1. Introduction
Commercial winemaking demands meticulous attention to fermentation, given the individual variations in the composition of small, segregated volumes from single cultivars in different geographical regions. Isolating the unique composition of each fermentation volume is essential for achieving the most distinctive aromas and flavors. This underscores the significance of monitoring and controlling the fermentation environment. Grapes are harvested and fermented in each hemisphere during a 6- to 8-week season in late summer. Generally, grape juice intended for high-value wine is not stored for fermentation after harvesting.
A wine business must navigate the tradeoff between investing in systems that will be primarily utilized during the short fermentation season and the importance of preventing undesirable or abnormal fermentation outcomes in high-value crops. Issues such as slow or stalled fermentations can limit tank turnover and hinder grape intake. Incomplete fermentation leads to a significant concentration of residual sugar, which can result in unwanted secondary yeast or bacterial growth and byproduct formation. Other negative outcomes may include the generation of volatile sulfur components (VSCs) and the production of sulfur dioxide, which binds to acetaldehyde and increases the bound sulfur dioxide concentration.
Wine fermentation is unique because it reflects the compositional aspects of each grape juice rather than a prepared medium like beer or fuel ethanol, where fermentable sugars usually come from malting or the enzymatic hydrolysis of starch polymers or polysaccharides. This natural variation in grape juice composition makes monitoring and controlling grape juice fermentation more critical and challenging.
Numerous alternative methods are available for monitoring wine fermentation. A recent review of sensors in biotechnology can be referenced elsewhere [
1]. This review will concentrate on the sensors used for monitoring, modeling, and controlling wine fermentation. The first and most common method involves manually collecting samples to assess density or conduct chemical analysis, utilizing established enzymatic, chromatographic, or spectral techniques such as UV-visible, NIR, MidIR, Raman, and fluorescence or ultrasound spectroscopy. Sampled systems yield small-volume and/or localized estimates, and their representativeness diminishes as the fermentation volume increases due to the rise in stratification and radial concentration gradients associated with scale. These methods typically necessitate clarified liquid samples, limiting their application in situ and inline.
A second monitoring approach involves measuring aggregate properties of the entire fermentation volume, such as weight, carbon dioxide evolution, or energy release. These methods minimize measurement variation caused by stratification and radial concentration gradients while providing overall values for the fermentation volume. A third method estimates solution properties, such as liquid density, by using density sensors or differential pressure systems installed in each fermentor. This approach delivers real-time data on the most commonly used measurements to track fermentation rates and their extent in commercial practice.
Measuring a representative liquid temperature during fermentation to determine control actions and to correct the temperature of the solution properties is essential. The primary aim of monitoring is to track the rate and extent of fermentation activity. The secondary aim is to use these measurements for control or intervention purposes and ultimately to incorporate them into a kinetic model for parameter estimation, identifying abnormal patterns, and predicting future fermentation outcomes. This review will focus on monitoring approaches suitable for large-scale commercial fermentations and how they can be integrated with a wine fermentation model for predictive, diagnostic, and control purposes. An existing model that uses density to interpret metabolic activity in both growing and non-growing cells, as well as the decrease in cell viability over time due to factors like temperature, cell age, and ethanol presence and exposure, is described elsewhere (Nelson and Boulton 2024) in Paper 1 of this Special Issue [
2]. The effectiveness of this model and density measurements in characterizing normal fermentations and in early detection of problematic fermentations in more than two hundred commercial wine fermentations can be found elsewhere (Nelson et al. 2024), as discussed in Paper 2 of this Special Issue [
3].
2. Wine Fermentation Characteristics
The term “wine fermentation” typically includes two distinct processes. The primary ethanol fermentation occurs during the harvest period, facilitated by yeast. This process involves converting hexose sugars into ethanol (and small amounts of glycerol and succinate) under anaerobic conditions. It lasts between 7 and 10 days for red wines and 18 to 24 days for white wines, influenced by fermentation temperature.
In the secondary process, lactic acid fermentation, bacteria transform malic acid into lactic acid, which is known as malolactic fermentation. These fermentations generally follow the primary fermentation process; however, they may occur or be induced simultaneously in certain wine styles. In other styles, malolactic fermentation is inhibited by maintaining lower temperatures and/or adding sulfur dioxide. This fermentation can take several weeks to complete.
The primary fermentation is divided into four phases based on yeast activity: the adaptation lag, the active cell mass growth, the non-growing maintenance of viable cell mass, and the decline in the viable (or actively fermenting) yeast population. During the lag phase, yeast acclimates to the temperature, osmotic pressure, pH, and redox potential of the grape juice/must environment. The growth phase is characterized by an exponential increase in the yeast population, which halts when essential nutrients are depleted. The transition to the stationary phase begins when cell division ceases. In the final phase, yeast cells start to die off due to aging and the concentration of ethanol, and/or the loss of fermentation capability. In most wine fermentations, growth has stopped by the midpoint of sugar consumption, with the cell mass, its viability, and activity influencing the tailing section of the fermentation curve.
During the lag phase, the density of the juice remains constant while the extracellular redox potential in the juice decreases as the conditions shift to anaerobic. The redox potential is a characteristic of the solution that is influenced by microbial activity [
4,
5,
6,
7,
8]. The observed decline in the redox potential during the lag phase occurs prior to any fermentation activity being detectable through changes in density, making it valuable for assessing the health and activity of yeast at the very early stages of wine fermentation. The duration of the lag phase typically depends on temperature, being shorter at higher temperatures, on the initial cell mass, being longer with lower cell concentrations, and on deficiencies in assimilable nitrogen or essential vitamins, which prolong the phase. Currently, no function exists to estimate the duration of the lag phase based on the initial composition.
During the active growth period, when the yeast population increases exponentially, a notable decrease in density and total sugars occurs, along with a more rapid decline in redox potential. The overall cell mass grows quickly, and ethanol is produced in proportion to sugar utilization. In red wine fermentations, the extraction of phenolic fractions continues while the juice remains in contact with the grape skins and solids. Winemakers use various mixing and contacting strategies to optimize the extent and nature of these extractions. The extraction of anthocyanins and low-molecular-weight phenolic compounds in red wines reaches a peak and levels off during this stage, but may decrease slightly due to reabsorption into the grape skins [
9,
10,
11]. There is no evidence that these phenolic components significantly impact the progress or extent of primary fermentation.
The stationary phase activity of a non-growing yeast population is usually referred to as the maintenance activity. The cessation of growth and the onset of the stationary phase represent a critical transition point and correspond to the maximum active cell mass, the peak fermentation rate, the highest CO2 and heat release rates, and the lowest redox potential. This point produces the highest rate of gas bubble release, resulting in the most intense bubble-driven liquid circulation. Temperature-controlled fermentations require the greatest energy transfer rate to maintain a constant temperature. This phase typically occurs between one-third and one-half of the fermentation period, depending on the fermentation temperature and growth conditions.
In the final phase, the population of active yeast declines, related to the rising ethanol concentration and the cells’ increasing age. From this point onward, the redox potential begins to rise slowly as the non-growing cells no longer export metabolites that contribute to lowering it. During this period, the viable cell mass declines until all the fermentable sugar has been consumed or the cells have died or become unable to ferment.
In the stationary and final phases of fermentation, the fermentation rate can slow down more than usual, leading to an extended tailing period or even a complete stop, with a high amount of unfermented sugars remaining, typically exceeding 2 g/L. These fermentations are described as sluggish if they proceed slower than expected or incomplete (or stuck) if the fermentation ceases before all fermentable sugars are consumed. Such scenarios are undesirable due to their susceptibility to secondary microbial spoilage, the prolonged time in the fermentor, and additional labor and energy inputs, and they often result in a lower final quality assessment of the wine.
White wines are typically made by fermenting slightly turbid juices at 15 to 20 °C range temperatures. In contrast, red wines may undergo a period of skin and juice contact before fermentation begins, typically at temperatures ranging from 25 to 30 °C, while in the presence of the skins and seeds. During red wine fermentations, the juice’s color intensifies due to the extraction of anthocyanins from the grape skins. This process occurs while the skins are in contact with the juice, known as maceration or skin contact. Maceration can start before fermentation (called a pre-soak) and usually continues during fermentation. Additionally, it can end before fermentation concludes (termed shortened maceration), and if desired, it can also occur after fermentation is complete (known as extended maceration).
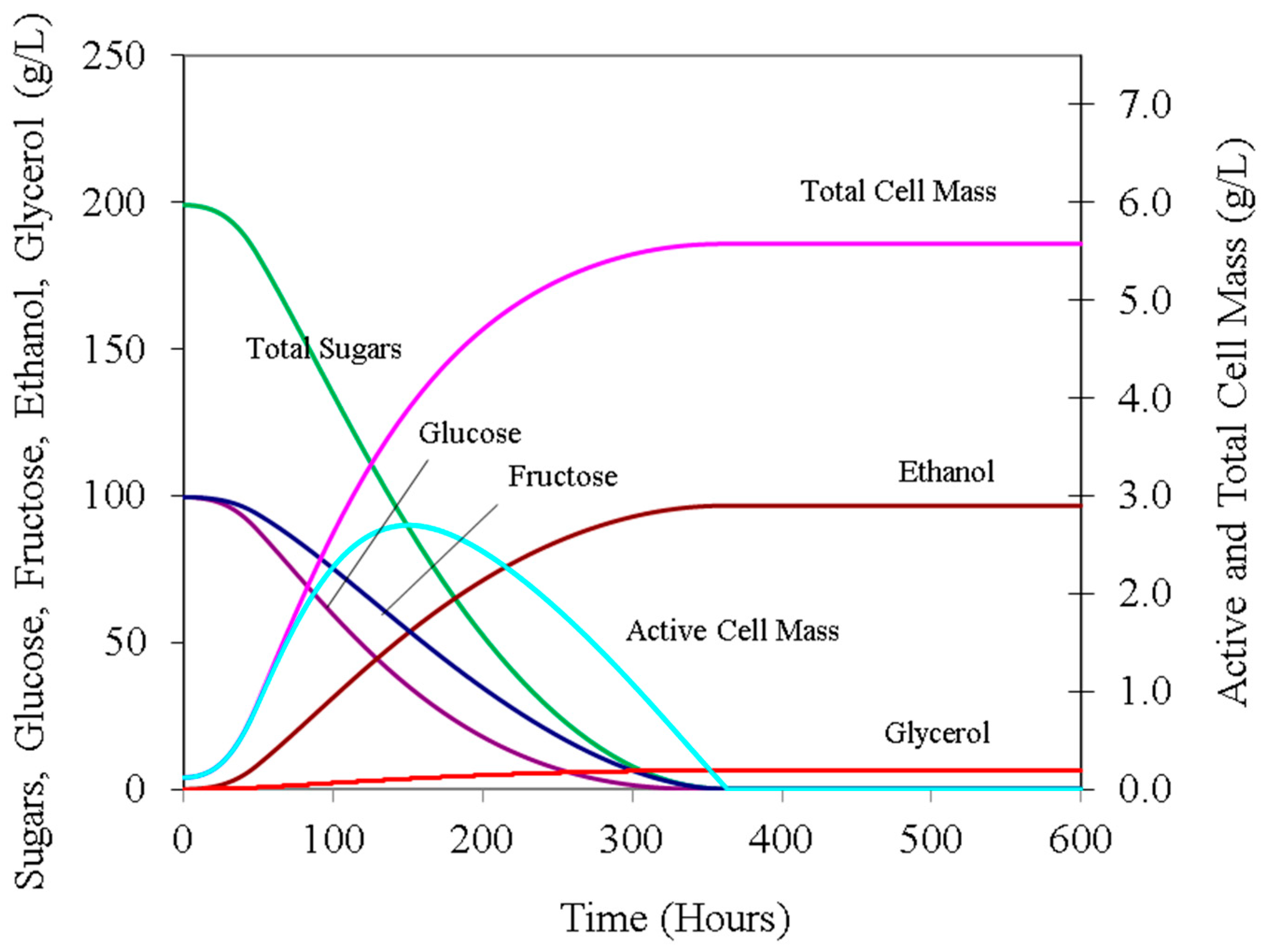
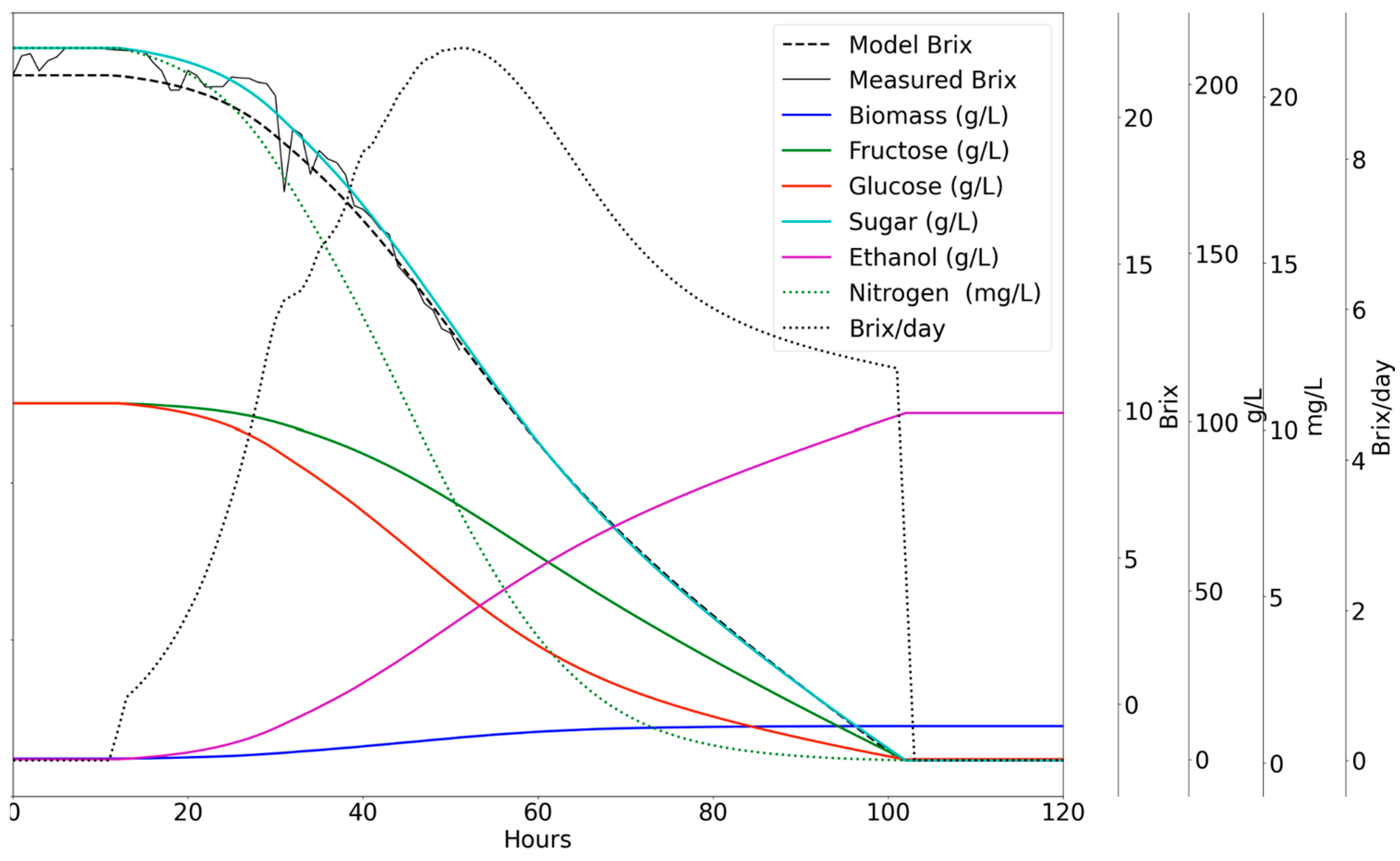
Figure 1 illustrates the trends in the concentrations of major components (total and viable cell mass, ethanol, glycerol, total sugars, and glucose and fructose) during wine fermentation.
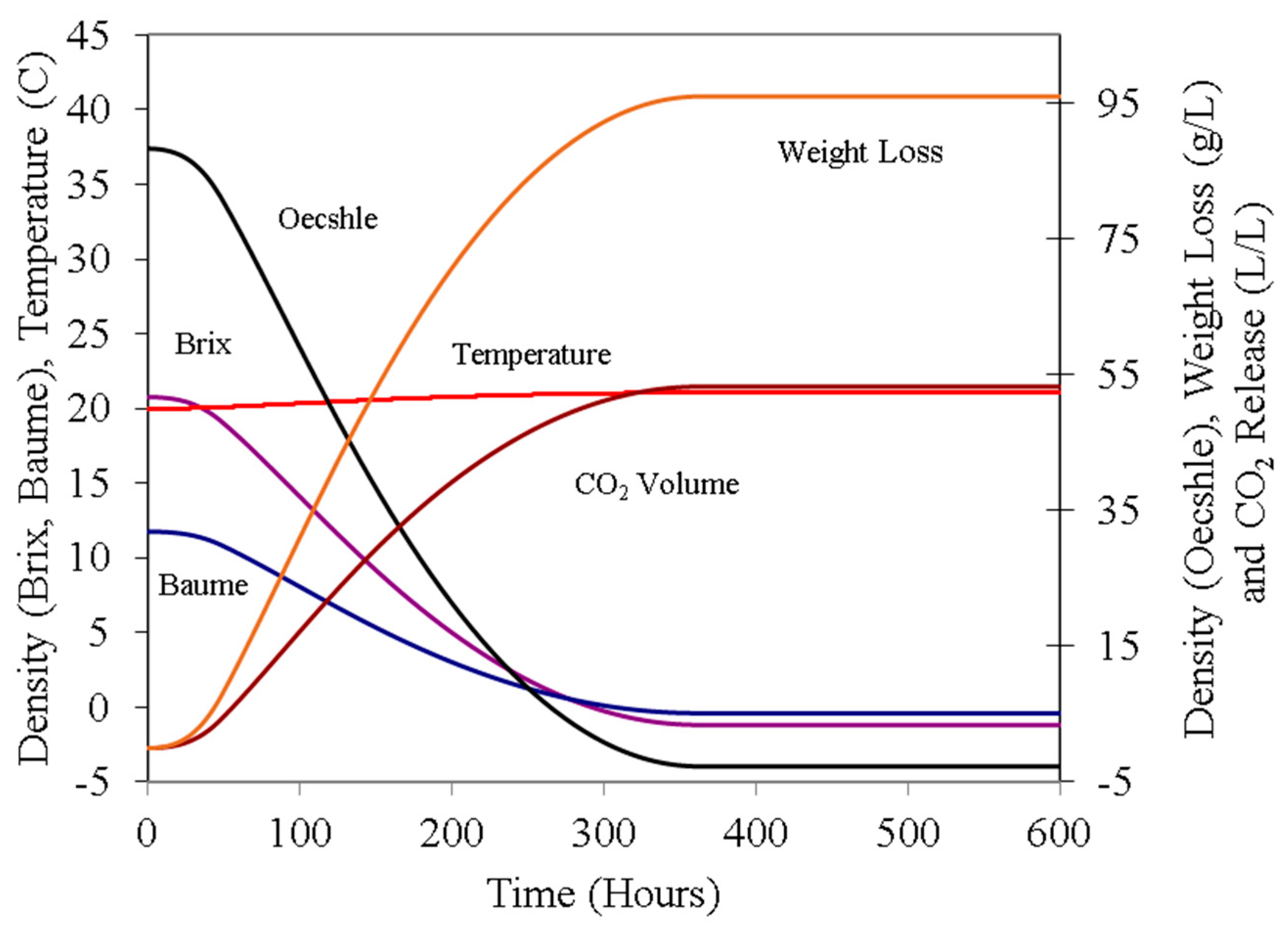
Figure 2 presents the corresponding density curve represented in various scales (°Brix, Baumé, and Oechsle), along with juice temperature and the volume of carbon dioxide released.
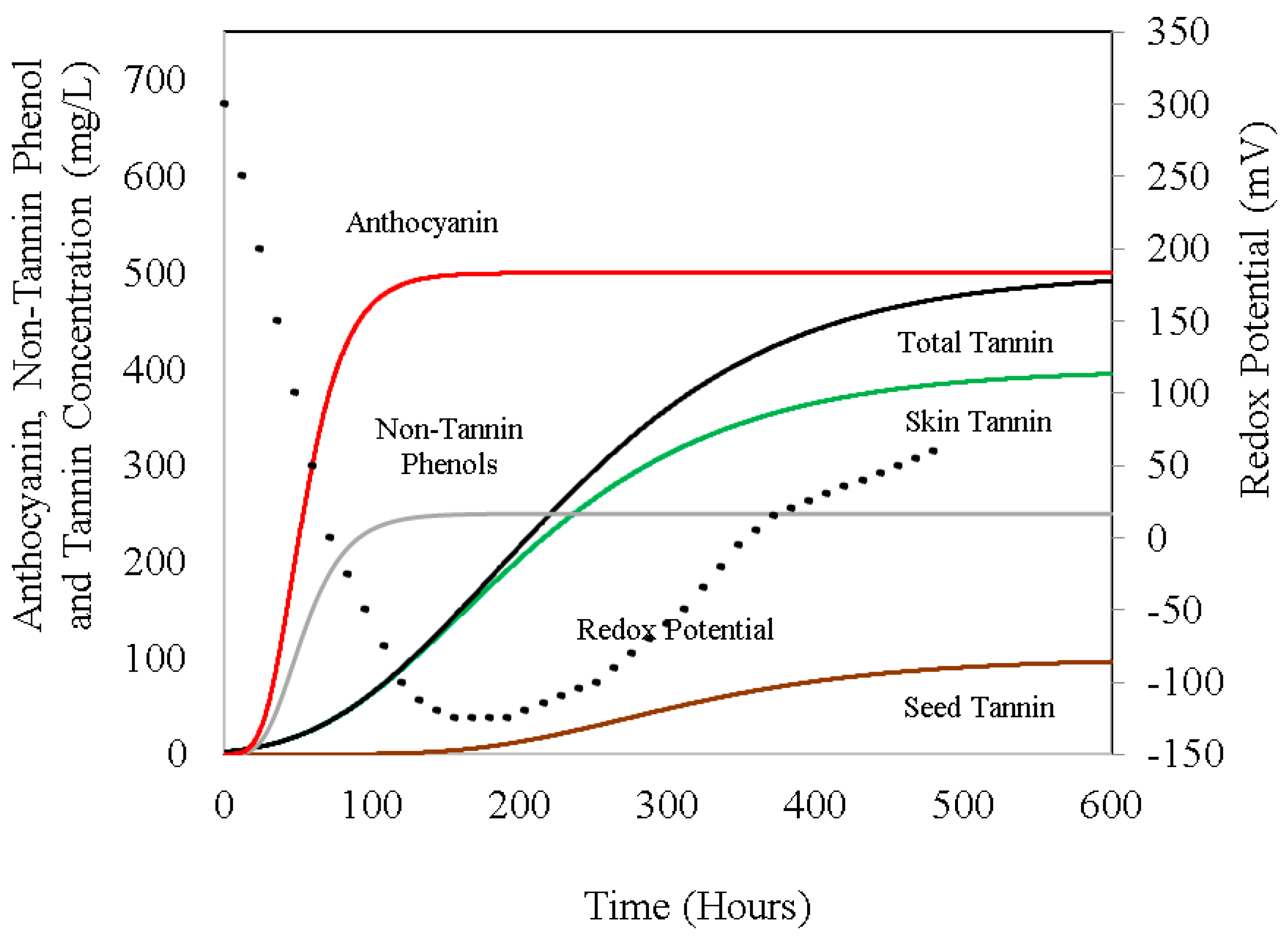
Figure 3 depicts the changes in the redox potential and the extraction patterns of several phenolic components in the red wine fermentation process. The rates of change in these and other components (total sugars, carbon dioxide, water, and ethanol vapor concentration), as well as the integrated release of ethanol and water vapor during a typical wine fermentation at controlled temperatures, are displayed in
Figure 4.
Any of the trends related to concentrations, rates, and solution properties of a typical wine fermentation, as outlined in
Figure 1,
Figure 2, and
Figure 4, can serve as the objective function for estimating model parameters using a comprehensive wine fermentation model that aims to estimate all of them and is based on widely known trends [
2]. The challenge lies in selecting the fermentation properties and sensors that effectively provide this dynamic information in real time. During the harvest period, numerous individual fermentations can proceed simultaneously, each at a different stage, displaying varying rates, patterns, and requirements for control or intervention. Effectively managing these fermentations manually, around the clock, to achieve desired outcomes poses a significant challenge, often one that remains unattainable. Currently, it is impossible to predict a wine fermentation trajectory or outcome based solely on the initial information available, which includes sugar, assimilable nitrogen, and temperature. The initial variation in natural juice chemistry and the winemaker’s choices regarding yeast strain and inoculum practices create a complex set of nonlinear trends and relationships among various fermentation properties. This highlights the need to digitize wine fermentation data for precise monitoring, modeling, control, and outcomes. On a larger scale, gathering data from multiple fermentors is crucial for better management and optimization of resource allocation involving people, energy, and water, refrigeration capacity, and ethanol vapor and CO
2 release into the environment or future carbon capture systems.
The secondary malolactic fermentation occurs in many wines, more commonly in red wines. This fermentation typically occurs in barrels or tanks after the primary fermentation is complete, when the new wine has been transferred from fermentation vessels (and the skins have been pressed in the case of red wines). The duration of this fermentation is often weeks rather than days and converts 2 to 3 g/L of malic acid into 1.3 to 2 g/L of lactic acid. This results in an insignificant change in density; the weight loss is less than 1 g/L, and the release of carbon dioxide is under 0.5 L/L, making it challenging to monitor using density, weight loss, or other aggregate methods. It is typically monitored by manual sampling, with acid concentrations determined through laboratory analysis rather than by any in situ sensor system.
3. Measurement Challenges at Commercial Scales
The accuracy of process measurements during wine fermentations relies on the fermentation volume, the sampling method, the sensor type and placement, as well as the extent to which concentration gradients in the fermentor affect them. The primary challenge in measuring wine fermentation is reconciling small sample measurements (mLs) with those from the entire fermentation volume (kLs), considering the radial and axial gradients and stratification that fluctuate as fermentation rates and progress vary.
3.1. Natural Convection Patterns During Unmixed Fermentations
In a typical commercial wine fermentor, the liquid properties show heterogeneity due to spatial and temporal fluctuations induced by bubble-driven circulation patterns. Understanding the mixing patterns within fermentors—whether from natural convection, applied mixing, or a combination of both—is important for placing sensors and extracting small samples for off-line measurements. The mixing within the fermentation volume occurs due to (a) natural convection from the momentum gained by rising bubbles and (b) density differentials from temperature and concentration gradients. While some wine fermentors are designed for periodic or continuous mixing using gas injection, forced swirling circulation, or impeller action, most are not. Even in the case of red wine fermentations, which are typically mixed by various means once or twice daily, usually for an hour or less, natural convection remains the primary contributor to mixing. The natural convection currents also vary as the corresponding radial and vertical gradients change with the fermentation rate. This mixing behavior is crucial to consider when positioning measurement sensors to assess fermentation progress. The liquid next to the inner wall will be undercooled and denser than the overall fermentation volume in jacketed fermentors. This density difference causes a downward flow adjacent to the wall, often leading to wall probe temperature measurements that underestimate the apparent temperature of the general fermentation volume. Therefore, the placement of liquid temperature probes is particularly vital if they are to be used for temperature control systems and for correcting the fluid properties of the fermentation volume.
The interaction among the bubble release rate, liquid depth, and fermentor shape determines the recirculation patterns within the fermentation volume. The natural convection caused by rising bubbles is significant, as between 50 and 60 L of CO
2 can be released per L of juice during a typical juice fermentation. The CO
2 release rate increases from zero, reaching a peak of between 5 and 10 L of CO
2/L of wine/hr at the maximum fermentation rate, as shown in
Figure 4, before returning to zero at the end of the fermentation. This peak usually occurs shortly after the maximum yeast growth rate. The time difference between these peaks depends on the fermentation temperature, being shorter at higher temperatures and longer at cooler temperatures. The intensity of the gas flux (along with the bubble plume supplying it) depends on the fermentation volume in relation to its cross-sectional area. This flux will be scaled by D
3 (volume)/D
2 (area) or D; that is, it increases in proportion to the fermentor’s length dimension, typically the diameter, D.
Reports of recirculation patterns during beer and wine fermentation have been measured or calculated in conical fermenters [
12], concrete eggs [
13], and upright cylinders [
14,
15,
16,
17]. The model predictions for the egg-shaped fermenters [
13] and cylindrical fermenters [
16] assume well-mixed liquid conditions and exclude the momentum contributions of rising CO
2 bubbles. Therefore, they do not accurately reflect the expected velocity profiles and recirculation patterns when these momentum contributions are present. Examples of natural convection patterns caused by bubble rise illustrate why sensor placement is a vital consideration in commercial wine fermenters; however, it is often overlooked in bench- and small-scale fermenters.
3.2. Temperature Gradients Within Red Wine Fermentations
The temperature gradients within commercial red wine fermenters can be viewed as two distinct fermentation zones, each with unique thermal characteristics. The first zone is the liquid below the skin cap, and the second is within the skin cap itself. This separation is established shortly after the first CO2 is released during active fermentation, causing the neutrally buoyant skins to rise to the surface due to the bubble plume. While it is common to take some liquid temperature measurements, it is less common to measure the skin cap temperatures or, even more so, to control them.
Accurate and representative temperature measurements are crucial for both control purposes and for compensating for temperature-dependent properties, such as liquid density. The size and geometry of the fermentor, along with the mixing techniques used, dictate the distribution of concentration and temperature gradients within the fermentor and the transient nature of the concentration gradients between the center and the walls or between the base and the upper surface. Established sensor cleaning protocols should be followed to prevent fouling over time.
In the case of red wine fermentations, a skin cap forms once fermentation begins, and CO2 bubbles are released. This cap becomes a floating stack of skins constrained laterally by the walls and compressed vertically by the weight of the skins above it. Once developed, it typically occupies the top third of the fermentation volume, with the juice below it making up the remainder. The skins are most compressed at the base of the cap due to the vertical load of the skins above. The top layers of the cap are the least compressed since they bear little vertical weight.
The purpose of mixing the liquid with the skins during the fermentation is to promote the extraction rate of components such as anthocyanins and tannins, among others. The ability of the liquid to permeate the skin cap during a pump-over event will depend on the vertical height of the skin cap, with taller (or thicker) skin caps becoming more compressed and restrictive of gas and liquid permeation. The skin cap in a short, wide fermentor behaves like a shallow floating disk, and in a tall, narrow one, it behaves like a thick cylindrical plug. For any fermentor geometry, the skin cap’s thickness varies with the fermentation volume’s height. Unfortunately, the compression of the cap due to its weight also varies with the height, creating a second-order effect on the permeability of the cap. This places a practical upper limit on the fermentor’s size in which the cap temperature can be effectively controlled, with a skin cap height of 3 m, by pumping over, to be of approximately 200 KL (52 Kgal). The alternative practice of punching the cap down into the fermenting liquid is limited to thinner skin caps, typically between 1 and 2 m thick, requiring shorter, smaller diameter fermentors that contain between 40 KL (10 Kgal) and 80 KL (20 Kgal) of juice and skins. These limits provide the height over which temperature probes need to be placed if skin cap temperature is to be monitored and used for control purposes.
The control of temperature for the entire red wine fermentation will depend on the permeability of the skin cap that determines the liquid distribution and flow rate and the interchange of thermal energy between the juice and the skins. This interchange varies with the pump-over’s frequency and volume or the punch-down operation’s efficiency. At larger scales, these become less effective due to compression of the skin cap due to its height and weight, and the ability to control the skin cap temperature is lost.
During wine fermentation, both radial and vertical temperature gradients develop within the liquid. The radial temperature gradients become more significant as the size of the fermentor increases. These gradients are especially pronounced when cooling is provided by a jacketed wall-exchanger; however, there are upper limits to the effectiveness of such cooling as the fermentor’s size increases. This occurs because the wall area of the jacket increases with the square of the diameter, while the volume of the fermentor increases with the cube of the diameter, and the energy removal rate varies with the area-to-volume ratio.
Pump-overs or punch-downs are typically repeated twice daily. While these processes can temporarily bring the cap temperature closer to that of the cooler juice, the effect is brief, as the cap temperature rises and returns to its peak within one to two hours. The frequency of these treatments can be increased to minimize the extent of the rise in cap temperature. Some wineries utilize temperature probes within the skin cap to regulate this process. Conversely, others opt for time-based methods such as adjusting pump-over durations and/or frequency or using inline sub-cooling to provide a colder pump-over liquid to the skin cap. Introducing moderate mixing or tangential swirling through impellers or liquid motion by pumping can help reduce the temperature gradients that wall-based sensors experience.
The maximum yeast growth rate occurs at a temperature of 35 °C. At temperatures above this, the net growth rate slows due to thermal death becoming a factor. At 40 °C, the death rate surpasses the growth rate, resulting in a decline in the viable population [
2]. Studies on the temperature within the skin cap of a 2000 L fermentor have shown that during peak fermentation, the center can be 8 °C warmer than the juice below [
18]. This indicates that most red wine fermentations with juice temperatures of 32 °C and higher will have a central core in the skin cap where yeast growth becomes slower, nonexistent, or in decline due to the increased temperature. Additionally, these elevated skin cap temperatures are anticipated to lead to lower redox potentials within the cap, which increases the likelihood of H
2S formation during fermentation. Another concern is the confusion that may arise regarding the impact of juice temperature on skin extraction when significant temperature differences exist between the skin cap and the juice. These factors underscore the conclusion that skin cap temperature is a critical measurement for red wine fermentations and should serve as the basis for a separate temperature control system to achieve more precise, reproducible, and desirable fermentation and extraction outcomes.
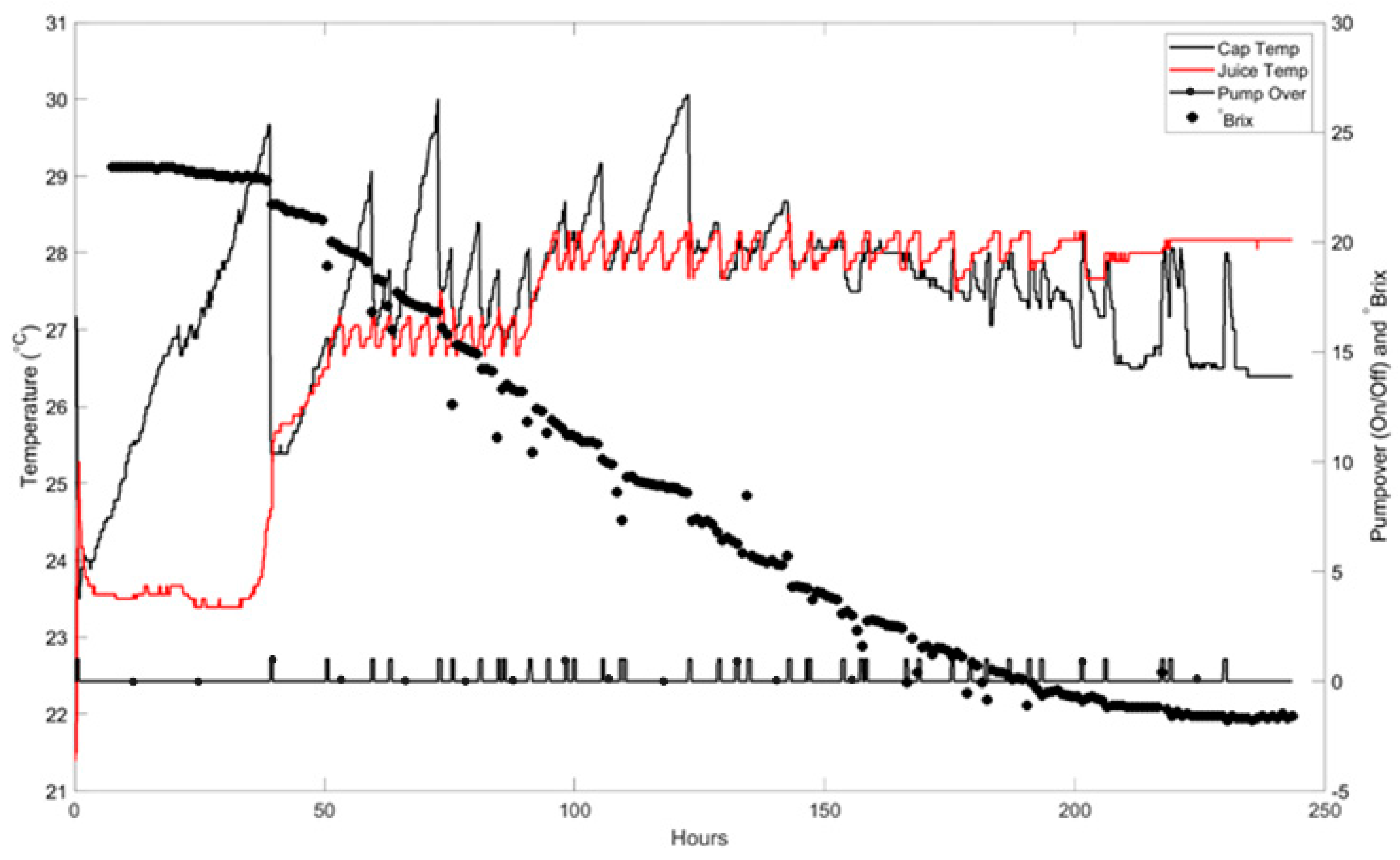
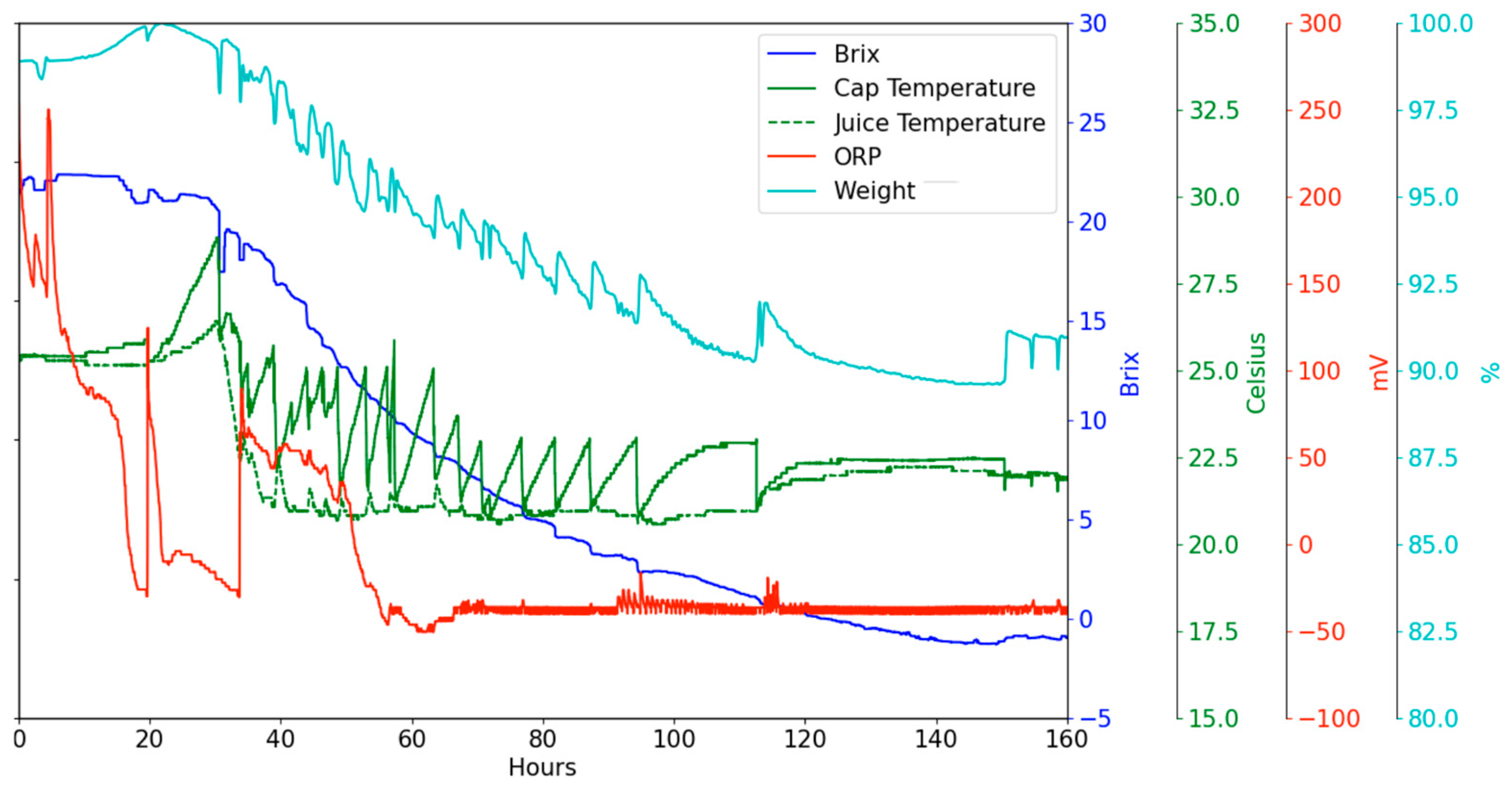
The temperature pattern in the juice for a commercial red wine fermentation is shown in
Figure 5. Inoculation occurs while the juice is still cold, and the juice temperature stays at 23 °C for nearly 40 h until the first pump-over. The temperature of the skin cap rises at approximately 2 °C per hour, reaching 29 °C before the first pump-over takes place. This initial pump-over causes the juice and skin cap to approach a common temperature of 10 °C. During the interval before the second pump-over, both temperatures rise to 15 °C due to heat released by fermentation. The saw-tooth pattern in juice temperature results from the on-off control of the coolant delivered to the cooling jacket. After 90 h, the juice temperature increases to 28 °C for the remainder of the fermentation. In contrast, the skin cap temperature is only lowered by the pump-over actions but can rise to between 1 °C and 5 °C higher than the juice temperature, depending on the pump-over frequency. After 150 h, the skin cap temperature generally remains below that of the juice, indicating that fermentation within the cap has slowed, and it only warms up through the pump-over action with warmer juice. This highlights the necessity for independent control of the skin cap temperature compared to the juice temperature below it. While more frequent pump-overs or punch-downs can minimize these temperature gradients in the short term, most commercial wineries cannot implement this due to fermentor dimensions and labor constraints. One approach is to perform the pump-over operation with a larger volume of juice; another is to sub-cool the pump-over liquid before applying it to the skin cap.
3.3. Bubble-Driven Recirculation Patterns in Wine Fermentor Geometries
Research on vertical bubble plumes in cylinders within air-water systems can reveal the recirculating flow patterns present in unmixed wine fermenters. These features are observable in commercial white wine fermentations and have been recently confirmed in pilot-scale fermentation systems and computational fluid dynamics models.
The studies conducted by Grevet in 1982 [
19] demonstrated the general characteristics of a recirculating flow pattern resulting from an upward central bubble plume, with the strongest upward liquid flow occurring at the outer boundary of the bubble plume and a downward liquid flow pattern along the outer radius, where the highest flow rate is adjacent to the wall. In 1986, Durst [
20] showed that bubbles increase in size and adopt a more “mushroom’ shape due to coalescence and a rise in volume caused by lower static pressure as they ascend through a liquid. These changes lead to higher drag coefficients, and the velocity of the rising bubbles decreases as the distance traveled increases. The height of the liquid increases as the bubble rises, and the pressure at the base elevates with the characteristic length (or the cube root of the volume) in larger fermenters. The outer downward wall current is redirected at the base towards the center of the fermenter, typically sweeping settled solids into this central area, causing them to gather there. In the later stages of fermentation, as the density approaches that of water, there is significant settling of yeast cells that were neutrally buoyant in the early stages, resulting in the base center becoming the largest radial region from which gas bubbles are released.
In 1994 [
21] and 1996 [
22], Castello-Branco and Schwerdtfeger demonstrated that the vertical bubble plume oscillates around the central axis as the bubbles rise. These oscillations commence at a height roughly equal to one diameter and become more pronounced as the fermentation volume height increases. These oscillations create recirculation zones that can shift and vary in size, showing minimal exchange with the adjacent liquid.
Sokolichin et al. (1999) [
23] captured the oscillations of the central bubble plume and illustrated the resulting liquid recirculation patterns. They indicated that three or four local recirculation zones can develop between the inner upward and outer downward flow patterns. Laupsien (2021) [
24] reconfirmed at least three of these local recirculation patterns through measurements in established bubble plumes.
Klembt (2021) [
17] observed the gas and liquid flow patterns in a cylindrical–conical beer fermentor during mid-fermentation. As expected, the central bubble plume is confined to the core of the fermentor, showing minimal evidence of bubble entrainment in the outer downward liquid flow. These results, taken at a liquid height of 1.4 m and a diameter of 0.7 m, indicate that the bubble rise velocity is 0.4 m/s from the base to mid-height, slowing to 0.3 m/s near the upper surface. The liquid pattern reveals three local recirculation zones driven by the inner upward and outer downward flow. The liquid velocity peaks at 0.15 m/s in the center of the fermentor and decreases to 0.08 m/s as it approaches the upper surface. The three recirculation zones have a vertical velocity ranging from 0.0 to 0.01 m/s, indicating local stationary patterns. While this fermentor design ensures that all settled yeast aggregates remain in the center of the cone, it serves as a preliminary model for an upright cylindrical wine fermentor.
3.4. Concentration Gradients During White and Red Wine Fermentations
The significance of these local recirculation patterns and overall flow directions is based on the gradients of the properties being measured. In a reaction system such as fermentation, the recirculation zones do not interact or exchange with the main fluid, representing local “dead zones.” This discrepancy causes measurements from single-point sensors in these local regions to differ from those of the bulk volume.
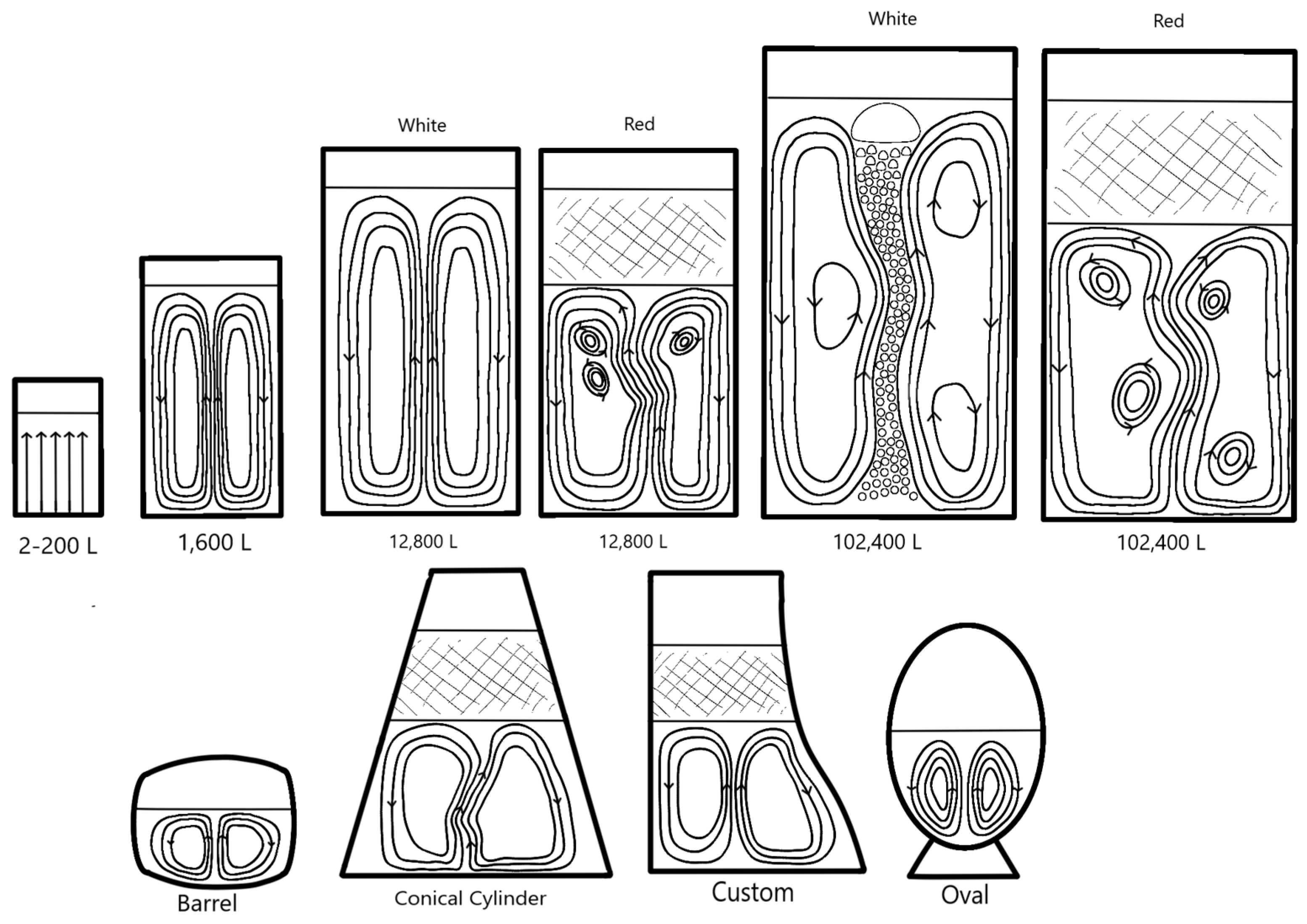
Figure 6 illustrates the expected natural convection circulation patterns in various wine fermentor configurations. The primary factor influencing the rise and shape of the circulation pattern is the liquid depth. The second factor is the cross-sectional area, while the third stems from curved or sloping walls.
In a small (1 to 20 L) upright cylindrical shapes, small spherical bubbles rise from the base, almost uniformly across the diameter, reaching the top surface with minimal wavering or significant bubble coalescence. The liquid depth determines the hydrostatic pressure at the base and the rise time that each bubble experiences. In horizontal barrels (200 to 600 L, typically half-filled), two major recirculation patterns develop from the center to the ends of the barrel, accompanied by two secondary patterns across the diameter. The general bubble pattern in large, unmixed fermentors, as shown in
Figure 6, is a central upward plume that transforms into a distributed outward flow once it reaches the upper surface (or the base of the skin cap in the case of red fermentations). This outward flow is redirected at the outer wall into a downward current that moves to the base, which is then directed to the center of the fermentor. This phenomenon can be observed in the collection of settled solids at the center of the base of a white wine fermentor. Such large-scale flow patterns create either a single toroidal swirling pattern or several patterns stacked vertically, depending on the liquid depth in proportion to the diameter, as seen in
Figure 6. Fermentors where the liquid height matches the radius typically exhibit a single spherical toroidal pattern. In contrast, those with several diameters in height can display multiple toroidal patterns within the depth, approximately related to the height-to-radius ratio. In tall fermentors, the bubble plume often oscillates, leading to fluctuations in the shape and position of these toroids. The development of the central plume and its ensuing oscillations and recirculation patterns becomes significant after about 2 m of bubble rise. This is why these effects are commonly observed in commercial fermentors but seldom in research-scale fermentors. Such effects are not seen in small, unmixed cylindrical glass fermentation vessels containing up to 1 m of liquid; however, they can be observed in small round-bottomed and conical fermentor shapes. The downward internal natural convection flow near the inside wall is intensified by density effects when cooling jackets are present, as the layers adjacent to the wall are considerably colder and denser than those in the general fermentation volume.
In all fermentor configurations, the circulation patterns will increase as fermentation develops and then decrease as the fermentation rate slows. This creates time-dependent variability in the effects, locations, concentrations, and temperature gradients of dead zones for most wall-mounted or sampled systems.
In very large fermentors, the rise time of the bubbles is roughly proportional to the liquid depth. Significant bubble merging and coalescing can occur, leading to larger, mushroom-shaped bubbles and bubble swarms (or clouds) that transfer their momentum to the surrounding liquid as they rise through a greater liquid depth. These bubble swarms can oscillate laterally as they rise, often resulting in secondary eddies and one or more local recirculation zones within the height of the fermentor.
3.5. Modification of Gradients by Swirling and Mixing Operations
Installing propeller mixers, gas injection, or liquid jets promotes swirling and modifies temperature and composition stratification. Their operation can be intermittent or continuous and does not need to be intense. Several commercial wine fermenters have been adapted to include swirling or mixing capabilities. Swirling to reduce radial gradients is especially important if sampling at the wall is used for density determinations, which are sensitive to both concentration and temperature gradients.
4. Sensors for Solution Properties
The progress of wine fermentation can be monitored in several ways. These methods range from the physical properties of the fermentation liquid (such as density, osmotic pressure, refractive index, or dielectric constant) to aggregate properties (including fermentation volume, liquid weight loss, carbon dioxide release, energy release, and the composition of the liquid’s sugar, ethanol, or vapor concentration).
Table 1 presents various properties and their changes during fermentation.
The extent of change in each property is the first consideration, the sensitivity and stability of the sensor is the second, and avoiding ambiguous conditions is the third.
Table 1 compares the fermentation properties that may be measured, their changes from the initial juice to the final fermented wine, and the commercial availability of corresponding sensors.
Monitoring temperature during fermentation is essential for accurate parameter estimation in temperature-dependent models and relies on well-developed temperature-sensing technology. Most properties of the solution, such as density, viscosity, refractive index, and volume, depend on temperature; therefore, their measurements must be accompanied by corresponding or nearby temperature readings for proper temperature compensation. This also applies to measurements like the volume of carbon dioxide evolved, which require corrections for the presence of water and ethanol vapors. The vapor pressures of the components have different temperature dependencies, with water concentration remaining essentially constant while ethanol’s concentration varies as it increases throughout fermentation. The gas released during red wine fermentations is typically at the temperature of the skin cap rather than that of the juice, necessitating the monitoring of gas temperature for these corrections.
Other measures, like weight loss, are independent of temperature but present challenges due to variations in the background contribution to weight from skins and seeds during red wine fermentations. Weight loss has been utilized at the benchtop scale for decades [
5], and at the commercial scale with white wine fermentations in at least one large winery since 2000. Weight loss is an aggregate property that has been shown to be effective for monitoring wine fermentations and estimating model parameters [
9,
25].
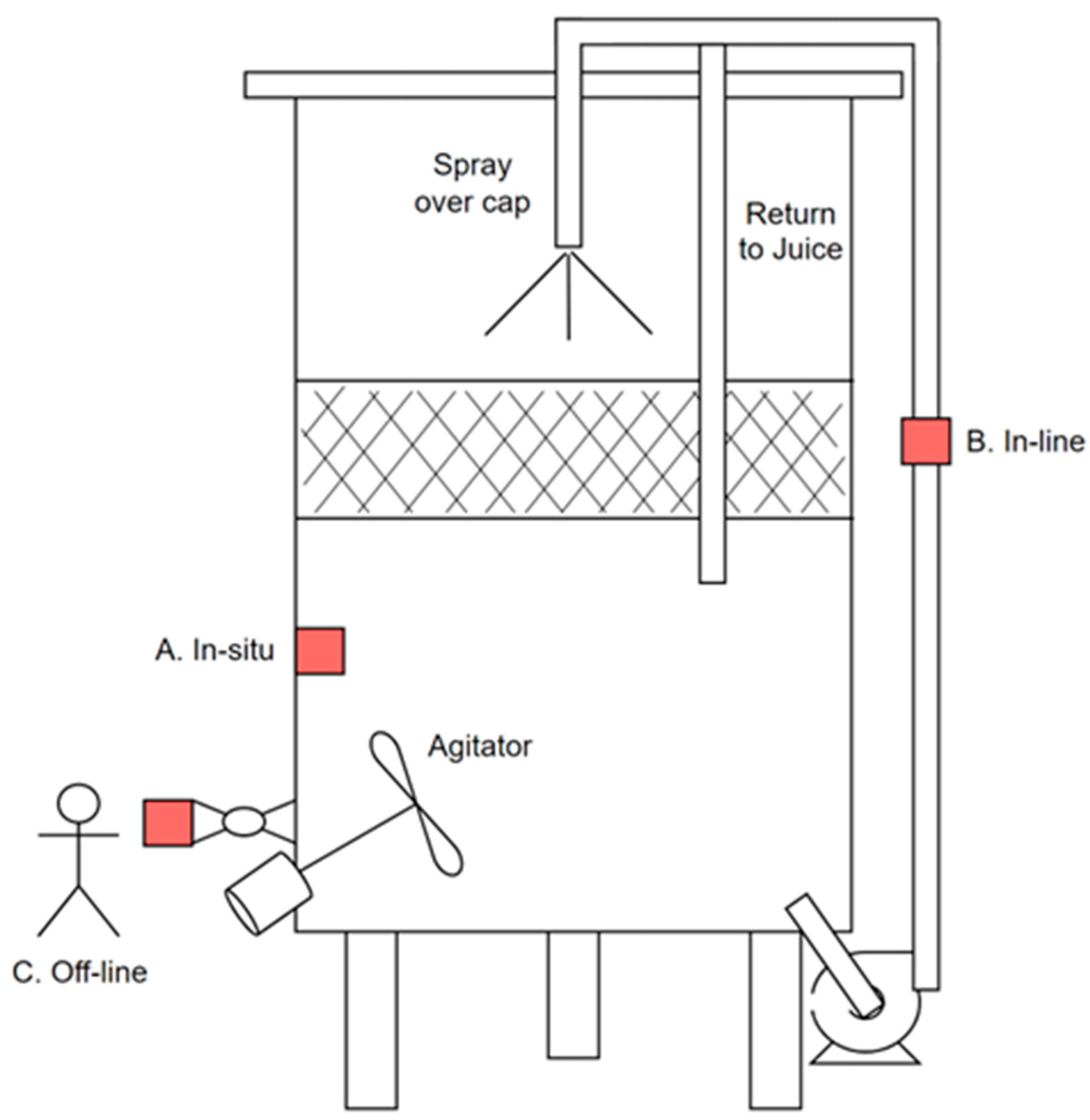
In Situ, In-Line and Off-Line Sensor Systems
Sensing can occur in situ, in-line, or off-line modes. In situ sensors provide real-time (e.g., by the second or minute) or near real-time (by the hour) data during wine fermentation, offering significant advantages for automated diagnosis, modeling, and control strategies. In situ measurements generate data that can be analyzed as a time series or used for parameter estimation, which facilitates real-time modeling of fermentation health and status. In situ sensors should be strategically positioned to avoid placement in stagnant areas of the tank and regions that do not provide representative measurements of the fermentation volume. For instance, when cooling jackets are used in small and mid-sized fermentors, temperature sensors should not be placed directly below the jacket, as they can be influenced by the refrigerant’s temperature circulating in the jacket, the colder juice in the descending current of the natural circulation pattern, or a colder-than-normal wall temperature. Unfortunately, such sensor placements are common in commercial fermentors and result in biased measurements rather than accurately reflecting juice temperature. While positioning in situ sensors at the center of the tank could yield a more representative juice temperature, it may also pose challenges if the tank needs to be filled from the top, potentially leading to sensor damage. The ideal scenario involves in situ sensors that can measure a significant volume and are easy to clean and sanitize.
In-line sensors are typically located within pump-over lines, which circulate juice from the bottom back to the top of the fermentor. This setup may be preferred when installing sensors directly into an existing fermentor presents challenges. The juice in the pump-over line continues to ferment similarly to the larger volume in the tank. However, the smaller volume-to-surface area ratio and lack of temperature control necessitate frequent pump-overs for reliable measurements. In-line sensors in a pump-over line rely on the mixing frequency and temperature control strategies. The frequency of pump-overs can be restrictive, as standard winemaking practices typically involve pump-overs twice a day. These features can be integrated into an external pump-over line and a dedicated pump suitable for liquid circulation in red and white fermentations, returning the juice below the skin cap or wine surface with a submerged outlet, as illustrated in
Figure 7. This prevents unwanted skin extraction if the returned liquid provides additional skin contact, effectively avoiding a pump-over if desired while ensuring reliable temperature data. The temperature can then be averaged over the total volume or a representative fraction.
Off-line measurements of drawn samples require significant personal intervention, exhibit low sample frequency, and may involve considerable delays in obtaining results (and incorporating them into a control strategy). Collecting samples can reveal vital information about the concentrations of essential components such as sugars, ethanol, yeast cell mass and viability measures, and the complex phenolic profile consisting of anthocyanins, tannins, and non-tannin fractions. While some of these can be analyzed using rapid methods, there is often a delay in posting results, and they are typically not used for process control applications. Although existing fermentation models can estimate parameters using concentration profiles of sugar, ethanol, and cell mass and viability information, we know of no instance where this has been implemented in wine fermentations. The detailed chemical analysis of the phenolic profile cannot yield useful information for parameter estimation in yeast behavior models or for the early detection of fermentation issues. Furthermore, manual sampling involves extracting only a few milliliters from thousands of liters, making it difficult to obtain a representative sample, particularly when drawn from a valve in the tank wall located in a stagnant corner at the base of the tank.
The fermentation properties for which food-grade, commercial sensors exist are limited to density, viscosity, weight, redox potential, temperature, and the flow rate (and thus volume) of carbon dioxide released. There does not seem to be a food-grade sensor for osmotic pressure. Changes in liquid volume could be measured for white wine fermentations; however, this does not apply to red fermentations due to the cap thickness of the skins, their compaction, and changes that occur during fermentation. The measurement of fermentation energy release necessitates several additional measurements to estimate removal by a coolant (flow rate and inlet and outlet temperatures) and is constrained by loss terms to the surrounding environment, which vary with scale, insulation, and shading. Moreover, corrections must consider the enthalpy contained in the carbon dioxide release, making it an approximation. The refractive index becomes an unsuitable measurement in the later stages of fermentation when its value starts to return to that of earlier, higher sugar concentrations. Measuring the liquid dielectric constant requires spectral measurements and is currently limited to research applications. The redox potential, like the pressure at the wall, is essentially independent of the concentrations of sugars and ethanol, and therefore is not influenced by radial concentration gradients that develop due to natural convection. This unique characteristic allows these two approaches to effectively capture the properties and status of the fermentation volume.
5. Liquid Density Using In Situ and In-Line Sensors
Liquid density is the primary variable indicating the conversion of sugars to ethanol during wine fermentation. As shown in the trends of wine fermentations in
Figure 1 and
Figure 3, changes in density relate to various other variables, such as total sugar and ethanol levels. Traditionally, the measurement of density in winemaking is performed using a hydrometer, which operates on the principle of buoyancy to float a glass rod in a graduated cylinder filled with the liquid being measured. Such off-line density measurements are typically conducted once or twice a day due to labor constraints. Since the introduction of the simple hydrometer, numerous attempts have been made to digitize density measurements. These methods are compiled in
Table 1 and are based on principles such as solution properties, optics, resonant frequency, CO
2 evolution, pressure, differential pressure, and ultrasonic frequency.
Single density sensors applicable to wine fermentation are limited in that they only assess properties of the small volume directly surrounding the sensor. As wine fermentations are generally not mixed, density gradients and vertical stratification can occur within fermentors, influencing the correlation between the density measured in a localized volume and that of the overall fermentation volume. In optical-based methods, the relationship between the index of refraction and the density of the juice is utilized to determine the density. However, this measurement is impacted by factors such as ethanol content, bubble formation, and solids present in the media. The measured fluid is introduced into a physical structure and excited with a piezoelectric device in resonant frequency measurements. Similarly to optical measurements, resonant frequency measurements only consider a small volume of juice and are affected by bubbles and suspended particles. Bubbles in the measurement chamber can shift the resonant frequency, resulting in errors in the measured density.
However, density measurements utilizing pressure, CO2, and ultrasonic velocity are influenced by a larger volume of juice, unlike optical and resonant frequency-based techniques that sample smaller volumes, making them aggregate measurements.
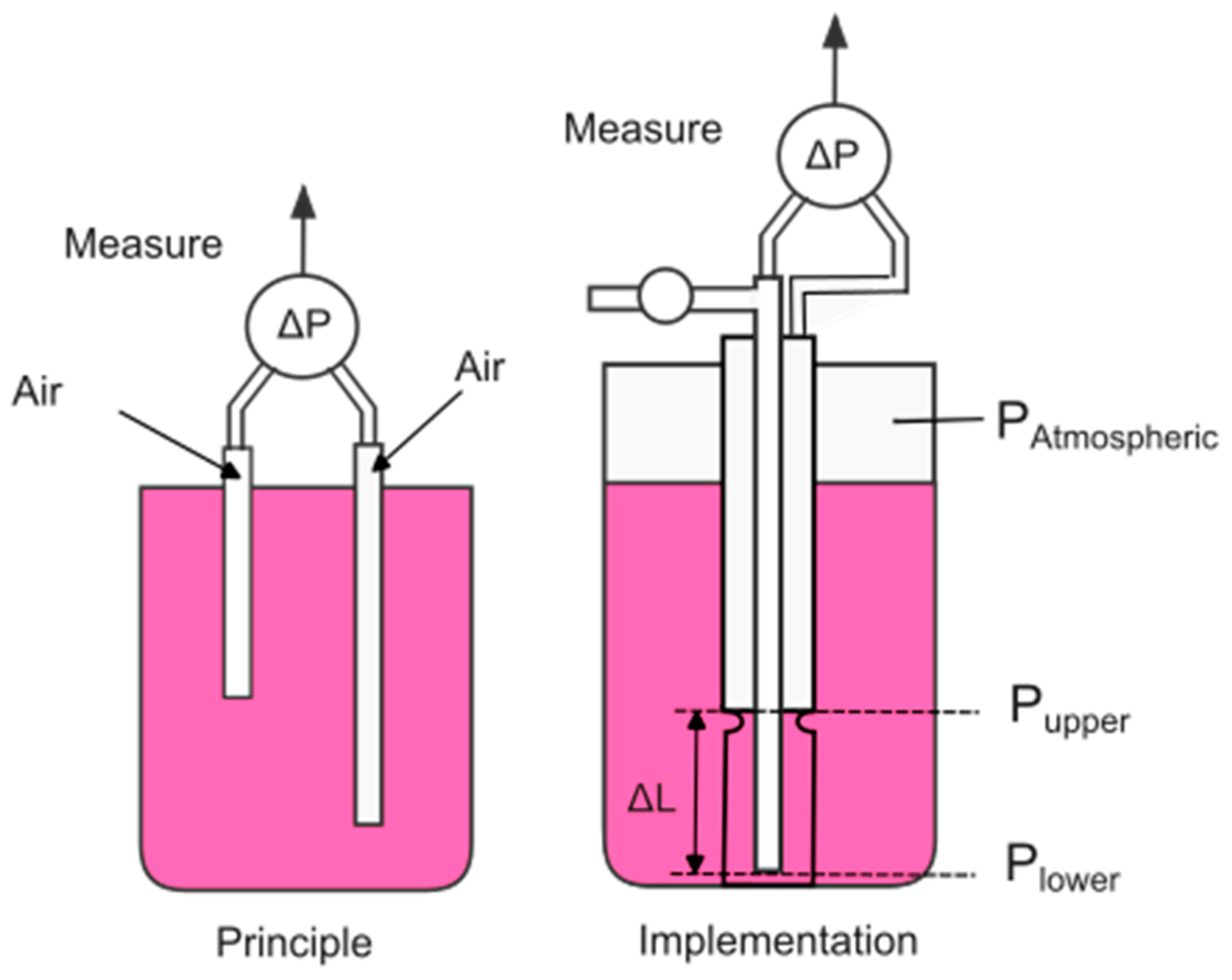
5.1. Density by the Differential Pressure
Pressure to measure density relies on the principle that the pressure at any point in a liquid is determined by four factors: the liquid’s density, the depth of the transducer relative to the surface, the atmospheric pressure, and the liquid’s temperature. Utilizing a single transducer allows for the measurement of weight loss, which can then be converted to a calculated density based on the initial density measurement. This weight loss measurement serves as a useful indicator of fermentation progress and rate. It has been used in white wine fermentation for over 20 years. However, it is not easily converted into density for red wines due to variations in the mass of the skin cap, which stem from differences in berry size and volume related to cultivar, irrigation, and cropping practices.
Employing two vertically separated pressure transducers addresses the limitations of a single transducer for weight loss [
26]. With a known distance between the transducers, the pressure difference between two vertical points in the liquid hinges on the liquid’s density and temperature, which is often measured by the pressure transducer. In red fermentations, the upper transducer must always remain below the cap. Differential pressure measurements also benefit from subtracting common noise sources, enhancing their resilience against ambient noise and diurnal variations. In a commercial pressure transducer, the membrane in contact with the liquid is sufficiently large that the transducer remains mostly unhindered by solids and bubbles present in the fermenting liquid.
By positioning the two transducers as far apart as possible (> 25 cm), differential pressure measurements can be performed with high accuracy (up to three significant figures), yielding sufficiently precise measurements, and an estimate based on as much as 80% of the fermentation volume, as the lower transducer captures contributions from all juice above it. This method averages out vertical and concentration gradients, resulting in a more representative measurement of fermentation progress [
26].
Density measurements using two pressure transducers installed on the side of a fermentor have been employed at the research scale (100 L volumes) since 2010 and in full-scale volumes (20 to 40 KL) at several wineries, making it the preferred method for monitoring both white and red wine fermentations. This approach has been implemented in at least 100 production fermentors. Furthermore, the decreasing cost of industrial pressure transducers (less than 1000 USD) makes pressure and differential pressure measurement an affordable and viable technique for commercial use.
In smaller commercial fermentations (<1500 L), typically utilized in medium-sized operations, installing sensors directly on the sides of the fermentor is generally avoided. This configuration facilitates easier grape processing within the tank and enhances cleanliness. In these setups, differential pressure can be measured from above. This method, shown in
Figure 8, pioneered by Dr. T.J. Rodgers in 2010 and successfully adopted across 150 research fermentors at the University of California, Davis, employs a small air pump to channel air through two concentric tubes placed into the juice, with the ends of these tubes approximately 19 cm apart. The differential back pressure created is then measured by a single low-cost microelectromechanical (MEMS) sensor to ascertain the juice’s density. While the air is injected, density measurements are performed with an accuracy of 0.1 °Brix, with the added benefit that the air conducts a self-cleaning operation, preventing the accumulation of bubbles and residues. This stands in contrast to fixed-location sensors, such as those developed by Jaywant [
27], which can suffer from residue buildup that affects measurement precision and necessitates thorough cleaning. Additionally, using a single sensor provides several advantages over designs that utilize multiple sensors. It minimizes errors linked to temperature, drift, and aging, which tend to increase with the number of sensors in use. The limited pressure range allows for the use of a more sensitive sensor, thereby reducing scale, noise, and error.
Measurements of CO
2 can also be used to track the fermentation rate and serve as an indirect measurement of density through the stoichiometric relationship of ethanol fermentation. In practice, measuring CO
2 during fermentation is challenging due to the necessity of capturing all the gas released. The variation in CO
2 production throughout fermentation also complicates the design of such systems, as fans for drawing and removing CO
2 and sensor measurement ranges must accommodate both small and large flows of CO
2. The difficulties in measuring CO
2 to monitor the progress of fermentation are illustrated by the fact that while several research studies [
28,
29,
30,
31,
32], this method does not seem to have been widely adopted in commercial practice.
5.2. Ultrasonic and Coriolis Sensors for Density Measurement
Ultrasonic velocity measurement of density has been demonstrated in wine and beer [
33,
34,
35,
36,
37,
38,
39,
40]. Ultrasonic velocity in a liquid depends on several factors, including the density and compressibility of the liquid, as well as temperature. The ultrasonic velocity density technique faces challenges due to temperature gradients and fluctuations, solids obstructing acoustic waves, changes in attenuation, and the presence of CO
2 bubbles—common features in commercial wine fermentation. Consequently, this technique has not been widely embraced in alcoholic fermentations, despite its potential as a noninvasive measurement method for simpler fluids.
Dual pressure transducers, a vibrating U-tube sensor [
41], and a Coriolis sensor [
42] can all be used for in-line density measurements in dedicated pump-over lines, typically found on modern red wine fermentors. A Coriolis sensor is an accurate method for measuring a fluid’s mass flow and density. Fluid flowing through one or more curved tubes within the sensor causes oscillations due to the Coriolis force. The phase shift between oscillations at the inlet and outlet of the tube determines the flow rate, while the resonance frequency provides insights into the fluid’s density. In a red wine fermentor, an in-line Coriolis sensor can measure the flow rate during pump-overs.
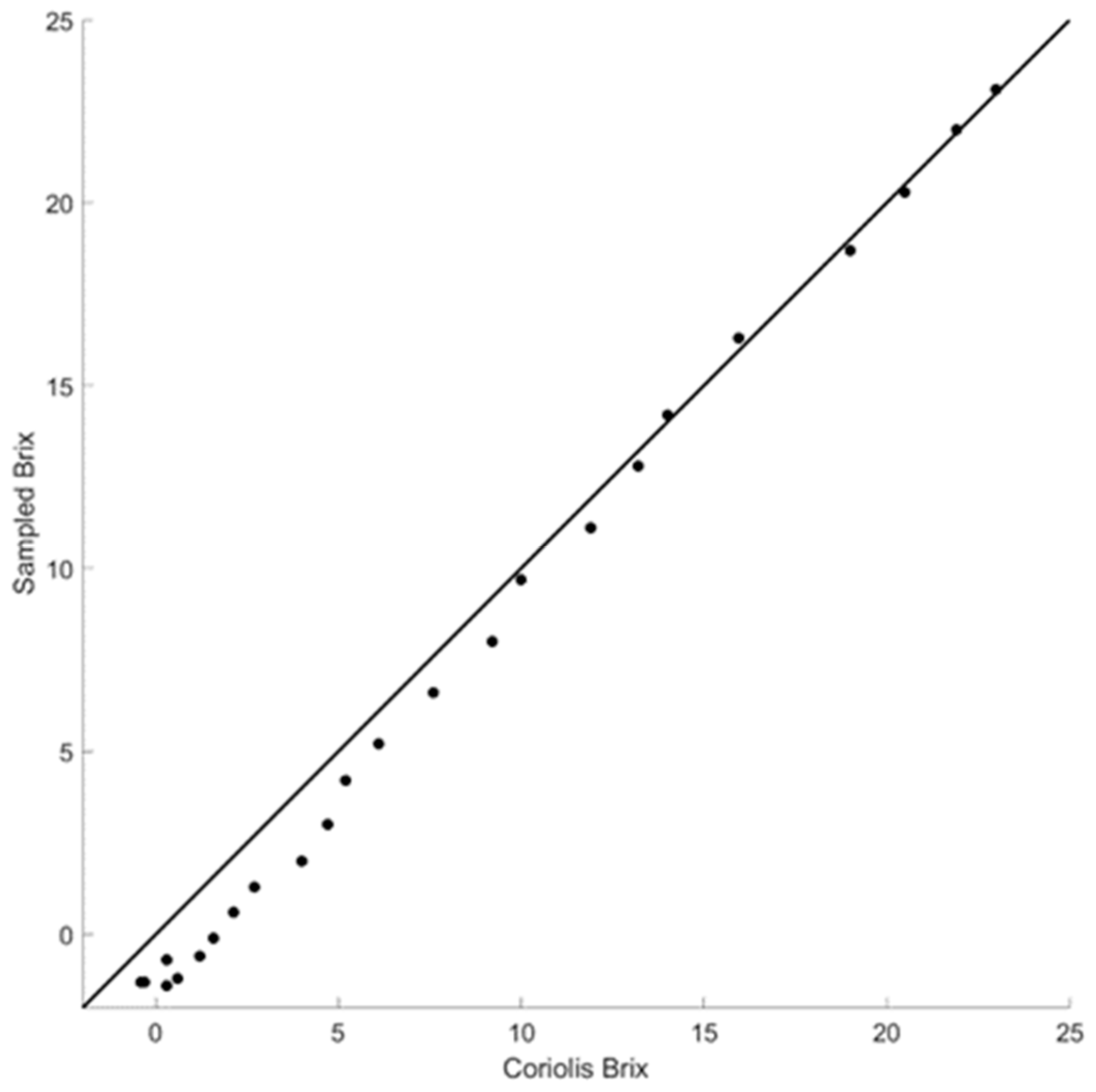
When placed in the pump-over line, this type of sensor can also provide a Coriolis density measurement during a pump-over. However, as shown in
Figure 9, the Coriolis density measurement diverges from the hand-held measurement at around 10 °Brix. It overestimates the density by 2 °Brix when the actual density is at 0 °Brix. If the flow rate is too low, these probes may develop bubble formation, leading to significant noise in the resonance frequency. This requires employing advanced signal processing techniques to effectively reduce the noise; we found that the inline dual pressure approach in the pump-over line is more accurate and simpler to implement in practice.
6. Sensors for Other Aggregate Measures of Fermentation
6.1. Carbon Dioxide Release
Measuring the release of CO
2 during fermentation requires flow measurements capable of covering a wide range of flow rates, temperature measurements for correcting gas volume, and estimating water and ethanol vapor pressures, which also depend on the ethanol concentration in the liquid. Losses in these estimates occur when fermenters are opened for additions or pump-over operations. This method is not suitable for open-top red wine fermenters. With adjustments for losses, it could be utilized in model parameter estimation, as illustrated in
Figure 2, or as the rate of fermentation in
Figure 4.
6.2. Energy Release
Capturing the energy release is challenging due to the complexities related to heat transfer and losses during fermentation. The generation term alone is proportional to the volume of CO
2 released, as shown in
Figure 2, and to the fermentation rate, indicated in
Figure 4. Estimating the removal term requires knowledge of the coolant flow rate, thermal properties, and the temperature change within the cooling jacket or heat exchanger. It also necessitates estimates of the radiant and convective losses from the fermentor wall that is not jacketed, the conductive transfer at the base of the fermentor, and the flow rate and temperature of the carbon dioxide released to calculate the enthalpy component lost in this stream. To the best of our knowledge, this method is not used commercially.
7. Sensors for Oxidation–Reduction Potential in Wine Fermentations
A less commonly measured fermentation property involves the liquid’s redox potential (also known as the Oxidation–Reduction Potential, ORP, or reduction potential) throughout wine fermentations. This concept is not new, but it has faded in interest, presumably due to the scarcity of reliable and robust commercial and food-grade probes. Graff (1950) [
43] described its application in brewing and winemaking, and winemaking texts from that time, in German and French, detail experiments that use it as a fermentation variable. The redox potential of a solution indicates its capacity for electron transfer and is measured potentiometrically. Depending on the solution’s composition, an electrode can function as either an electron donor or acceptor. In oxidizing conditions, the electrode loses electrons to the solution, resulting in a positive potential. Conversely, the electrode gains electrons from the solution in a reducing environment, producing a negative voltage. The voltage generated during wine fermentations typically ranges from +500 mV to −200 mV, using the standard hydrogen electrode as the reference.
De Graef et al. (1999) [
44] suggested that internal NADH/NAD ratios are related to the external redox potential and correlated with cellular adaptation in
Escherichia coli. This implies that the same might be true for
Saccharomyces cerevisiae and other organisms, establishing a link between the controlled redox potential during fermentation and cellular changes in response to it, such as cell viability and maintenance rates in non-growing cells, according to Killeen et al. [
45].
Although there is some confusion in publications regarding the interpretation of redox values, particularly their relationship to dissolved oxygen concentration, we view it as an important unmeasured variable necessary to define the medium environment in which viable cells are growing and metabolizing. A recent review of the chemical and biological basis of redox potential in grape juice, wine, and wine fermentation can be found elsewhere [
46], along with examples of the changes in redox potential caused by yeast growth and commercial fermentations where it is controlled.
The redox potential changes during fermentation due to the export of reductive compounds, such as glutathione, which are synthesized by the yeast during growth [
4,
47]. The level at which the potential reaches or is controlled can positively influence fermentation outcomes, such as faster completion [
45,
48] and limit H
2S formation, particularly in the presence of residual elemental sulfur on the grapes [
48].
The potential falls shortly after inoculation, decreasing from +250 to 300 mV to a minimum of −100, −150, or −200 mV before recovering slowly as fermentation is completed. The steepest descent occurs during yeast growth, with the minimum value reached when growth ceases. In monitored systems, this indicates the end of yeast growth and can signify a problem with fermentation if it happens too early. Since the fermentation rate and temperature determine the rate of change in potential and the depth to which it falls, monitoring allows for actions to prevent undesirable fermentation tailing or product formation. Controlling the potential at a desired set point is achieved through frequent and periodic addition of air, oxygen, or hydrogen peroxide.
An example commercial redox probe commonly used in winemaking is shown in
Figure 10. The reference electrode generally consists of a silver (Ag) electrode in a silver chloride (AgCl) electrolyte solution, along with a junction that maintains contact between the electrolyte and the wine without leaking the electrolyte into the wine. The components are typically housed in glass to prevent electrical or chemical interaction with the solution.
The redox potential is primarily determined by the substances exhibiting the greatest current exchange density, indicating their proficiency in electron exchange with the platinum surface. Consequently, the measured redox potential is mainly influenced by the kinetics of the rate-controlling redox reaction in the solution rather than being contingent on the concentration of all possible reactants [
49,
50]. In the context of wine fermentations, it seems that the iron-based autoxidation of tartaric acid establishes a reaction sequence involving iron and copper complexes and glutathione [
46,
51]. The redox potential during fermentation is generally understood not to represent an equilibrium measure of any single half-cell reaction due to the changing redox reactive components in the medium and those introduced by yeast activity or added dissolved oxygen. The rate-limiting redox reaction couple is likely to change during fermentation, depending on the components within the medium.
The unique composition of grape juices and wines determines their prevailing redox potential and their ability to buffer against changes introduced by the addition of oxygen. As previously mentioned, controlling the skin cap temperature to limit the decline in redox potential in the skin cap is a winemaking objective that aims to reduce the possibility of H2S formation in the cap. Therefore, the measurement and control of the redox potential represent a new frontier for establishing reproducible and precise outcomes in commercial wine fermentations.
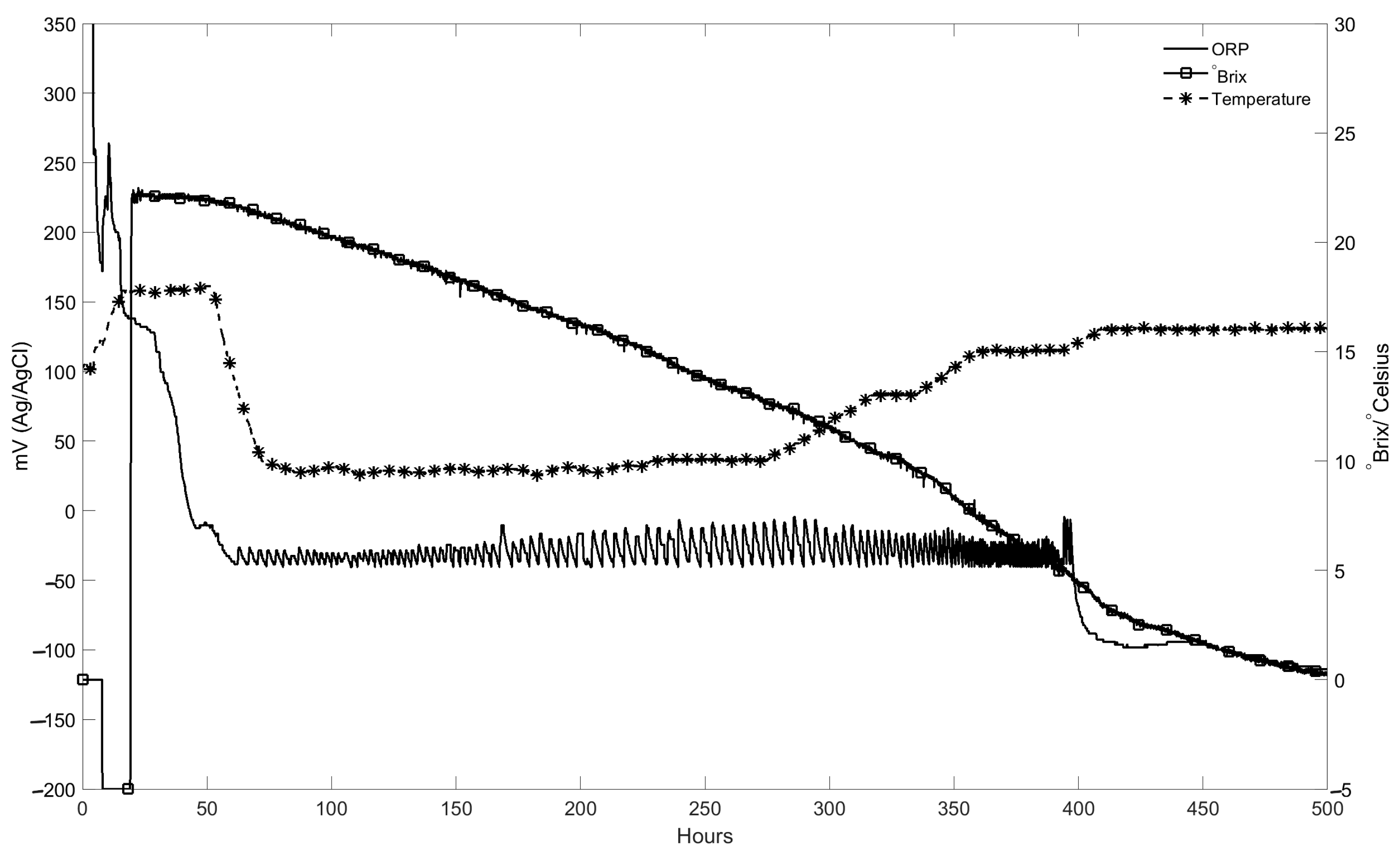
Example of Controlled Redox Potential in a Commercial White Wine Fermentation
An example of the control of the redox potential during the fermentation of 140 KL of white wine is shown in
Figure 11. In this fermentation, the temperature set point is changed after 250 h from 10 to 16 °C in three steps, causing the corresponding °Brix curve to become steeper. After 40 h into the fermentation, the redox potential is maintained at −40 mV before control was removed at 380 h. The potential, similar to the juice temperature, was measured with an in situ redox probe mounted in a side arm fitting.
The availability of sensors for monitoring the redox potential of wine opens new research frontiers for overseeing and managing bacterial and malolactic fermentation in both research and commercial winemaking. The influence of continuous, periodic, and no aeration, exhibiting high, intermediate, and low redox potentials, has been shown to affect the speed and completion of malolactic fermentation in bench-scale trials [
52]. The fermentation with higher redox potential was the most active, essentially reaching completion after 12 days, whereas the fermentation with lower redox potential converted less than 10% of the malic acid during this time.
Although there has been minimal association in the wine literature between the wine’s redox potential and the rate of growth or malic acid metabolism, it is expected to reflect a similar pattern to that of other bacterial growth in yogurt and milk fermentations [
46]. Establishing a favorable fermentation environment, tracking microbial activity, and ultimately controlling the potential for desirable outcomes—whether in terms of speed of completion or byproduct formation—remains a future research objective.
The ability to manage redox potential during commercial-scale wine fermentations, along with the implications of reducing H2S formation when it occurs and achieving earlier completion through controlled potential, represents one of the most significant advancements in wine fermentation technology. Additionally, controlling the redox potential during fermentation opens exciting research opportunities related to yeast viability and maintenance activity during the non-growing phase of their life cycle, as well as the metabolic effects of altered electron preferences and end product formation, particularly in dehydrogenase reactions, glycerol and succinate production, and sulfite and sulfide generation.
8. In Situ and In-Line Sensing of Cell Mass and Cell Viability
The representative and reproducible sampling for cell mass and compositional analysis presents challenges in unmixed fermentors, such as most commercial wine fermentors, since the degree of stratification varies throughout the fermentation process. These variations introduce inconsistencies and uncertainty between the sampled volume measurements and the overall fermentation volume. For these reasons, the sampled measurements and approaches discussed below pertain to well-mixed, research-scale fermentation monitoring rather than unmixed commercial fermentations. This section aims to demonstrate that significant advancements in monitoring and modeling techniques can and should be made at the research scale with clarified juices.
The issue with utilizing all spectral and counting approaches is the small sample volume analyzed relative to the total fermentation volume, along with the presence of bubbles and suspended pulp fragments that lead to light scattering and shadowing.
The interest in total cell mass and viable cell mass (and their ratio, or cell viability) arises from the insights they provide for parameter estimation in wine fermentation models, potentially enhancing the mathematical representation of the dynamics of the viable population during wine fermentation.
Harris and Kell (1985) [
53] thoroughly reviewed cell mass measurement techniques. While methods exist to quantify cell mass in clear fermentation solutions, such as absorbance measurements in the 600 to 650 nm range (or 780 or 920 nm in the NIR band [
54]) and dielectric spectroscopy, these techniques are seldom employed in research, let alone in commercial wine fermentations. Chandra et al. (2017) [
55] recently reviewed a variety of sensors and spectral fermentation methods.
In the case of turbid wine fermentations, advanced methods like counters for estimating cell size distributions and more sophisticated spectral analyses are essential to differentiate between suspended materials, including yeast and bacterial cells, grape cells in pulp fragments, and carbon dioxide bubbles.
8.1. Absorbance and Nephelometric Measurements of Total Cell Mass
The absorbance measurements of in situ optical cells at a wavelength of 660 nm showed a linearity limit of 3 g/L with
Saccharomyces cerevisiae, as noted by Ohashi et al. (1978) [
56], even when employing a flow path to eliminate bubble interference. This limit could potentially be extended to the range of cell mass observed during wine fermentations, specifically 4 to 8 g/L, though the bubble interference would also increase proportionately. We are not aware of any instances where this or other absorbance or turbidimetric sensors have been utilized to quantify total cell mass in commercial wine fermentations. Ji et al. (2024) [
57] successfully monitored at 890 nm instead of 600 nm for estimating the cell mass of yeast and three different bacteria at concentrations up to 2 g/L. While this concentration is below the yeast cell mass during primary fermentation, it falls within the range of bacterial cell mass during secondary fermentation and could be relevant for monitoring bacterial growth and cell concentrations.
8.2. Fluorescence Measurement of Cellular Activity
While measuring total cell mass and viable cell mass is important for parameter estimation, total cell fermentation activity—especially during the non-growing phase—could serve as an alternative measure for the early detection of problematic fermentations. The use of fluorescence to monitor metabolic activity through cellular NADH concentrations was first proposed by Harrison and Chance in 1970 [
58]. This method was investigated with S. cerevisiae by Zabriskie in 1978 [
59] and further explored by Zabriskie and Humphrey [
60]. They used a 366 nm wavelength for excitation and a 460 nm wavelength for emission, targeting internal cellular NADH. Their studies assessed cell concentrations up to 20 g/L, but they concluded that this method could only reliably estimate cell concentration when nutrient levels were high and the growth rate remained constant. Unfortunately, neither of these conditions holds true during wine fermentations. Fluorescence has proven suitable in cellulose suspensions and complex media [
61] and recognized as an indicator of cell activity or vitality, but only applicable to modeling in limited fermentation scenarios, not specifically in wine fermentations.
Volesky (1991) [
62] examined cultivation conditions using on-line fluorescence measurements with Clostridium acetobutylicum, while Anton (2010) [
63] provides a comprehensive review of single and multiple-wavelength fluorescence applications to estimate cell mass for various bacterial, yeast, and mammalian cell types. Bogomolov et al. (2011) [
64] illustrate in-line monitoring of fluorescence during fermentations using
Saccharomyces cerevisiae.
The measurement of cellular fluorescence has been interpreted as “cellular activity,” which is a product of active cell concentration multiplied by the maintenance rate in the non-growing, viable population. In this context, it could prove beneficial for detecting slow or incomplete fermentations once yeast growth has halted. However, interpreting its value is challenging since it is a compounded variable that can show varying values of either component over time or at different fermentation stages. It may be advantageous if paired with a fermentation model that can separately estimate the maintenance rate and cell viability [
2]. The pattern of “cellular activity” during a wine fermentation is expected to align with the fermentation rate, as illustrated in
Figure 4. Interferences from quenching and scattering of fluorescent signals due to suspended particles and certain wine solutes are anticipated. Currently, there appears to be no application of these measurements in wine fermentations.
8.3. Total and Viable Cell Mass by Dielectric Spectroscopy
The application of dielectric spectroscopy to fermentations involves measuring the frequency-dependent permittivity and conductivity between two electrodes immersed in a cell suspension. The typical frequency range employed is 10 kHz to 10 MHz for yeast and other cell types (such as bacteria, mammalian, and plant cells). Some applications use a single frequency to monitor changes in capacitance, while others scan 25 or more frequencies to develop a more comprehensive response curve. Dielectric spectroscopy, also referred to as impedance or capacitance measurement, can provide estimates of the total cell mass, the growth rate, and possibly the viable cell mass by distinguishing the responses of intact and dead cells [
65]. It is important to note that any wall-mounted or in-line sensor systems are influenced by the flow of fluid over the probe, necessitating some form of tank mixing to ensure that an in situ probe provides useful information.
Kell et al. (1990) [
66] described their measurement property as “biovolume” based on the permittivity measured in the frequency range of 0.1 to 10 MHz. These measurements were shown to be independent of bubble and light scattering components in the solution. The recorded capacitance was linear to cell concentrations of 15 g/L, with a slope of 2 pF per g/L cell mass. Fehrenbach et al. (1992) [
67] established linear correlations between capacitance measurements and total cell mass for
Saccharomyces cerevisiae,
Pichia pastoris, and
Streptomyces virginiae in bioreactors ranging from 20 to 2000 L. Austin et al. (1994) [
68] presented a linear relationship between viable cell mass, determined by the methylene blue staining technique, and capacitance measurements for a brewing strain of the yeast
Saccharomyces cerevisiae. Siano (1997) [
69] used frequencies ranging from 75 KHz to 30 MHz to investigate yeast, bacteria, and mammalian cells separately, finding that spectral characteristics change with cell type and concentration, along with overlapping relaxation times for the yeast (
Saccharomyces cerevisiae) and bacteria (
Escherichia coli) studied. He reported that cell size and its distribution were major factors in the characteristic frequency, while cell shape played only a minor role. Junker (1994) [
70] compared several commercial NIR probes based on wavelengths from 800 to 1100 nm with a capacitance probe and found the capacitance probe to provide the most linear signal with cell concentration. November et al. (2000) [
71] reported impedance measurements in suspensions of
Candida utilis, demonstrating a good correlation with dry cell mass up to 11 g/L. Elvira (2001) [
72] employed the 1 kHz to 10 MHz range for quantifying wine yeast and bacteria (
Escherichia coli). Krommenhoek et al. (2004) [
73] investigated capacitance measurements of
Saccharomyces cerevisiae suspensions using two frequency ranges: 60 to 80 kHz and 1 to 2 MHz. They reported a linear response up to 9 g/L cell mass, with a characteristic frequency of 2.8 MHz for this yeast.
Patel and Markx (2008) [
65] provide a review of capacitance spectroscopy and its application to estimating viable cells and cell volume in culture and fermentation systems. They note that exclusion dyes such as trypan blue and methylene blue accurately measure viable cells only when cell death accompanies membrane injury. They emphasize that intact cells that are inhibited or injured will be included in viable cell estimates using capacitance spectroscopy. This is a significant consideration in estimating cell viability by spectral methods due to the potential for ethanol injury or inhibited sugar transport during the non-growing phase of wine fermentation. We observed a cell viability of 50%, based on the methylene blue assay, when all fermentation activity ceased in stuck fermentations. This aspect merits further investigation if this approach is to be adopted for monitoring wine fermentation. The review by Kiviharju et al. (2008) [
74] considered 18 studies involving 20 cell types to evaluate the performance of 12 commercial in situ sensors for biomass. The approaches reviewed include optical density, NIR and Mid-IR, capacitance, and fluorescence spectroscopy. They highlight the limitations posed by suspended matter in the medium and the advancements in capacitance spectroscopy over the previous decade. A recent comprehensive review focusing on cellular physiology and capacitance measurements during cell culture and fermentation is by Flores-Cosío et al. (2020) [
75]. They revisit the assumption that all intact cells are viable and thus capable of fermentation.
Given that one of the factors influencing impedance measurements is the resistance (or conductivity) of the medium in which the cells are measured, applying this to wine fermentation raises several questions. These include the impact of higher sugar and ethanol concentrations (2.2% and 4.4% m/m, respectively) on the conductivity of the solution, changes in solute gradients across the yeast or bacterial membranes, and likely changes in cell volume throughout the fermentation process. The capacitance measurements below the critical frequency vary with cell mass, while the critical frequency shifts to higher values as cellular volume decreases [
76,
77,
78].
Yardley et al. (2000) [
79] suggested that dead cells and non-biomass solids lack intact plasma membranes and, therefore, do not contribute to the capacitance signal. However, they also reference the work of Markx et al. (1991) [
80], who employed capacitance measurements to estimate the biomass of
Festuca arundinacea in plant cell cultures. There is anecdotal evidence from unpublished work that grape cells in pulp behave similarly and contribute to the capacitance signal of these probes during wine fermentations. Sources of variation in the conductivity component of the capacitance measurements across different juices include the high ionic strength and low pH of grape juice. The effects of these variables, along with ethanol and osmotic pressure on yeast cell size during wine fermentation, as well as the impact of bacterial cells or intact plant cells on the capacitance measurements, have yet to be established. Horta et al. (2015) [
81] described an online application of capacitance measurements for determining cell mass concentrations in yeast and bacterial cultures. Kiss and Nemeth (2016) [
82] investigated the use of capacitance spectroscopy for monitoring cell mass with seven cell types, including
Saccharomyces cerevisiae and a
Lactobacillus strain. They observed that the conductivity component of the signal varied with the formation of lactic acid during lactic acid fermentation. They also monitored microalgae cells, confirming that intact plant cells contribute to the signal in this measurement. Slouka et al. (2017) [
83] utilized low-frequency capacitance measurements to monitor yeast growth in a brewing application, and Metze et al. (2020) [
84] applied multivariate capacitance analysis to monitor viable mammalian cells online.
8.4. Monitoring Total Cell Mass During a Wine Fermentation
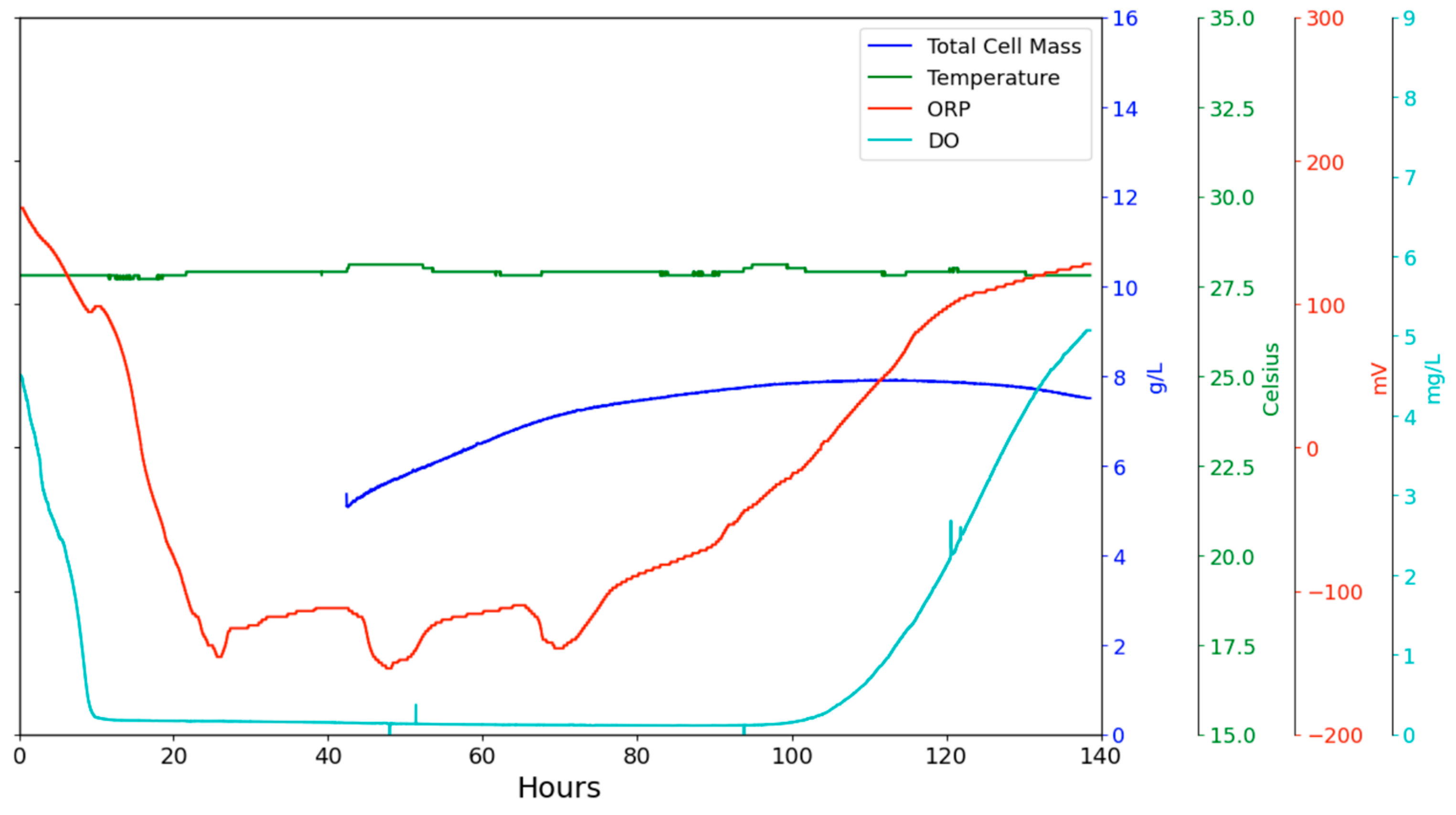
The experimental measurement of total cell mass using a commercial optical probe is illustrated in a wine fermentation depicted in
Figure 12. However, the signal is sensitive to the intensity of mixing in the probe’s vicinity. This indicates that the probe operates within a sphere of fluid for measurement, and this fluid must not be stationary to ensure reproducible results, as shown in
Figure 12. The optical total cell density probe provided stable values once the stirrer speed was increased to 300 rpm in a 1 L vessel at around 40 L. Therefore, in situ probes at a commercial scale will require local agitation to achieve reliable and reproducible measurements from wall-mounted setups. For in-line measurements, it will be necessary to establish a threshold fluid velocity.
Cell mass and viability are essential variables that could be included in parameter estimation calculations for suitable wine fermentation models, as shown in
Figure 1. They would offer more specific insights to examine and develop the relationships among cell viability over time, temperature, cell age, and medium properties. Comprehensive studies on cell viability and cell fermentation activity using reference methods and currently available probes throughout wine fermentation are necessary to enhance the understanding of the non-growth stage of wine fermentations from both monitoring and modeling perspectives. While some portion of the total cell mass in a red wine fermentation will remain within the skin cap, liquid measurements, even if not entirely precise, may still prove useful for parameter estimation and modeling of wine fermentations.
8.5. Other Spectral Systems for Total or Viable Cell Mass
There have been applications of ultrasound velocity methods for estimating cell mass, utilizing pulses of 50 MHz and backscattering to monitor
Saccharomyces cerevisiae [
85]. Other spectral methods include Raman spectroscopy (in mammalian cell culture [
86] and
Saccharomyces cerevisiae, [
87,
88,
89], near-infrared (NIR) [
90,
91], and mid-infrared (MIR) spectroscopy [
89]. These techniques have been developed to estimate cell mass in clear media and, in some cases, to quantify glucose, ethanol, and glycerol concentrations. Ji et al. (2024) [
57] describe an online application of NIR spectroscopy to measure cell mass across four different microorganisms. A more detailed review is beyond the current scope.
9. In-Line and Off-Line Spectral Methods for Chemical Components During Wine Fermentation
The interest in these sensing systems lies in capturing the composition of components that can display the progress of phenolic extraction or malolactic fermentation and estimate parameters for future extraction and fermentation models in real-time. The primary objective is to provide information for winemaking actions or automate control using temperature and redox potential as the environmental control variables for optimal outcomes. Clearly, all future applications of these systems will require pre-filtration of samples to remove suspended matter in commercial fermentations. However, there may be significant advancements in modeling and optimization using some of these spectral methods for biomass and cell viability in research fermentations with clarified juices.
Currently, several measurement approaches, ranging from fiber-optic devices to diode arrays, can be applied to measure ranges or bands of the UV-visible, NIR, MIR, Raman spectrum, and fluorescence in an off-line mode. The applicable analytes for parameter estimation in models include fermentable sugars (specifically, glucose and fructose), ethanol concentration, and measures of organic and inorganic nitrogen components for estimating yeast assimilable nitrogen (YAN).
Other informative analytes can also be used for monitoring and display, and may support parameter estimation in lactic acid fermentation models and comprehensive phenolic extraction models in the future. Quantitative measurements of organic acids, particularly malate and lactate, would enable monitoring the progress of malolactic fermentation, regardless of whether it occurs concurrently or sequentially with ethanol fermentation.
9.1. Sugar and Ethanol Concentrations
The decline in sugar concentration and the rise in ethanol concentration are crucial elements in any structured fermentation model. The rates of change in these concentrations during fermentation hold value only if they can be converted into density equivalents, as most winemakers adhere to the density pattern during fermentation. This conversion has been a significant difference among wine fermentation models and their suitability for parameter estimation in commercial fermentations [
2,
3]. Information about the substrate preference of wine yeast necessitates measuring glucose and fructose separately; this is a distinguishing feature of yeast strain fermentation that can be integrated into models recognizing these substrates [
2], as depicted in
Figure 1. Unfortunately, most spectral methods do not effectively distinguish glucose from fructose, limiting in-line systems to total or hexose-sugar concentration.
9.2. Amino Acids, Ammonia, and YAN Concentrations
The widespread use of yeast assimilable nitrogen (YAN) concentration as a composite nitrogen source for yeast growth in wine and beer makes estimating its components—ammonia and alpha-amino acids—crucial for both juice preparation and fermentation modeling. Initial juice concentrations are sometimes supplemented with ammonium salts to achieve target YAN values. Monitoring its depletion during yeast growth will aid in parameter estimation for all nitrogen-based fermentation models. Understanding cell yield based on nitrogen and substrate preference within the nitrogen pool would provide additional insights for current and future wine fermentation models. Future studies may develop correlations for YAN values from separate ammonia and major amino acid analyses. Examples of spectral methods for determining ammonia and amino acids include the application of nonlinear NIR, as described by Pontius in 2020 [
92], for measuring glucose, ethanol, glycerol, acetate, ammonium, and phosphate. Wu et al. (2015) [
93] analyzed glucose and 20 amino acids in rice wine using NIR and MIR, while Hubli et al. (2023) [
94] developed an FT-IR quantification method for 20 amino acids in mammalian cell culture. Banerjee et al. (2024) [
95] employed NIR spectra analyzed with a convolutional neural network for real-time monitoring of 20 amino acids and glucose, lactose, and acetate in an
Escherichia coli fermentation.
9.3. Color and Phenolic Components
The following are parallel extraction patterns that are also temperature dependent. Informational trends are not utilized as control variables for temperature, pump-over action, or contact time between the wine and the skins in commercial winemaking practice. We are not aware of any instance in commercial wine fermentations where any phenolic component is monitored as the objective variable in a control loop. The extraction patterns illustrated in
Figure 2 may be used to determine the timing of skin separation from the fermentation juice. Consequently, this monitoring serves an informational purpose rather than for feedback control and holds no value in parameter estimation for a fermentation model. While studies have examined the kinetics of the extraction patterns, there is no comprehensive model available for parameter estimation in individual fermentations, given the range of initial concentrations, state of the skins, and skin cap or juice temperature. As a result, quantitatively controlling the extraction rate and extent during wine fermentations remains elusive.
The fractions that can be monitored through off-line methods include tannin and non-tannin phenols, red color, color density, anthocyanins, and polymeric pigments. Notable examples of clarified spectral analyses using multivariate methods include anthocyanins and tannins assessed with a few wavelengths in the UV-Vis spectrum [
96], tannins in red wine analyzed using FT-IR [
97], color parameters assessed with FTIR [
98], as well as color parameters, total phenols, and tannins evaluated via MIR [
99], and Vis-NIR techniques [
100]. One method identifies the tannin and non-tannin fractions alongside the anthocyanins and polymeric pigments in the Harbertson–Adams assay [
101], while other studies analyze phenolics in berry juice prior to fermentation using Vis-NIR [
102]. Utilizing approximate arrays based on a few characteristic wavelengths and narrower sections of the spectrum may yield trending data with lower precision, but still offer valuable insights into the rates and extent of extraction, enabling important winemaking decisions and control actions to be made. Such an application has been developed for monitoring extraction patterns in red wine fermentations using diodes and a sampled system [
103].
While advancements in optics and electronics allow these measurements to be made at relatively low costs, the continuous filtration of the liquid throughout the fermentation period remains a limitation of these approaches for commercial wine fermentations.
10. Sensors for Monitoring the Malolactic Fermentation
The time scale for malolactic fermentation spans weeks rather than days. Knowing the start and end times is crucial, as the progress and rate are important for understanding fermentation patterns, given that minimal temperature control is needed due to low heat release. Currently, no kinetic model for growth and biomass in this fermentation exists that allows for parameter estimation to enable quantitative interpretation, early problem diagnosis, or optimized control using temperature or redox potential. A quantitative grasp of the complex nutrient requirements for growth and environmental sensitivities remains elusive [
104]. Existing kinetic descriptions have mainly concentrated on the conversion of malate to lactate [
105,
106] or the transport of malate into the cells [
107]. The fermentation’s progress can be tracked by malate consumption or lactate formation; however, the relationship between cell mass or growth of lactic acid bacteria and malate content is still unclear [
108]. Given that this fermentation typically occurs in the presence of suspended yeast from the primary fermentation, it is essential to differentiate between bacterial and yeast cells amidst grape pulp fragments and CO
2 bubbles. The analytical challenge lies in tracking malate consumption and lactate formation against a backdrop of tartaric acid, ethanol, and glycerol. Secondary components of interest include citric acid and glucose, which seem to serve as carbon sources for growth [
108]. Regarding cell mass estimation, NIR spectra have been employed to estimate the cell biomass of
Escherichia coli at concentrations of up to 16 g/L [
109]. Similar examples with lactic acid bacteria seem to be lacking. The application of ultrasonic velocity using 50 MHz pulses has been used for monitoring [
37], while 2 MHz pulses have tracked the cell mass of
Lactobacillus casei [
110]; these methods may offer potential for real-time tracking of malolactic fermentation. The spectra of malic acid in the IR band [
111] and lactic acid in the IR and Raman bands [
112] allow for monitoring bacterial fermentation in association with lactic acid, glucose, and bacterial cell mass during lactic acid fermentation utilizing MIR [
113]. Vodnar et al. (2010) [
114] employed FT-IR to track lactic acid and specific markers of individual lactic acid bacteria based on carbohydrates, nucleic acids, and proteins found in
Lactobacillus plantarum,
Bifidobacterium infantis,
Lactobacillus casei, and
Bifidobacterium breve. Grassi (2013) [
115] used FT-NIR to monitor lactic acid concentrations during milk fermentation involving
Streptococcus thermophilus and
Lactobacillus delbrueckii. Fan et al. (2016) [
116] utilized a light scattering sensor for the online monitoring of lactic acid production by
Bacillus coagulans, achieving cell mass concentrations of up to 2.2 g/L. Fluorescence spectroscopy has been applied to observe malolactic fermentation [
117], and recently, a 2D fluorescence method involving 440 to 480 nm excitation and 480 to 520 nm emission has estimated glucose, lactic acid, and biomass of lactic acid bacteria [
118]. Both methods appear promising for monitoring the progress of malolactic fermentations and may contribute to the future development of growth and fermentation models for this process. Future capacitance spectroscopy investigations are likely to provide more insights about cell size and activity than other physical and spectral techniques, particularly in making measurements in small volumes through immersion or flow-through cells for in-line monitoring applications and in the presence of suspended cell mixtures of varying sizes.
11. Status of Wine Fermentation Monitoring, Modeling, and Control
Figure 13 shows a state-of-the-art display of fermentation in a medium-sized commercial winery. The status of sensing systems in commercial wine fermentation involves monitoring the density, temperature of the juice and skin caps, and the redox potential of the juice. Limitations to adopting other available sensor systems stem from the presence of suspended pulp with intact plant cells, conditions in which both yeast and bacterial populations are significant simultaneously, and stratification due to natural convection rather than swirled or mixed liquid within the fermenters.
Despite these limitations, density and redox potential can be easily measured in ways that minimize the effects of stratification in commercial fermenters, as shown in
Figure 12. The use of temperature probes that measure skin cap temperature provides actionable insights into the need for cooling the cap through either pump-over or punch-down operations. Controlling skin cap temperature is essential for achieving reproducible and desired outcomes of phenolic extraction and in preventing the formation of hydrogen sulfide at high temperatures.
As shown in
Figure 13, the raw measurements of static pressure at two heights enable the estimation of the average density, and the temperature within the skin cap provides information regarding the differential temperature between the juice and the cap of a red wine fermentation. The redox potential is also shown and was controlled to −40 mV. The weight loss calculated from the bottom pressure transducer shows a large influence of pump-over operations, while the density is relatively unaffected due to the subtraction of the top and bottom transducers.
Figure 14 shows a complete model-based visualization of the same fermentation using measurements down to 15 °Brix. The display shows the states of a fermentation model (biomass, fructose, glucose, sugar, ethanol, nitrogen, and °Brix) and the fermentation rate.
The current sensor systems for wine fermentation lay the groundwork for controlling juice and fermentation temperatures. They may also monitor skin cap temperature if connected to a pump-over setup and redox potential if measurements are incorporated into an air addition system. Additionally, these systems provide the density information necessary for parameter estimation in wine fermentation models, enabling predictions of future fermentation rates and trajectories and heat release for each fermentor. Wine fermentation models are now being used at commercial scales in model predictive control strategies to achieve desired fermentation profiles. The summation of heat release from all active fermentations determines the cooling load required from the refrigeration system, which could operate optimally based on the time of day, demand charge structure for purchased electricity, ambient air properties that influence condenser performance, and other cooling demands unrelated to fermentation. Purchasing electricity constitutes the largest production expense in wine fermentation, and optimal management depends on estimating cooling loads, their temporal patterns, and the site-specific, time-of-use costs associated with acquiring the necessary electricity.
12. Conclusions
Wine fermentation monitoring faces a fundamental challenge: ensuring that sensor measurements accurately represent the entire fermentation volume. In unmixed fermentors, bubble-driven natural convection creates concentration gradients that vary with scale, fermentation rate, and geometry. These dynamics significantly impact sensor placement decisions and can cause fluctuating discrepancies between measured and actual fermentation conditions.
Our findings indicate that solution density monitoring using pressure transducers embedded in tank walls or vertical pump-over lines offers the most reliable commercial-scale method for parameter estimation within existing fermentation models. This approach enhances fermentation diagnosis and enables faster-than-real-time assessment of alternative control actions or interventions. By eliminating the need for manual sampling, it provides a robust foundation for regulating fermentation temperature and/or redox potential.
For red wine fermentations, separate control of the skin cap temperature is essential to limit volatile thiol formation due to low redox potentials while ensuring reproducible and quantitative phenolic extraction during pump-over and punch-down operations. Critical to this control is the comprehensive monitoring of skin cap temperature profiles—both vertically and radially—to enable continuous thermal management using cooler juice rather than relying on conventional twice-daily contacting practices.
A significant research gap exists in developing in situ or in-line techniques for assessing yeast cell mass and viability within the complex matrix of grape pulp fragments, bacteria, and bubbles. Such measurements would enhance parameter estimation in fermentation models, particularly by monitoring viability decline during the non-growing phase and tracking growth dynamics during the growth phase. These measurements would provide crucial validation data regarding assimilable nitrogen availability and uptake, refining current mathematical models of wine fermentation. The persistent challenge of distinguishing between yeast and bacterial cells in mixed suspensions remains unresolved, yet quantifying cell growth is essential for advancing fermentation models, especially for malolactic fermentation.
Several off-line techniques can be adapted for compact in-line applications, including cell mass, viability, and assimilable nitrogen concentration, which can facilitate comprehensive monitoring of yeast growth and parameter estimation related to substrate preference, nutrient uptake, and cell yield in wine fermentation models.
Spectral methods, which are particularly well-suited for in-line applications, should be further developed to monitor the changes in phenolic components during red wine fermentations for information and/or control purposes.
Sensor systems for monitoring malolactic fermentation represent another promising direction, with controlled redox potential potentially enhancing secondary product formation and expediting completion. The ability to monitor cell growth during malolactic fermentation or detect undesirable bacterial populations alongside yeast cells would mark significant progress. These techniques merit further investigation to facilitate their implementation in both research and commercial fermentation settings.
As monitoring technology advances, the wine industry stands to benefit from more precise, data-driven fermentation management approaches that can enhance product consistency, quality, and innovation while reducing resource requirements and environmental impacts.