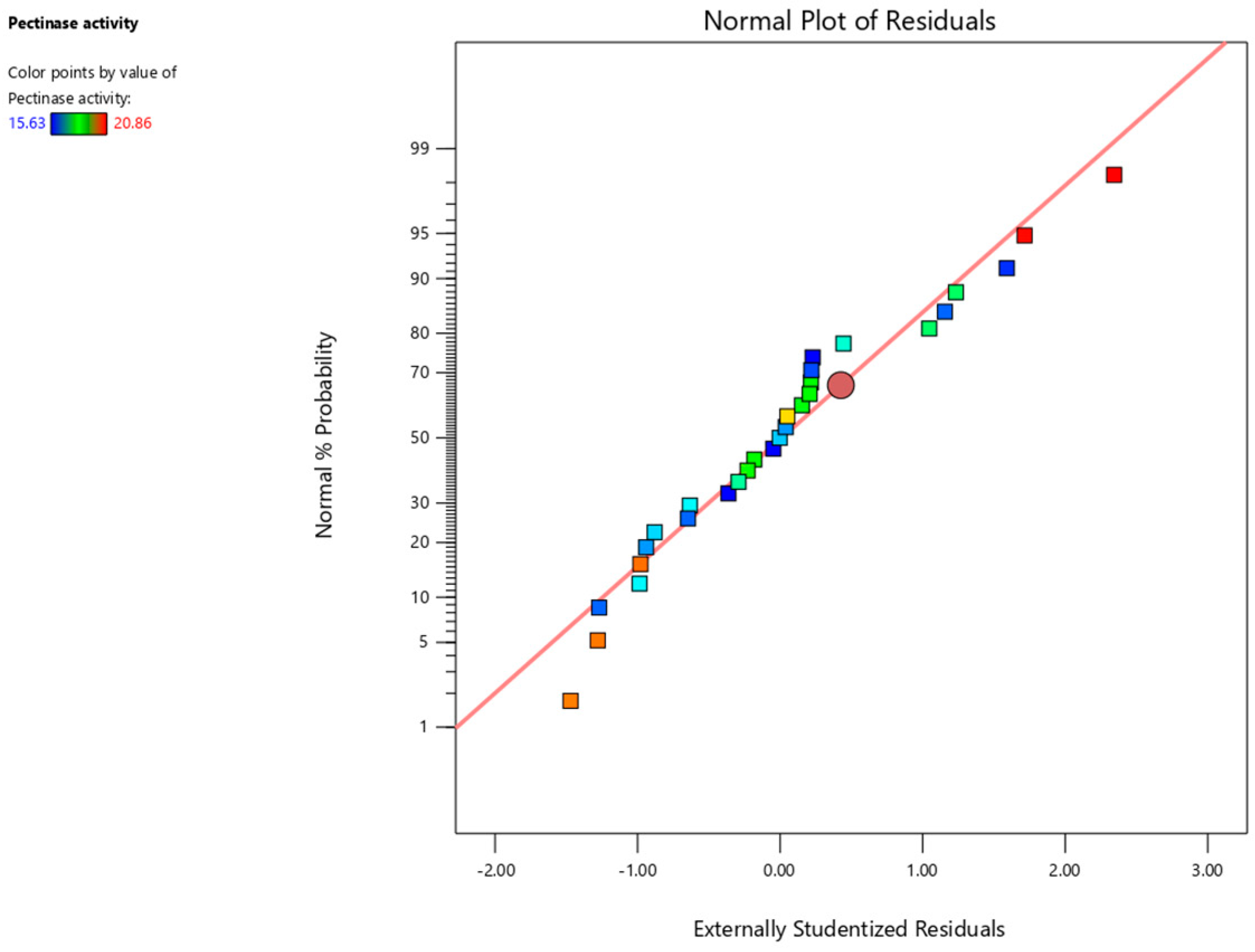
3.3. Validation of the RSM Model
4. Discussion
4.1. Optimization of Fermentation Medium Components
4.2. Interaction Analysis and Model Validation
4.3. Practical Applications and Environmental Sustainability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Bian, P.; Huang, H.; Zhong, K.; Huang, D.; Nong, X.; Mao, J.; Zhang, C. The mechanism of silkworm excrement organic fertilizer to reduce the Cd availability in paddy soil. Soil Sediment Contam. 2022, 31, 1–14. [Google Scholar] [CrossRef]
- Shen, X.; He, J.; Zhang, N.; Li, Y.; Lei, X.; Sun, C.; Muhammad, A.; Shao, Y. Assessing the quality and eco-beneficial microbes in the use of silkworm excrement compost. Waste Manag. 2024, 183, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Lu, F.; Yang, L.; Li, T. Cultivation of earthworms and analysis of associated bacterial communities during earthworms’ growth using two types of agricultural wastes. Bioresour. Bioprocess. 2024, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yang, J.; Lin, M.; Chen, Q.; Wang, B.; Zhao, J.; Rensing, C.; Liu, H.; Fan, Z.; Feng, R. Using silkworm excrement to restore vegetation and soil ecology in heavily contaminated mining soils by multiple metal (loid)s: A recyclable sericultural measure. J. Hazard. Mater. 2023, 459, 132184. [Google Scholar] [CrossRef]
- Fan, W.; Kong, Q.; Chen, Y.; Lu, F.; Wang, S.; Zhao, A. Safe utilization and remediation potential of the mulberry-silkworm system in heavy metal-contaminated lands: A review. Sci. Total Environ. 2024, 927, 172352. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Zhang, Y.; Xu, X.; Zhao, Y.; Jiang, X.; Zhang, R.; Gui, Z. Characterization of cellulose-degrading bacteria isolated from silkworm excrement and optimization of its cellulase production. Polymers 2023, 15, 4142. [Google Scholar] [CrossRef]
- He, H.; Luo, C.; Li, F.; Lu, S.; Qin, Y. Optimization of processing conditions for the production of pectinase by fermentation of silkworm sand. China Feed 2014, 13, 22–24. [Google Scholar]
- He, H.; Li, F.; Tu, X.; Qin, Y. Study on the optimum condition of pectinase production by Aspergillus aculeatus with silkworm faeces fermentation. China Food Addit. 2015, 3, 92–96. [Google Scholar]
- Zion Market Research. Global Pectinase Market Report. Available online: https://www.zionmarketresearch.com/news/global-pectinase-market (accessed on 27 April 2023).
- Gonçalves, D.B.; Teixeira, J.A.; Bazzolli, D.M.S.; de Queiroz, M.V.; de Araújo, E.F. Use of response surface methodology to optimize production of pectinases by recombinant Penicillium griseoroseum T20. Biocatal. Agric. Biotechnol. 2012, 1, 140–146. [Google Scholar] [CrossRef]
- Garai, D.; Kumar, V. A Box–Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L. Ind. Crop. Prod. 2013, 44, 352–363. [Google Scholar] [CrossRef]
- Das, A.; Paul, T.; Halder, S.K.; Jana, A.; Maity, C.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Production of cellulolytic enzymes by Aspergillus fumigatus ABK9 in wheat bran-rice straw mixed substrate and use of cocktail enzymes for deinking of waste office paper pulp. Bioresour. Technol. 2013, 128, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.C.; Nai, C.; Meyer, V. How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol. Biotechnol. 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Chen, X.; Pan, B.; Yu, L.; Wang, B.; Pan, L. Enhancement of protein production in Aspergillus niger by engineering the antioxidant defense metabolism. Biotechnol. Biofuels 2024, 17, 91. [Google Scholar] [CrossRef]
- Esawy, M.A.; Gamal, A.A.; Kamel, Z.; Ismail, A.-M.S.; Abdel-Fattah, A.F. Evaluation of free and immobilized Aspergillus niger NRC1ami pectinase applicable in industrial processes. Carbohydr. Polym. 2013, 92, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Ketipally, R.; Ram, M.R. Optimization of pectinase production by Aspergillus oryzae RR 103. Curr. Agric. Res. J. 2018, 6, 37. [Google Scholar] [CrossRef]
- Abd Rahman, N.H.; Rahman, R.A.; Rahmat, Z.; Jaafar, N.R.; Puspaningsih, N.N.T.; Illias, R.M. Innovative biocatalyst synthesis of pectinolytic enzymes by cross-linking strategy: Potentially immobilized pectinases for the production of pectic-oligosaccharides from pectin. Int. J. Biol. Macromol. 2024, 256, 128260. [Google Scholar] [CrossRef]
- Abd-Elhalim, B.T.; Gamal, R.F.; El-Sayed, S.M.; Abu-Hussien, S.H. Optimizing alpha-amylase from Bacillus amyloliquefaciens on bread waste for effective industrial wastewater treatment and textile desizing through response surface methodology. Sci. Rep. 2023, 13, 19216. [Google Scholar] [CrossRef]
- de Alencar Guimarães, N.C.; Glienke, N.N.; Contato, A.G.; Galeano, R.M.; Marchetti, C.R.; Rosa, M.P.; de Sa Teles, J.S.; de Oliveira Simas, A.L.; Zanoelo, F.F.; Masui, D.C.; et al. Production and biochemical characterization of Aspergillus japonicus pectinase using a low-cost alternative carbon source for application in the clarification of fruit juices. Waste Biomass Valorization 2024, 15, 177–186. [Google Scholar] [CrossRef]
- Abd-Elhalem, B.T.; El-Sawy, M.; Gamal, R.F.; Abou-Taleb, K.A. Production of amylases from Bacillus amyloliquefaciens under submerged fermentation using some agro-industrial by-products. Ann. Agric. Sci. 2015, 60, 193–202. [Google Scholar] [CrossRef]
- Abdel Hamid, E.M.; Mohamed, A.E.; Mohamed, A.A.; Galal, A.A.; Mekhemr, A.A.; Saleh, E.S.; Hassan, M.I.; Ahmed, M.H.; Elgendy, S.K. Optimization of corn starch/glycerol, acetic acid, and cellulose fibers ratio on biodegradable plastic synthesis by Box–Behnken design (BBD). Clean Technol. Environ. Policy 2025, 1–23. [Google Scholar] [CrossRef]
- Li, Q.; Ray, C.S.; Callow, N.V.; Loman, A.A.; Islam, S.; Ju, L.-K. Aspergillus niger production of pectinase and alpha-galactosidase for enzymatic soy processing. Enzyme Microb. Technol. 2020, 134, 109476. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.; Patel, H.; Patel, N.; Vamkudoth, K.R.; Ajmera, S. Pectinase production by Aspergillus niger and its applications in fruit juice clarification. J. Pure Appl. Microbiol. 2022, 16, 2724–2737. [Google Scholar] [CrossRef]
- Esawy, M.A.; Gamal, A.A.; Kamel, Z. Optimization of Aspergillus niger NRC1ami pectinase using citrus peel pectin, purification, and thermodynamic characterization of the free and modified enzyme. Waste Biomass Valorization 2022, 13, 4823–4837. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics–A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Shrestha, S.; Khatiwada, J.R.; Kognou, A.L.M.; Chio, C.; Qin, W. A comparative study of Cellulomonas sp. and Bacillus sp. in utilizing lignocellulosic biomass as feedstocks for enzyme production. Arch. Microbiol. 2023, 205, 130. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.; Ayele, A. Pectinase from microorganisms and its industrial applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent advances in the production strategies of microbial pectinases—A review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef]
- Laswai, F.C.; Matofari, J.W.; Nduko, J.M. Pectinolytic enzyme production from orange processing waste using Aspergillus brasiliensis strain. Biomass Convers. Biorefin. 2024, 14, 25173–25186. [Google Scholar] [CrossRef]
- Hanif, A.; Ejaz, U.; Ansari, I.; Sohail, M.; Samma, M.K.; Siddiqi, M.; Suleman, F.; Karim, M. Potential application of Suaeda fruticosa and Cressa cretica biomass as a substrate for pectinase production by Geotrichum candidum. Arab. J. Sci. Eng. 2024, 49, 59–66. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Zhang, M.; Zhang, Y.; Zhao, Y.; Jiang, X.; Xin, X.; Zhang, Z.; Zhang, R.; Gui, Z. Accelerated degradation of cellulose in silkworm excrement by the interaction of housefly larvae and cellulose-degrading bacteria. J. Environ. Manag. 2022, 323, 116295. [Google Scholar] [CrossRef]
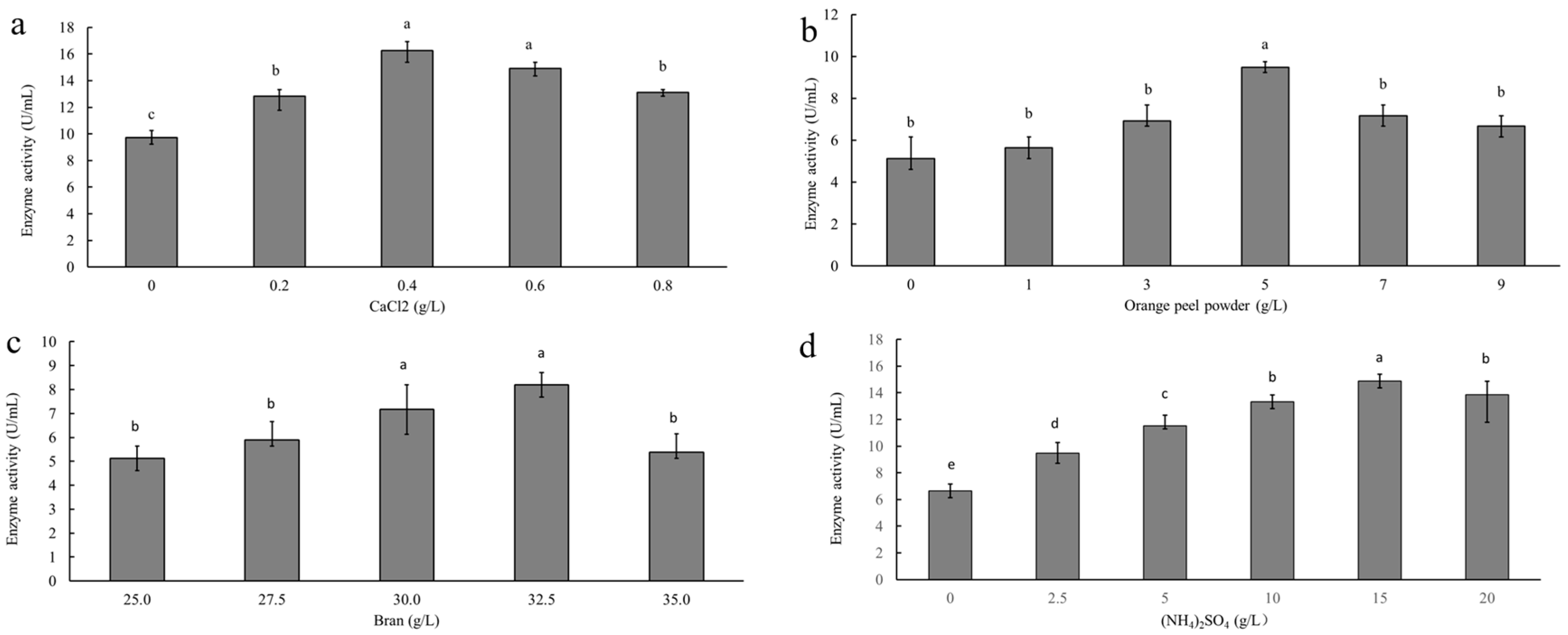

| Factor | Additive Amount (g/L) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Calcium chloride | 0 | 0.2 | 0.4 | 0.6 | 0.8 | - |
| Orange peel powder | 0 | 1 | 3 | 5 | 7 | 9 |
| Ammonium sulfate | 0 | 2.5 | 5 | 10 | 15 | 20 |
| Bran | 25 | 27.5 | 30 | 32.5 | 35 | - |
| Code | Variables | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Calcium chloride | 0.2 | 0.4 | 0.6 |
| B | Orange peel powder | 3 | 5 | 7 |
| C | Ammonium sulfate | 10 | 15 | 20 |
| D | Bran | 30 | 32.5 | 35 |
| Run | A | B | C | D | Pectinase (U/mL) | |
|---|---|---|---|---|---|---|
| Experimental | Predicted | |||||
| 1 | 1 | 0 | 0 | 1 | 17.94 | 17.92 |
| 2 | 0 | 1 | −1 | 0 | 16.74 | 16.86 |
| 3 | −1 | 0 | 0 | 1 | 17.17 | 17.11 |
| 4 | 0 | 1 | 1 | 0 | 16.15 | 16.00 |
| 5 | 0 | 0 | 0 | 0 | 20.86 | 20.48 |
| 6 | 1 | 0 | 1 | 0 | 16.15 | 16.32 |
| 7 | 0 | 0 | 1 | −1 | 15.64 | 15.61 |
| 8 | 1 | 0 | 0 | −1 | 17.43 | 17.47 |
| 9 | −1 | 0 | 1 | 0 | 16.41 | 16.54 |
| 10 | 0 | 0 | 0 | 0 | 20.25 | 20.48 |
| 11 | 0 | −1 | 0 | −1 | 16.4 | 16.39 |
| 12 | 0 | −1 | −1 | 0 | 16.91 | 17.04 |
| 13 | 0 | 0 | −1 | 1 | 16.15 | 16.24 |
| 14 | 0 | −1 | 1 | 0 | 17.68 | 17.54 |
| 15 | 1 | 1 | 0 | 0 | 15.63 | 15.64 |
| 16 | 0 | −1 | 0 | 1 | 19.74 | 19.73 |
| 17 | −1 | −1 | 0 | 0 | 15.64 | 15.69 |
| 18 | 1 | 0 | −1 | 0 | 17.69 | 17.53 |
| 19 | 0 | 0 | 0 | 0 | 20.22 | 20.48 |
| 20 | 1 | −1 | 0 | 0 | 18.71 | 18.68 |
| 21 | −1 | 0 | 0 | −1 | 16.66 | 16.66 |
| 22 | 0 | 0 | −1 | −1 | 18.71 | 18.74 |
| 23 | −1 | 0 | −1 | 0 | 15.89 | 15.69 |
| 24 | 0 | 0 | 0 | 0 | 20.78 | 20.48 |
| 25 | 0 | 0 | 0 | 0 | 20.30 | 20.48 |
| 26 | 0 | 1 | 0 | −1 | 18.45 | 18.42 |
| 27 | 0 | 1 | 0 | 1 | 16.01 | 15.98 |
| 28 | 0 | 0 | 1 | 1 | 18.97 | 19.00 |
| 29 | −1 | 1 | 0 | 0 | 16.92 | 17.01 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 79.93 | 14 | 5.71 | 132.90 | <0.0001 | significant |
| A | 1.97 | 1 | 1.97 | 45.82 | <0.0001 | |
| B | 2.24 | 1 | 2.24 | 52.05 | <0.0001 | |
| C | 0.0990 | 1 | 0.0990 | 2.30 | 0.1512 | |
| D | 0.6030 | 1 | 0.6030 | 14.04 | 0.0022 | |
| AB | 4.75 | 1 | 4.75 | 110.62 | <0.0001 | |
| AC | 1.06 | 1 | 1.06 | 24.70 | 0.0002 | |
| AD | 0.0000 | 1 | 0.0000 | 0.0000 | 1.0000 | |
| BC | 0.4624 | 1 | 0.4624 | 10.76 | 0.0055 | |
| BD | 8.35 | 1 | 8.35 | 194.42 | <0.0001 | |
| CD | 8.67 | 1 | 8.67 | 201.89 | <0.0001 | |
| A2 | 26.88 | 1 | 26.88 | 625.64 | <0.0001 | |
| B2 | 18.59 | 1 | 18.59 | 432.82 | <0.0001 | |
| C2 | 24.14 | 1 | 24.14 | 562.03 | <0.0001 | |
| D2 | 8.68 | 1 | 8.68 | 202.06 | <0.0001 | |
| Residual | 0.6014 | 14 | 0.0430 | |||
| Lack of Fit | 0.2142 | 10 | 0.0214 | 0.2212 | 0.9758 | not significant |
| Pure Error | 0.3873 | 4 | 0.0968 | |||
| Corr. Total | 80.53 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Tan, C.; Li, H.; Qian, F. Optimization of Pectinase Production from Silkworm Excrement Using Aspergillus niger. Fermentation 2025, 11, 333. https://doi.org/10.3390/fermentation11060333
Lu F, Tan C, Li H, Qian F. Optimization of Pectinase Production from Silkworm Excrement Using Aspergillus niger. Fermentation. 2025; 11(6):333. https://doi.org/10.3390/fermentation11060333
Chicago/Turabian StyleLu, Fuzhi, Caimei Tan, Huizhen Li, and Feng Qian. 2025. "Optimization of Pectinase Production from Silkworm Excrement Using Aspergillus niger" Fermentation 11, no. 6: 333. https://doi.org/10.3390/fermentation11060333
APA StyleLu, F., Tan, C., Li, H., & Qian, F. (2025). Optimization of Pectinase Production from Silkworm Excrement Using Aspergillus niger. Fermentation, 11(6), 333. https://doi.org/10.3390/fermentation11060333






