Comparison of the Effect of Different Microbial Agents on the Decomposition of Rice Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Determination Items and Methods
2.3.1. Apparent Color Record
2.3.2. Determination of the pH Value, Electrical Conductivity Value (EC Value), and Humus Polymerization Degree (E4/E6 Value)
2.3.3. Determination of Lignocellulose
2.3.4. Determination of Nutrient Content
2.4. Statistical Analysis
3. Results
3.1. Variation in Color
3.2. Variation in pH Value
3.3. Variation in EC Value
3.4. Variation in Humus Polymerization Degree (E4/E6 Value)
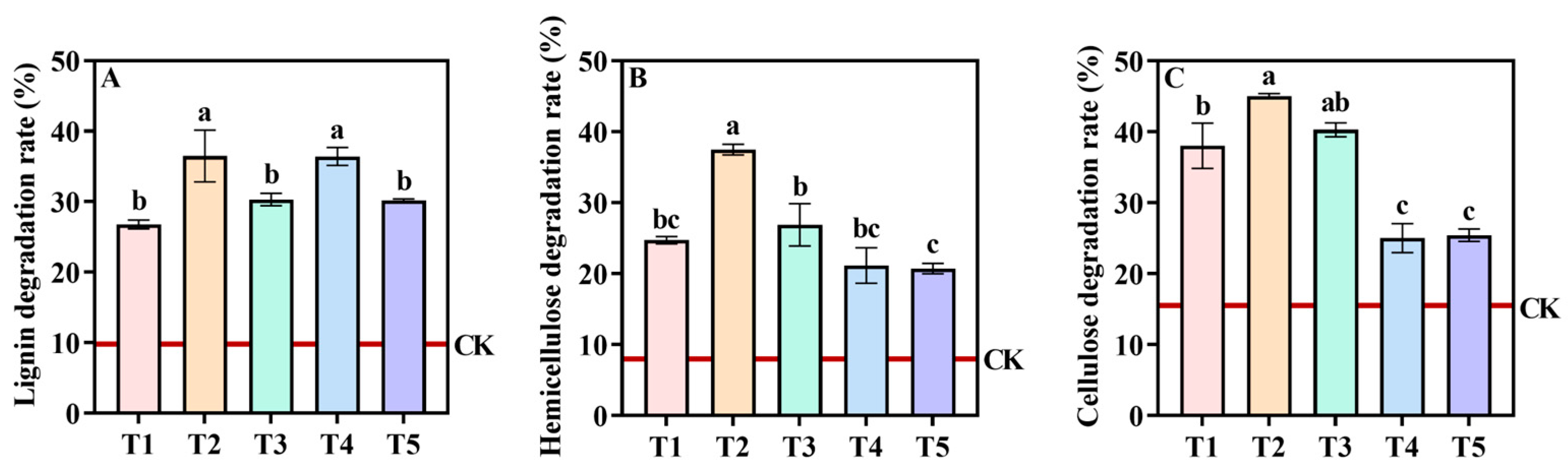
3.5. Variation in Lignocellulose Degradation Rate
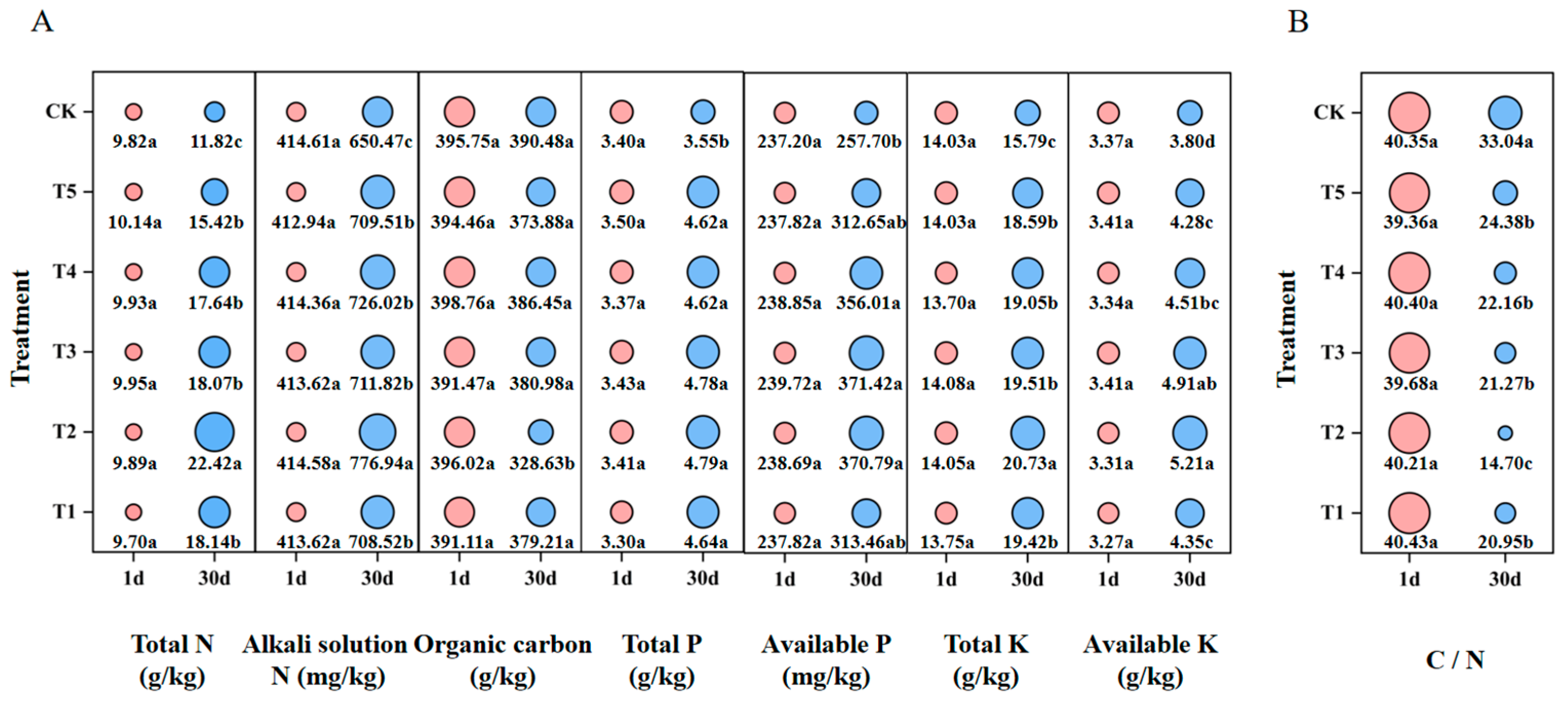
3.6. Variation in Nutrient Content
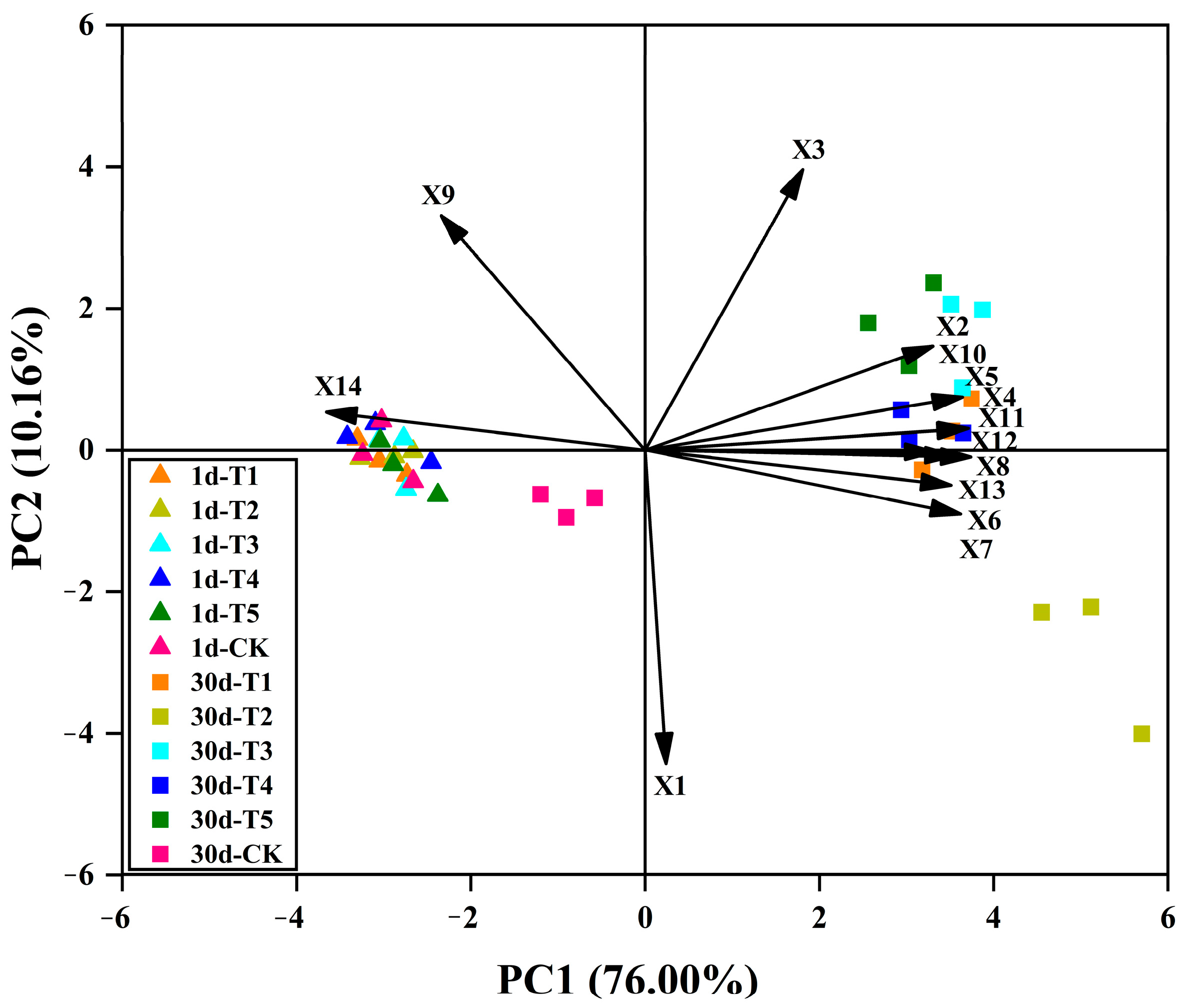
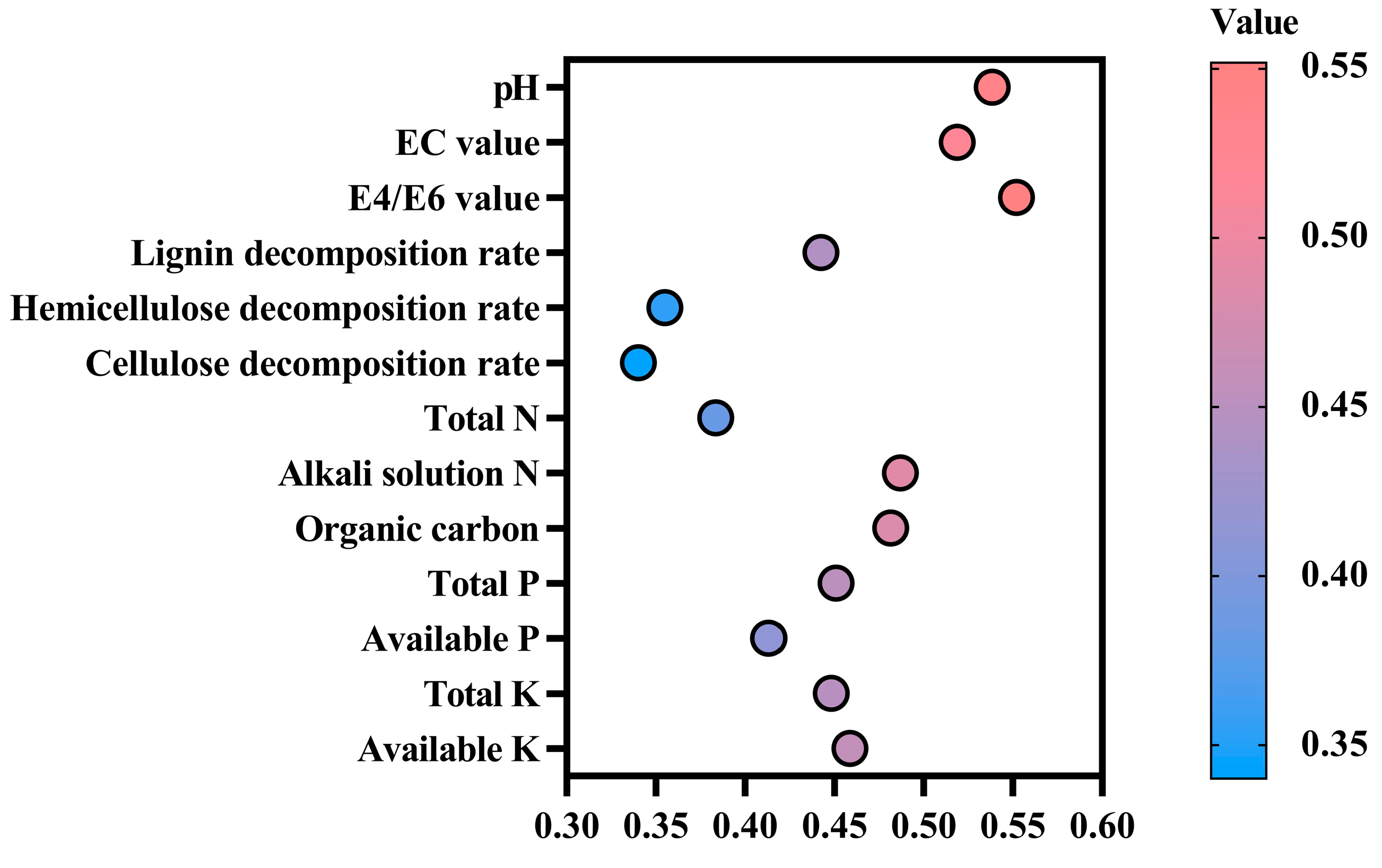
3.7. Principal Component Analysis
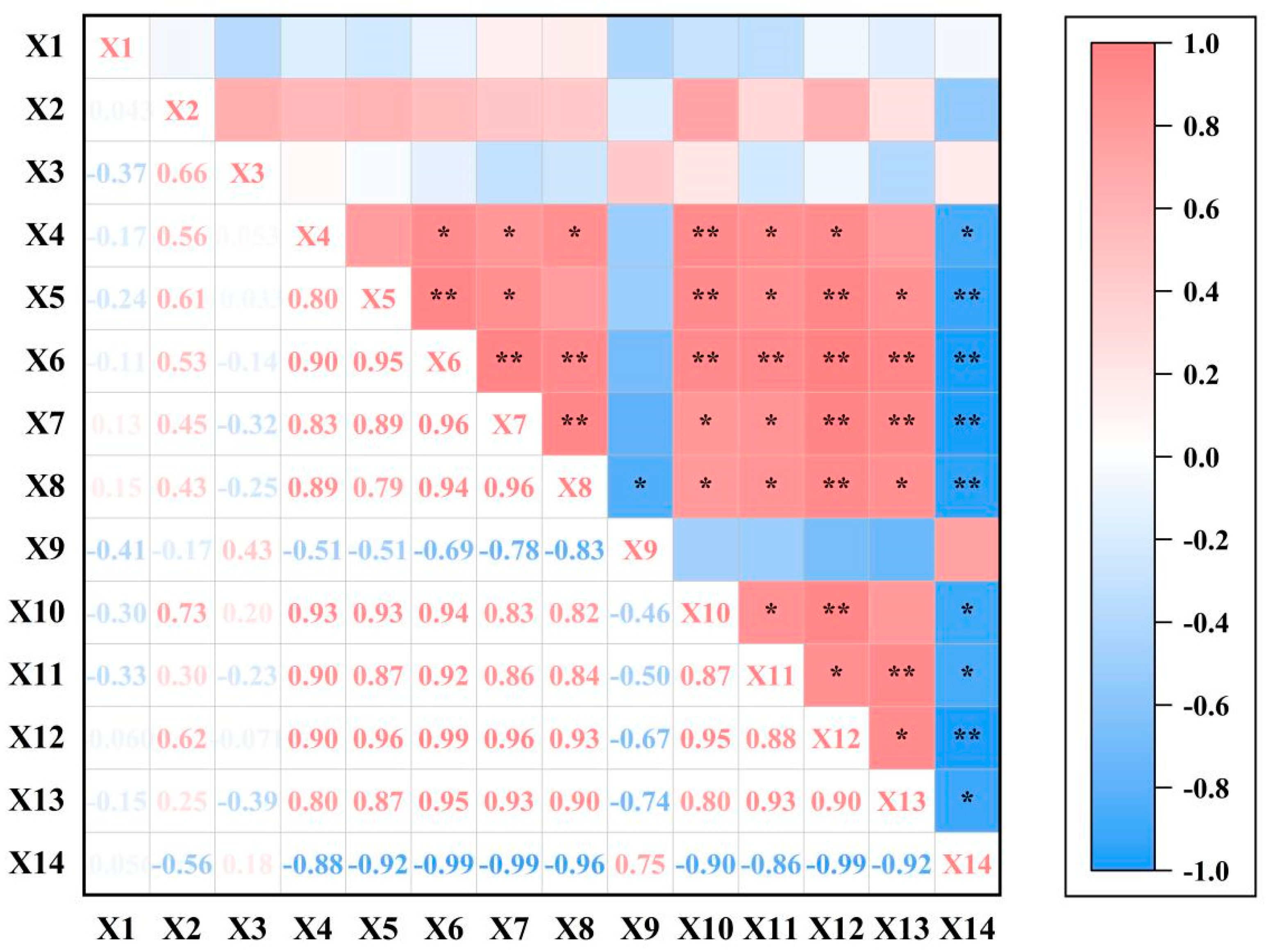
3.8. Correlation Analysis
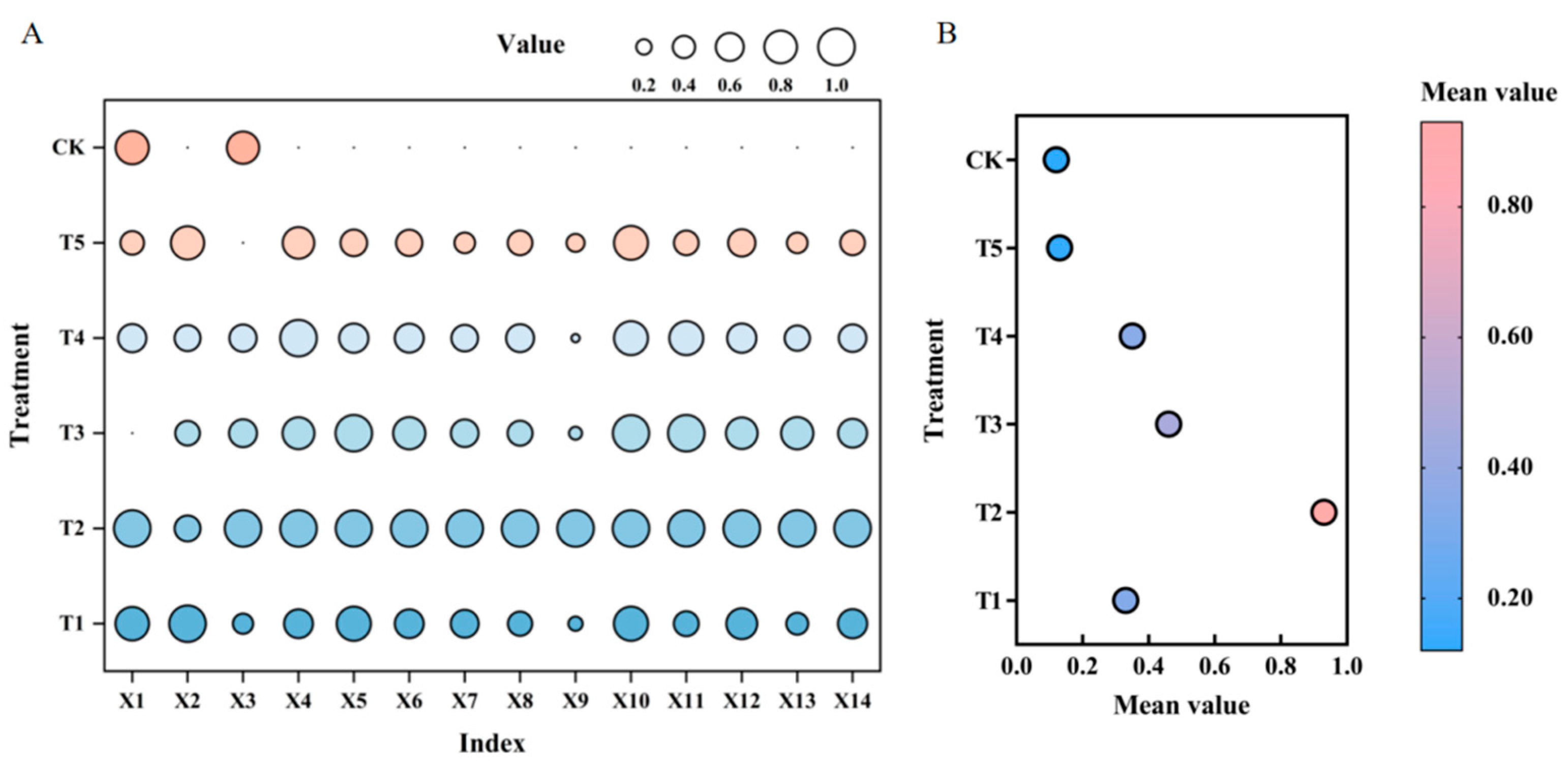
3.9. Gray Analysis Between Different Processing C/N Ratios and Other Indices
3.10. Comprehensive Evaluation of Microbial Agents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Qin, W.; Zhang, Q.; Wang, X.; Ma, Y.; Chen, Q. Evaluation of crop residues and manure production and their geographical distribution in China. J. Clean. Prod. 2018, 188, 954–965. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huang, G.; Xiao, W.; Han, L. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef]
- Nur, A.R.; Noor, F.F.; Kamarul, Z.Z.; Azrin, S. Biological and chemical composition related to particulate matter in open rice straw burning event: A mini review. AIP Conf. Proc. 2023, 2785, 030011. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Dou, S.; Ndzelu, B.S.; Ma, R.; Zhang, D.D.; Zhang, X.W.; Ye, S.F.; Wang, H.R. Effects of returning corn straw and fermented corn straw to fields on the soil organic carbon pools and humus composition. Soil 2022, 8, 605–619. [Google Scholar] [CrossRef]
- Zhan, Y.S.; Liu, W.J.; Bao, Y.Y.; Zhang, J.W.; Evangelos, P.; Li, Z.P.; Lin, X.G.; Feng, Y.Z. Fertilization shapes a well-organized community of bacterial decomposers for accelerated paddy straw degradation. Sci. Rep. 2018, 8, 7981. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Sun, S.L.; Yao, B.; Peng, Y.T.; Gao, C.F.; Qin, T.; Zhou, Y.Y.; Sun, C.R.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Geng, P.; Yang, Q.; Chen, K.; Liu, N.; Fan, Y.; Zhan, X.; Han, X. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci. Rep. 2021, 11, 15495. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z. Chemical Composition and Structure of Natural Lignocellulose. In Biotechnology of Lignocellulose; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. [Google Scholar] [CrossRef]
- Chang, J.; Lu, M.; Yin, Q.Q.; Zhen, Q.H.; Guo, H.W.; Fan, C.G. Progress of research on pretreatment of corn stover. Chin. Agric. Sci. Bull. 2012, 28, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.B.; Zhang, L.H.; Liu, D.H. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Barley husk and coconut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Compos. Sci. Technol. 2010, 70, 840–846. [Google Scholar] [CrossRef]
- Xie, C.X.; Sun, J.Z.; Li, C.L.; Zhu, D.C. Exploring the lignin degradation by bacteria. Microbiol. China 2015, 42, 1122–1132. [Google Scholar] [CrossRef]
- Fidio, D.N.; Carmassi, L.; Kasmiarti, G.; Fulignati, S.; Licursi, D.; Galletti, A.M.R.; Antonetti, C. Chemical and enzymatic hydrolysis of waste wheat bran to sugars and their simultaneous biocatalytic conversion to valuable carotenoids and lipids. Catal. Today 2024, 442, 114941. [Google Scholar] [CrossRef]
- Fidio, D.N.; Federico, T.; Marco, M.; Domenico, L.; Sara, F.; Claudia, A.; Maria, R.G.A. Sustainable Valorisation and Efficient Downstream Processing of Giant Reed by High-Pressure Carbon Dioxide Pretreatment. ChemPlusChem 2022, 87, e202200189. [Google Scholar] [CrossRef]
- Fidio, D.N.; Domenico, L.; Monica, P.; Sandra, V.; Maria, R.G.A. Closing a biorefinery cycle of giant reed through the production of microporous and reusable activated carbon for CO2 adsorption. J. Clean. Prod. 2023, 428, 139359. [Google Scholar] [CrossRef]
- Chu, X.; Awasthi, M.K.; Liu, Y.; Cheng, Q.; Qu, J.; Sun, Y. Studies on the degradation of corn straw by combined bacterial cultures. Bioresour. Technol. 2021, 320, 124174. [Google Scholar] [CrossRef]
- Sarnklong, C.; Cone, J.W.; Pellikaan, W.; Hendriks, W.H. Utilization of Rice Straw and Different Treatments to Improve Its Feed Value for Ruminants: A Review. Asian-Australas. J. Anim. Sci. 2010, 23, 680–692. [Google Scholar] [CrossRef]
- Zeng, J.; Singh, D.; Laskar, D.D.; Chen, S. Degradation of native wheat straw lignin by Streptomyces viridosporus T7A. Int. J. Environ. Sci. Technol. 2013, 10, 165–174. [Google Scholar] [CrossRef]
- Piao, M.; Li, A.; Du, H.; Sun, Y.; Du, H.; Teng, H. A review of additives use in straw composting. Environ. Sci. Pollut. Res. Int. 2023, 30, 57253–57270. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, T.F.; Xing, Z.J.; Zhang, Q.F.; Niu, X.H.; Yu, Y.S.; Teng, Z.J.; Chen, J.X. Enhanced lignocellulose degradation and composts fertility of cattle manure and wheat straw composting by Bacillus inoculation. J. Environ. Chem. Eng. 2023, 11, 109940. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.G.; Li, J.M.; Fan, W.; Wang, C.Y.; Yao, Y.Y. Effects of cellulose-degrading bacteria on degradation of corn stalk. J. Jilin Agric. Univ. 2019, 41, 402–407. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, S.S.; Wang, Y.; Lu, C.L.; Liu, A.M. Screening and identification of a cellulase- producing strain and its degradation of rice straw. J. South China Agric. Univ. 2019, 40, 84–91. [Google Scholar] [CrossRef]
- Wu, Q.; Nie, C.E.; Zhu, Y.Y.; Huang, W.; Ma, C.; Jiang, Y.; Zhu, L.; Fao, H.J. Isolation, identification of a cellulose degrading bacterium with IAA-producing ability and optimization of its culture conditions. Biotechnol. Bull. 2020, 36, 54–63. [Google Scholar] [CrossRef]
- Lin, L.; Kan, X.; Wang, D.N. Characterization of extracellular cellulose-degrading enzymes from Bacillus thuringiensis strains. Semant. Sch. 2012, 15, 2. [Google Scholar] [CrossRef]
- Sreena, C.P.; Sebastian, D. Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J. Genet. Eng. Biotechnol. 2018, 16, 9–16. [Google Scholar] [CrossRef]
- Karim, A.; Nawaz, M.A.; Aman, A.; Ul Qader, S.A. Hyper production of cellulose degrading endo (1,4) β-d-glucanase from Bacillus licheniformis KIBGE-IB2. J. Radiat. Res. Appl. Sci. 2015, 8, 160–165. [Google Scholar] [CrossRef][Green Version]
- Ghorbani, F.; Karimi, M.; Biria, D.; Kariminia, H.R.; Jeihanipour, A. Enhancement of fungal delignification of rice straw by Trichoderma viride sp. to improve its saccharification. Biochem. Eng. J. 2015, 101, 77–84. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Z.; Wang, F.; Li, J.; Hu, K.; Du, Z. Lignin degradation in corn stover catalyzed by lignin peroxidase from Aspergillus oryzae broth: Effects of conditions on the kinetics. Renew. Energy 2019, 130, 32–40. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Agrochemical Analysis of Soil; China Agriculture Press: Beijing, China, 2000; pp. 272–282. (In Chinese) [Google Scholar]
- Gu, P.Y.; Luo, F.F.; Tao, W.Q.; Li, Y.; Wang, D.J.; Wu, X.; Ju, X.X.; Chao, L.; Zhang, Y.L. Higher nitrogen content and auxin export from rice tiller enhance low-ammonium-dependent tiller outgrowth. J. Plant Physiol. 2022, 268, 153562. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 30–107. (In Chinese) [Google Scholar]
- Wang, Z.H.; Wu, X.S.; Chang, X.P.; Li, R.Z.; Jing, R.L. Chlorophyll content and chlorophyll fluorescence kinetics parameters of flag leaf and their gray relational grade with yield in wheat. Acta Agron. Sin. 2010, 36, 217–227. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Q.; Hu, J.J. Research Technology of Plant Physiology; Northwest University of Agriculture and Forestry Science and Technology Press: Yangling, China, 2005; p. 67. (In Chinese) [Google Scholar]
- St Martin, C.C.; Bekele, I.; Eudoxie, G.D.; Bristol, D.; Brathwaite, R.A.I.; Campo, K.R. Modelling response patterns of physico-chemical indicators during high-rate composting of green waste for suppression of Pythium ultimum. Environ. Technol. 2014, 35, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Carmine, A.; Del, V.L.; Silvano, S.; Antonio, C.; Gianluca, C. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Hortic. Sci. 2017, 44, 91–98. [Google Scholar] [CrossRef]
- Marouani, E.; Benzina, N.K.; Ziadi, N.; Bouslimi, B.; Abouda, A.; Koubaa, A. Deinking sludge compost stability and maturity assessment using Fourier transform infrared spectroscopy and thermal analysis. Waste Manag. Res. 2019, 37, 1043–1057. [Google Scholar] [CrossRef]
- Chen, F.R.; Zeng, G.M.; Yu, H.Y.; Xi, X.M. Biodegradation of lignin in compost environment. J. Microbiol. 2008, 28, 88–93. [Google Scholar] [CrossRef]
- Salmén, L. On the organization of hemicelluloses in the wood cell wall. Cellulose 2022, 29, 1349–1355. [Google Scholar] [CrossRef]
- Barapatre, S.; Rastogi, M.; Khosla, B.; Nandal, M. Synergistic effect of fungal consortia and C/N ratio variation on rice straw degradation. Nat. Environ. Pollut. Technol. 2020, 19, 1831–1839. [Google Scholar] [CrossRef]
- Wang, X.; Selvam, A.; Chan, M.; Wong, J. Nitrogen conservation and acidity control during food wastes composting through struvite formation. Bioresour. Technol. 2013, 147, 17–22. [Google Scholar] [CrossRef]
- Xu, C.; Su, X.; Wang, J.; Zhang, F.; Wang, W. Characteristics and functional bacteria in a microbial consortium for rice straw lignin-degrading. Bioresour. Technol. 2021, 331, 125066. [Google Scholar] [CrossRef]
- Jiménez, E.I.; García, V.P. Composting of domestic refuse and sewage sludge. I. Evolution of temperature, pH, C/N ratio and cation-exchange capacity. Resour. Conserv. Recycling. 1991, 6, 45–60. [Google Scholar] [CrossRef]
- Nakasaki, K.; Yaguchi, H.; Sasaki, Y.; Kubota, H. Effects of pH control on composting of garbage. Waste Manag. Res. 1993, 11, 117–125. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, X.Q.; Qi, X.G.; Li, J.; Ren, Y.P.; Wang, X.H.; Xia, Q.Q. Optimization of Sporulation Parameters for Aerobic Composting Bacillus subtilis GX2 Strain. J. Food Sci. Biotechnol. 2021, 40, 91–99. [Google Scholar] [CrossRef]
- Gaind, S. Effect of fungal consortium and animal manure amendments on phosphorus fractions of paddy-straw compost. Int. Biodeterior. Biodegrad. 2014, 94, 90–97. [Google Scholar] [CrossRef]
- Xu, M.Y.; Yang, M.; Sun, H.S.; Meng, J.; Li, Y.S.; Gao, M.; Wang, Q.H.; Wu, C.F. Role of multistage inoculation on the co-composting of food waste and biogas residue. Bioresour. Technol. 2022, 361, 127681. [Google Scholar] [CrossRef]
- Wu, D.; Gao, W.F.; Zhao, Y.; Wei, Z.M.; Song, C.H.; Qu, F.T.; Wang, F. Elaborating the microbial mechanism of humic substance formation during lignocellulosic biomass composting by inoculation with different functional microbes. Ind. Crops Prod. 2024, 208, 117838. [Google Scholar] [CrossRef]
- Zhang, J.; Ying, Y.; Li, X.; Yao, X. Physical and chemical properties of Camellia oleifera shell composts with different additives and its maturity evaluation system. Environ. Sci. Pollut. Res. 2020, 27, 35294–35302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Yang, R.P.; Yang, P.; Fu, H.B.; Yang, Y.C.; Liu, C.W. Construction of Microbial Consortium to Enhance Cellulose Degradation in Corn Straw during Composting. Agronomy 2024, 14, 2107. [Google Scholar] [CrossRef]
- Wang, S.C.; Long, H.C.; Hu, X.R.; Wang, H.; Wang, Y.C.; Guo, J.M.; Zheng, X.F.; Ye, Y.L.; Shao, R.X.; Yang, Q.H. The co-inoculation of Trichoderma viridis and Bacillus subtilis improved the aerobic composting efficiency and degradation of lignocellulose. Bioresour. Technol. 2024, 394, 130285. [Google Scholar] [CrossRef]
- Dash, P.K.; Padhy, S.R.; Bhattacharyya, P.; Pattanayak, A.; Pathak, H. Efficient Lignin Decomposing Microbial Consortium to Hasten Rice-Straw Composting with Moderate GHGs Fluxes. Waste Biomass Valorization 2022, 13, 481–496. [Google Scholar] [CrossRef]
- Zhang, C. Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting. Horticulturae 2021, 7, 482. [Google Scholar] [CrossRef]
- Qu, F.t.; Cheng, H.P.; Han, Z.Y.; Wei, Z.M.; Song, C.H. Identification of driving factors of lignocellulose degrading enzyme genes in different microbial communities during rice straw composting. Bioresour. Technol. 2023, 381, 129109. [Google Scholar] [CrossRef]
- Duan, M.L.; Zhang, Y.H.; Zhou, B.B.; Qin, Z.L.; Wu, J.H.; Wang, Q.J.; Yin, Y.N. Effects of Bacillus subtilis on carbon components and microbial functional metabolism during cow manure—Straw composting. Bioresour. Technol. 2020, 303, 122868. [Google Scholar] [CrossRef] [PubMed]
- Siu-Rodas, Y.; de los Angeles Calixto-Romo, M.; Guillén-Navarro, K.; Sánchez, J.E.; Zamora-Briseno, J.A.; Amaya-Delgado, L. Bacillus subtilis with endocellulase and exocellulase activities isolated in the thermophilic phase from composting with coffee residues. Rev. Argent. Microbiol. 2017, 50, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Shu, L.L.; Yang, Y.Y.; Kumar, S.; Tripathi, P.; Mishra, S.; Yang, Z.C. Microbial agents obtained from tomato straw composting effectively promote tomato straw compost maturation and improve compost quality. Ecotoxicol. Environ. Saf. 2024, 270, 115884. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sun, X.; Zhou, W.; Zhang, W.; Che, F.; Li, S. The effects of green waste compost on soil N, P, K, and organic matter fractions in forestry soils: Elemental analysis evaluation. RSC Adv. 2021, 11, 31983–31991. [Google Scholar] [CrossRef]
- Cardoso, E.; Santos, R.; Soares, S.; Ribeiro, M.; Silva, F. Substrate in the initial development of pajeu seedlings. Sci. Agrar. Parana. 2020, 19, 72–76. [Google Scholar] [CrossRef]
 ” represents the degree of apparent color change of straw. (↑), (↓), (|), and other symbols represent upregulation and downregulation, respectively, and there were no significant changes in any parameter.
” represents the degree of apparent color change of straw. (↑), (↓), (|), and other symbols represent upregulation and downregulation, respectively, and there were no significant changes in any parameter.
 ” represents the degree of apparent color change of straw. (↑), (↓), (|), and other symbols represent upregulation and downregulation, respectively, and there were no significant changes in any parameter.
” represents the degree of apparent color change of straw. (↑), (↓), (|), and other symbols represent upregulation and downregulation, respectively, and there were no significant changes in any parameter.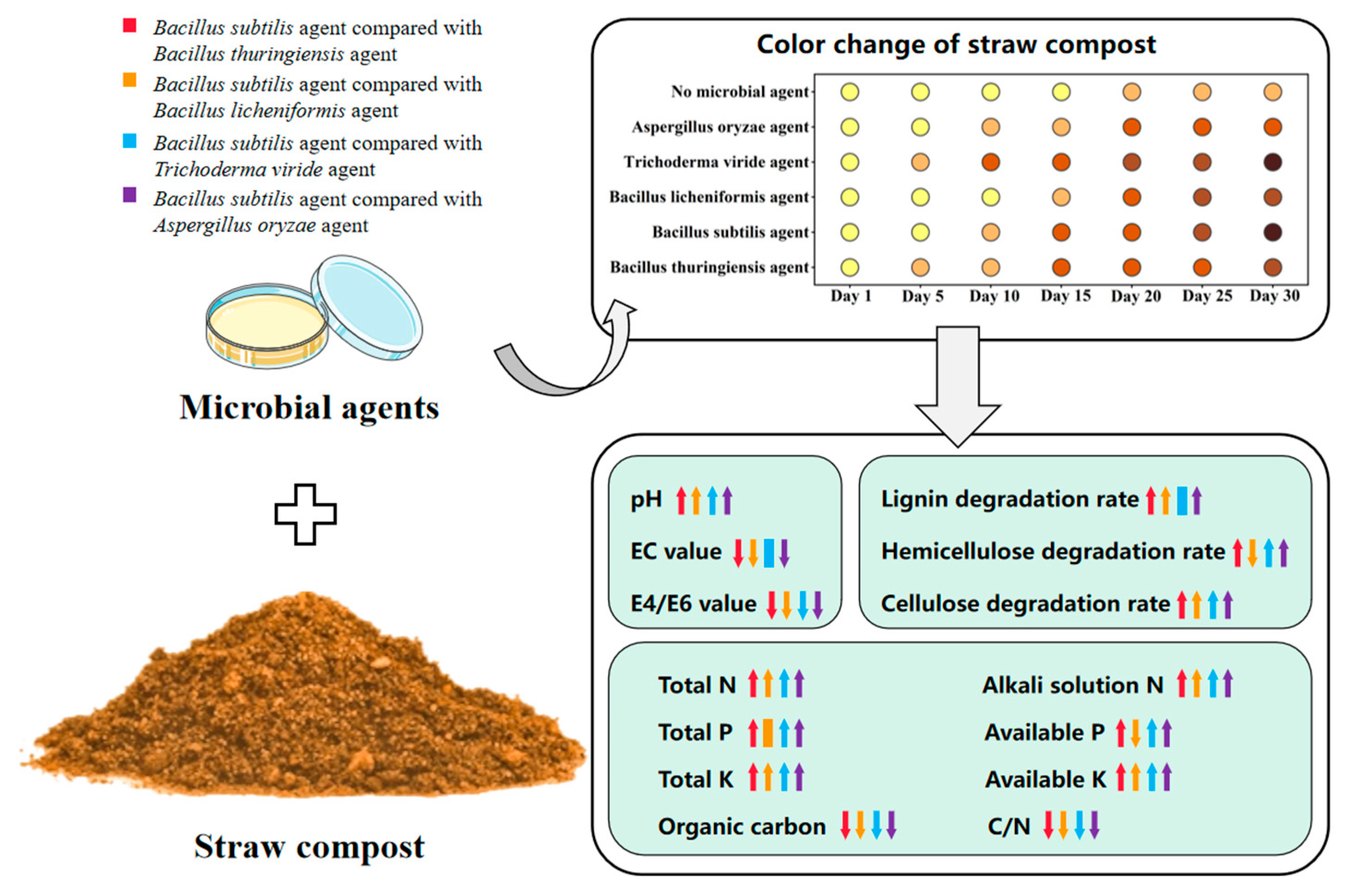
| Time/d | T1 | T2 | T3 | T4 | T5 | CK |
|---|---|---|---|---|---|---|
| 1 | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow |
| 5 | Dark yellow | Light yellow | Light yellow | Dark yellow | Light yellow | Light yellow |
| 10 | Dark yellow | Dark yellow | Light yellow | Yellowish brown | Dark yellow | Light yellow |
| 15 | Yellowish brown | Yellowish brown | Dark yellow | Yellowish brown | Dark yellow | Light yellow |
| 20 | Yellowish brown | Yellowish brown | Yellowish brown | Brown | Yellowish brown | Dark yellow |
| 25 | Yellowish brown | Brown | Brown | Brown | Yellowish brown | Dark yellow |
| 30 | Brown | Dark brown | Brown | Dark brown | Yellowish brown | Dark yellow |
| Treatment | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|
| T1 | 7.62 ± 0.05a | 7.65 ± 0.03b | 6.56 ± 0.01d | 6.78 ± 0.05d | 7.61 ± 0.01b | 6.59 ± 0.03f | 7.81 ± 0.02b |
| T2 | 7.62 ± 0.05a | 7.65 ± 0.03b | 6.55 ± 0.05d | 6.74 ± 0.04d | 7.20 ± 0.08d | 7.39 ± 0.01b | 7.90 ± 0.01a |
| T3 | 7.60 ± 0.01a | 7.66 ± 0.01b | 6.84 ± 0.02c | 7.00 ± 0.03c | 7.25 ± 0.04d | 7.18 ± 0.09c | 7.25 ± 0.04e |
| T4 | 7.60 ± 0.01a | 7.66 ± 0.01b | 6.82 ± 0.10c | 7.31 ± 0.05b | 7.47 ± 0.04c | 6.91 ± 0.03d | 7.65 ± 0.02c |
| T5 | 7.67 ± 0.01a | 7.73 ± 0.01a | 7.47 ± 0.01b | 6.74 ± 0.01d | 7.43 ± 0.03c | 6.75 ± 0.03e | 7.54 ± 0.01d |
| CK | 7.61 ± 0.01a | 7.73 ± 0.01a | 7.65 ± 0.05a | 7.73 ± 0.04a | 7.76 ± 0.02a | 7.63 ± 0.04a | 7.80 ± 0.03b |
| Treatment | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|
| T1 | 3.09 ± 0.04a | 4.01 ± 0.11a | 4.34 ± 0.07a | 4.71 ± 0.09a | 3.71 ± 0.04d | 4.31 ± 0.06b | 4.65 ± 0.2a |
| T2 | 3.05 ± 0.07a | 3.95 ± 0.1a | 4.03 ± 0.02b | 4.13 ± 0.14b | 4.58 ± 0.12ab | 4.39 ± 0.07ab | 3.91 ± 0.05b |
| T3 | 2.98 ± 0.11a | 3.66 ± 0.03b | 3.93 ± 0.04b | 4.32 ± 0.26ab | 4.32 ± 0.04c | 4.42 ± 0.03ab | 3.83 ± 0.04b |
| T4 | 2.98 ± 0.05a | 3.58 ± 0.03b | 3.92 ± 0.01b | 4.42 ± 0.17ab | 4.37 ± 0.1bc | 4.31 ± 0.01b | 3.92 ± 0.06b |
| T5 | 2.94 ± 0.08a | 3.56 ± 0.01b | 3.74 ± 0.06c | 4.43 ± 0.04ab | 4.62 ± 0.05a | 4.58 ± 0.09a | 4.39 ± 0.07a |
| CK | 2.93 ± 0.06a | 2.86 ± 0.05c | 2.98 ± 0.02d | 3.04 ± 0.04c | 3.12 ± 0.05e | 3.03 ± 0.09c | 3.09 ± 0.08c |
| Treatment | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|---|
| T1 | 1.76 ± 0.05a | 1.82 ± 0.07b | 1.89 ± 0.09bc | 2.12 ± 0.18ab | 2.45 ± 0.12abc | 2.99 ± 0.21a | 2.64 ± 0.2ab |
| T2 | 1.78 ± 0.02a | 2.21 ± 0.18ab | 2.39 ± 0.12a | 2.72 ± 0.1a | 2.06 ± 0.24bcd | 2.57 ± 0.13ab | 1.51 ± 0.02d |
| T3 | 1.73 ± 0.03a | 2.41 ± 0.24a | 2.49 ± 0.18a | 2.48 ± 0.36a | 2.55 ± 0.17ab | 2.53 ± 0.23ab | 2.18 ± 0.29bc |
| T4 | 1.75 ± 0.05a | 2.25 ± 0.09ab | 2.52 ± 0.22a | 2.49 ± 0.12a | 2.55 ± 0.03ab | 2.23 ± 0.51ab | 2.24 ± 0.19bc |
| T5 | 1.75 ± 0.04a | 1.81 ± 0.1b | 2.23 ± 0.07ab | 2.24 ± 0.16ab | 2.92 ± 0.16a | 1.95 ± 0.42b | 3.2 ± 0.26a |
| CK | 1.72 ± 0.04a | 1.75 ± 0.22b | 1.73 ± 0.01c | 1.79 ± 0.08b | 1.87 ± 0.09d | 1.88 ± 0.03b | 1.89 ± 0.13cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shuai, K.; Li, J.; Hu, X.; Chen, G. Comparison of the Effect of Different Microbial Agents on the Decomposition of Rice Straw. Fermentation 2025, 11, 332. https://doi.org/10.3390/fermentation11060332
Li Y, Shuai K, Li J, Hu X, Chen G. Comparison of the Effect of Different Microbial Agents on the Decomposition of Rice Straw. Fermentation. 2025; 11(6):332. https://doi.org/10.3390/fermentation11060332
Chicago/Turabian StyleLi, Yufei, Kaifeng Shuai, Juan Li, Xinyu Hu, and Guanghui Chen. 2025. "Comparison of the Effect of Different Microbial Agents on the Decomposition of Rice Straw" Fermentation 11, no. 6: 332. https://doi.org/10.3390/fermentation11060332
APA StyleLi, Y., Shuai, K., Li, J., Hu, X., & Chen, G. (2025). Comparison of the Effect of Different Microbial Agents on the Decomposition of Rice Straw. Fermentation, 11(6), 332. https://doi.org/10.3390/fermentation11060332





