Application of Transcriptome Analysis for the Exploration of the Mechanism of Methionine Promoting the Synthesis of Cephalosporin C in Acremonium chrysogenum by Employing a Chemically Defined Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
2.2. Cultivation Methods
2.3. Determination of CPC Titer
2.4. Data Analysis Method
2.5. Transcriptome Sequencing
3. Results
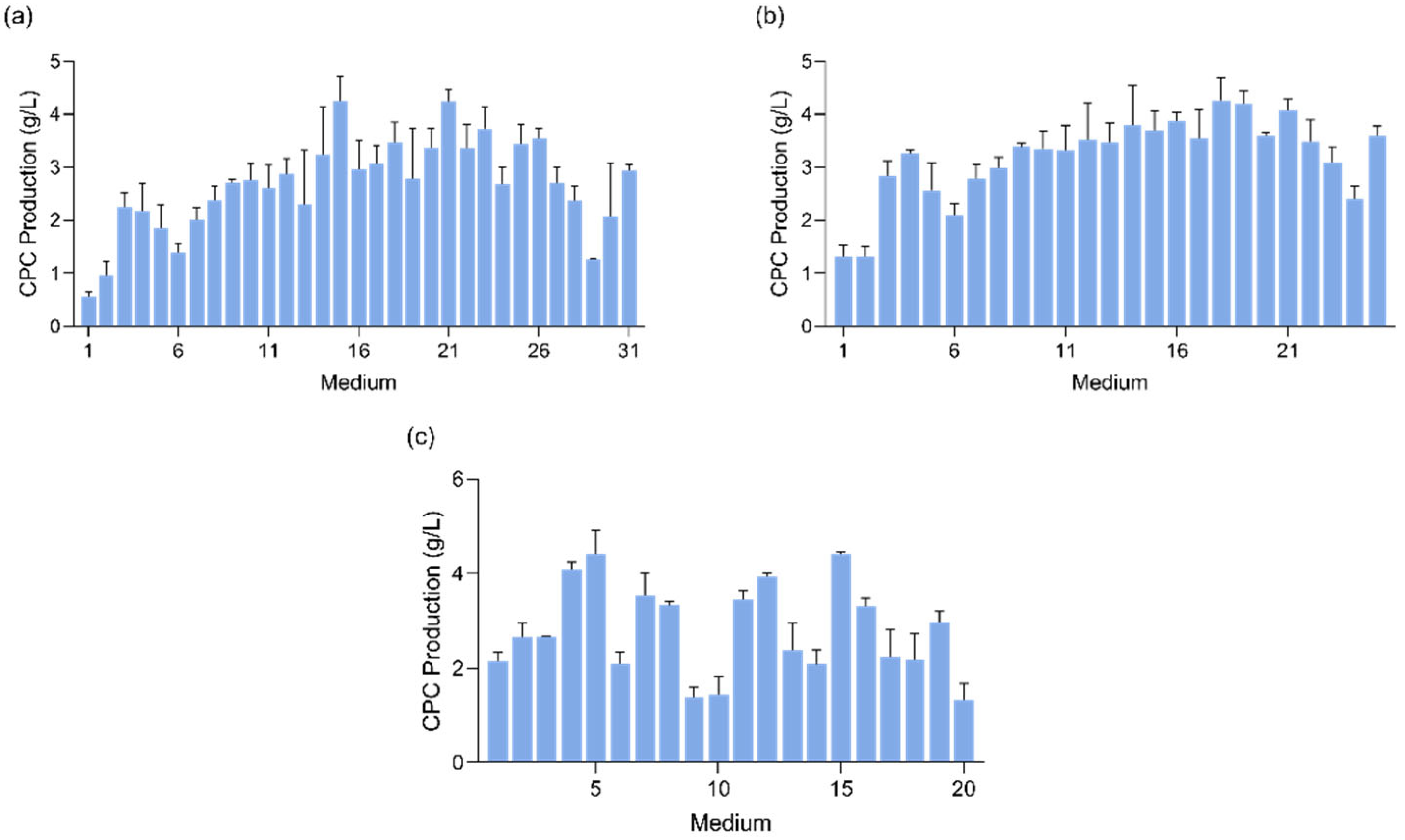
3.1. Single-Factor Omission Experiment
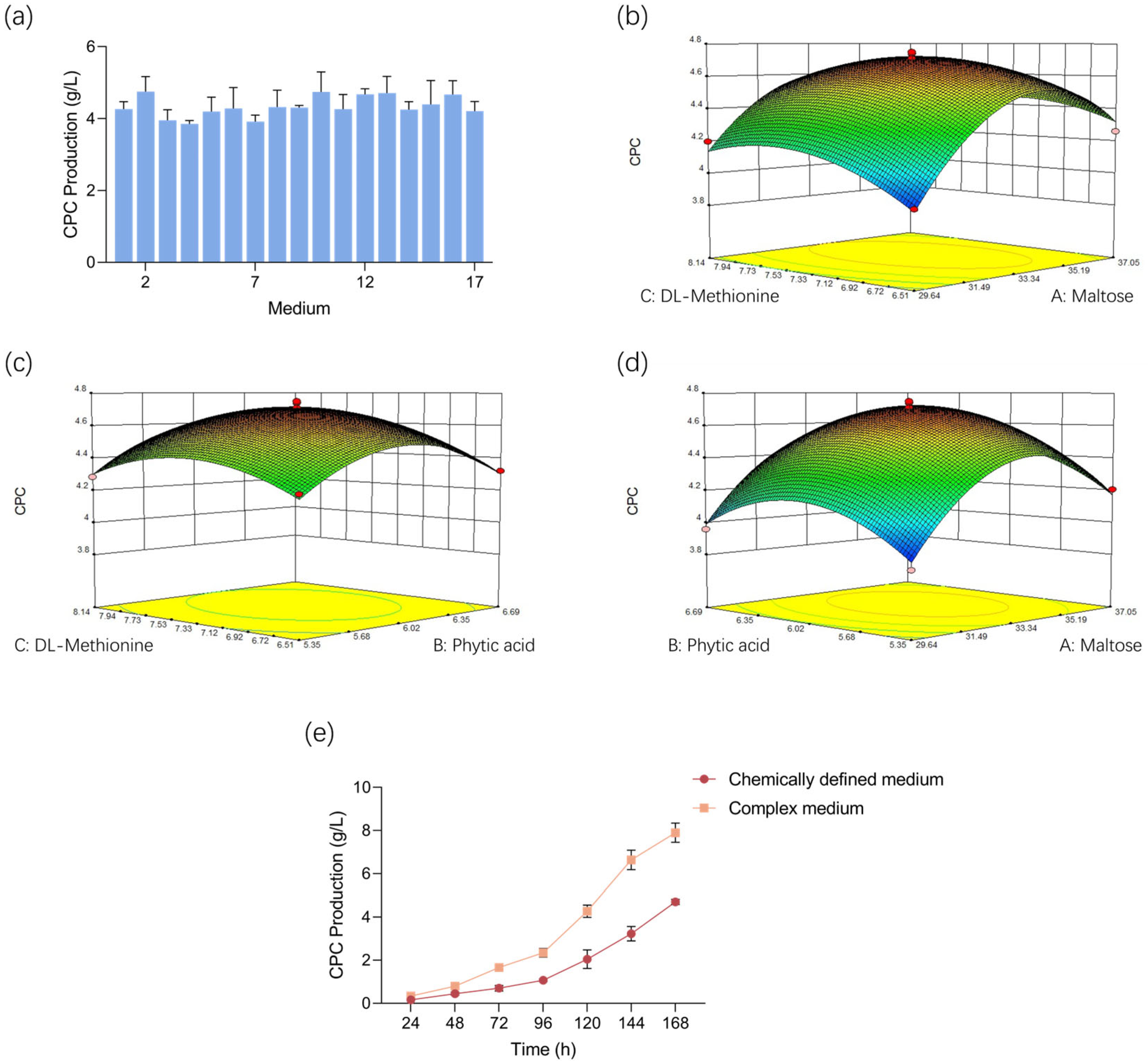
3.2. PB Design and Medium Optimization
3.3. RSM for Optimal Concentration Determination
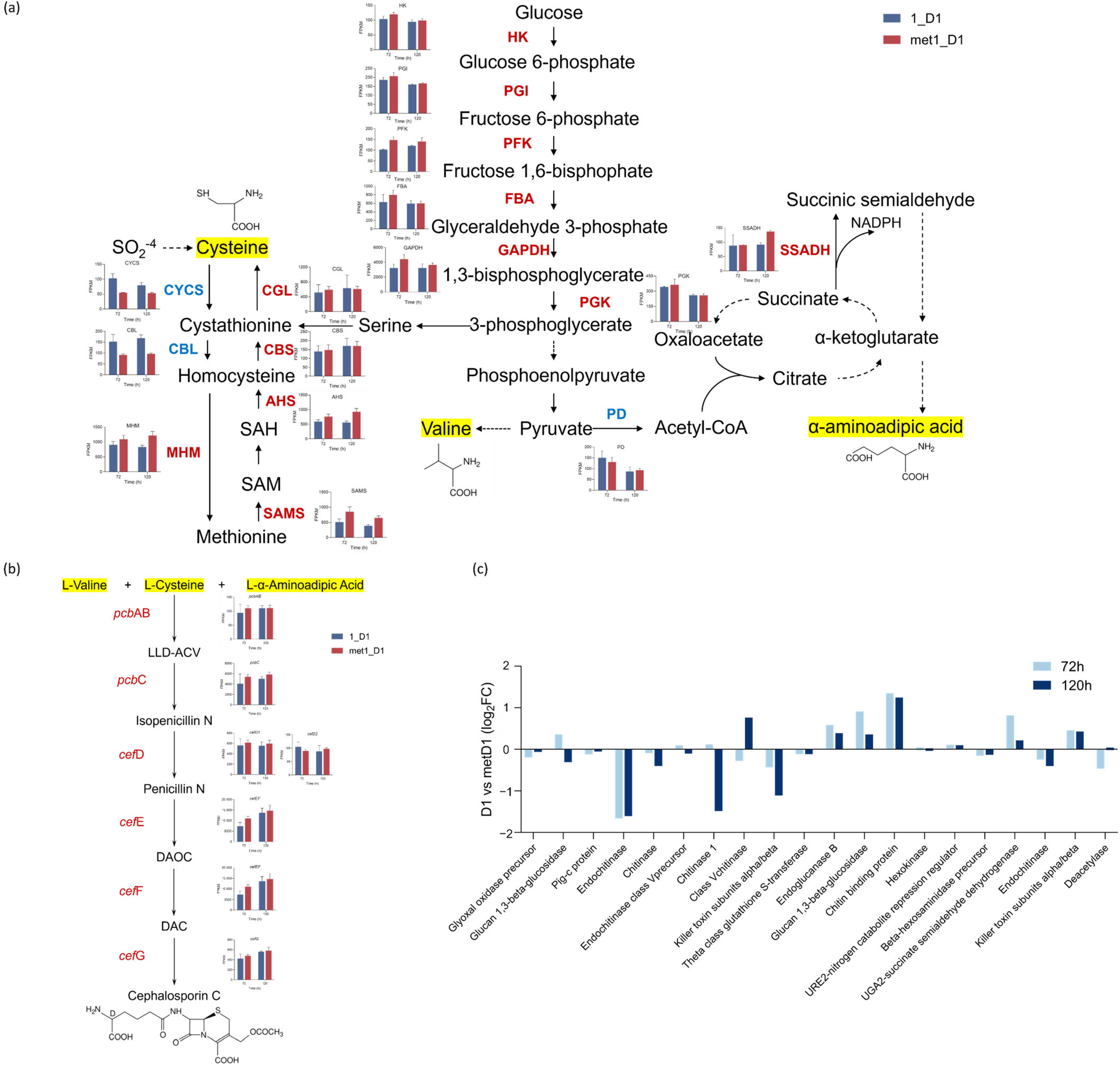
3.4. Transcriptomic Analysis of Methionine-Mediated CPC Biosynthesis Enhancement
3.5. Differential Expression of Genes in Key Metabolic Pathways Involved in CPC Biosynthesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPC | Cephalosporin C |
| PB | Plackett–Burman |
| RSM | Response surface methodology |
| BBD | Box–Behnken Design |
| SIN | Septation initiation network |
| BSSS | Bud site selection system |
References
- Li, X.; Wang, J.; Su, W.; Li, C.; Qu, G.; Yuan, B.; Sun, Z. Characterization and Engineering of Cephalosporin C Acylases to Produce 7-Aminocephalosporanic Acid. Mol. Catal. 2023, 550, 113595. [Google Scholar] [CrossRef]
- Grand View Research. Antibiotics Market Size, Share & Trends Analysis Report by Action Mechanism (Cell Wall Synthesis Inhibitors, RNA Synthesis Inhibitors), by Drug Class (Penicillin, Cephalosporin), by Type (Branded, Generic), and Segment Forecasts, 2024–2030; Grand View Research: San Francisco, CA, USA, 2023. [Google Scholar]
- Tan, Q.; Qiu, J.; Luo, X.; Zhang, Y.; Liu, Y.; Chen, Y.; Yuan, J.; Liao, W. Progress in One-Pot Bioconversion of Cephalosporin C to 7-Aminocephalosporanic Acid. Curr. Pharm. Biotechnol. 2018, 19, 30–32. [Google Scholar] [CrossRef]
- Gross, L.; Mohn, F.; Moll, N.; Meyer, G.; Ebel, R.; Abdel-Mageed, W.M.; Jaspars, M. Organic Structure Determination Using Atomic-Resolution Scanning Probe Microscopy. Nat. Chem. 2010, 2, 821–825. [Google Scholar] [CrossRef]
- Marín-Candón, A.; Rosso-Fernández, C.M.; Bustos de Godoy, N.; López-Cerero, L.; Gutiérrez-Gutiérrez, B.; López-Cortés, L.E.; Pulido, L.B.; Borreguero, I.B.; León, M.J.; Merino, V.; et al. Temocillin Versus Meropenem for the Targeted Treatment of Bacteraemia Due to Third-Generation Cephalosporin-Resistant Enterobacterales (ASTARTÉ): Protocol for a Randomised, Pragmatic Trial. BMJ Open 2021, 11, e049481. [Google Scholar] [CrossRef]
- Yang, Y.M.; Xia, J.Y.; Li, J.H.; Chu, J.; Li, L.; Wang, Y.H.; Zhuang, Y.P.; Zhang, S.L. A Novel Impeller Configuration to Improve Fungal Physiology Performance and Energy Conservation for Cephalosporin C Production. J. Biotechnol. 2012, 161, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F. Transport Systems, Intracellular Traffic of Intermediates and Secretion of β-Lactam Antibiotics in Fungi. Fungal Biol. Biotechnol. 2020, 7, 6. [Google Scholar] [CrossRef]
- Zhgun, A.A. Fungal BGCs For Production of Secondary Metabolites: Main Types, Central Roles in Strain Improvement, and Regulation according to the Piano Principle. Int. J. Mol. Sci. 2023, 24, 11184. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Yuan, G.Q.; Zhao, Y.L.; Ni, W.J.; Luo, H.Z.; Shi, Z.P.; Liu, F. Simulation of Computational Fluid Dynamics and Comparison of Cephalosporin C Fermentation Performance with Different Impeller Combinations. Korean J. Chem. Eng. 2013, 30, 1097–1104. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, X.H.; Liu, C.; Hang, H.F.; Chu, J.; Guo, M.J.; Zhuang, Y.P. Method for Improving Penicillin Fermentation Production Unit. China Patent CN113388658A, 12 March 2020. [Google Scholar]
- Liu, J.J.; Liu, G. Advances in the Regulation of Cephalosporin C Biosynthesis—A Review. Acta Microbiol. Sin. 2016, 56, 461–470. [Google Scholar]
- Chen, J.; Yao, R.; Yu, W.; Zhan, J.; Zhu, H.; Wang, H. Optimization of Culture Medium for Cephalosporin C Production from Rice Straw by Cephalosporium acremonium Fermentation Using Response Surface Methodology. China Brew. 2020, 39, 131–136. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.R.; Oh, H.J.; Lee, K.; Um, Y.; Kim, D.W.; Seo, S.W.; Park, C.; Kim, S.W. Undefined Xylose Media Extracted from Biorefinery Waste for Enhanced and Eco-Friendly Production of Cephalosporin C By Acremonium chrysogenum M35. GCB Bioenergy 2023, 15, 731–742. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Zhang, S.; Feng, T.; Yu, N. Optimization of Fermentation Medium for Cephalosporin C Production by Acremonium chrysogenum. Chem. Ent. Manag. 2024, 20, 131–135. [Google Scholar]
- Mapar, M.; Rydazk, T.; Groves, R.A.; Lewis, L.A. Biomarker Enrichment Medium: A Defined Medium for Metabolomic Analysis of Microbial Pathogens. Front. Microbiol. 2022, 13, 957158. [Google Scholar] [CrossRef]
- Harris, D.M.; van der Krogt, Z.A.; van Guilk, W.M.; van Dijken, J.P.; Pronk, J.T. Formate as an Auxiliary Substrate for Glucose-Limited Cultivation of Penicillium chrysogenum: Impact on Penicillin G Production and Biomass Yield. Appl. Environ. Microbiol. 2007, 73, 5020–5025. [Google Scholar] [CrossRef]
- Jami, M.S.; Barreiro, C.; García-Estrada, C.; Martín, J.F. Proteome Analysis of the Penicillin Producer Penicillium chrysogenum: Characterization of Protein Changes During the Industrial Strain Improvement. Mol. Cell. Proteom. 2010, 9, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, L.; Chen, Z.; Tian, X.W.; Chu, J. The Arthrospore-Related Gene Acaxl2 is Involved in Cephalosporin C Production in Industrial Acremonium chrysogenum by the Regulatory Factors AcFKH1 and CPCR1. J. Biotechnol. 2021, 347, 26–39. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, L.P.; Gong, G.H.; Zhang, W.; Zhu, B.Q.; Hu, Y.J. De Novo Comparative Transcriptome Analysis of Acremonium chrysogenum: High-Yield and Wild-Type Strains of Cephalosporin C Producer. PLoS ONE 2014, 9, e104542. [Google Scholar] [CrossRef]
- López-Calleja, A.C.; Cuadra, T.; Barrios-González, J.; Fierro, F.; Fernández, F.J. Solid-State and Submerged Fermentations Show Different Gene Expression Profiles in Cephalosporin C Production by Acremonium chrysogenum. J. Mol. Microbiol. Biotechnol. 2012, 22, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Dumina, M.V.; Zhgun, A.A.; Novak, M.I.; Domratcheva, A.G.; Petukhov, D.V.; Dzhavakhiya, V.V.; Eldarov, M.A.; Bartoshevitch, I.E. Comparative Gene Expression Profiling Reveals Key Changes in Expression Levels of Cephalosporin C Biosynthesis and Transport Genes Between Low and High-Producing Strains of Acremonium chrysogenum. World J. Microbiol. Biotechnol. 2014, 30, 2933–2941. [Google Scholar] [CrossRef]
- Kluge, J.; Kück, U. AcAxl2 and AcMst1 Regulate Arthrospore Development and Stress Resistance in the Cephalosporin C Producer Acremonium chrysogenum. Curr. Genet. 2018, 64, 713–727. [Google Scholar] [CrossRef]
- Matsumura, M.; Liu, L.Y. Regulatory Effects of Endogenous Methionine on Cephalosporin C Production in Acremonium chrysogenum. Foreign Pharm. (Antibiot. Sect.) 1981, 2, 22–29. [Google Scholar] [CrossRef]
- Kern, B.A.; Inamine, E. Cystathionine γ-Lyase Activity in the Cephamycin C Producer Streptomyces Lactamdurans. J. Antibiot. 1981, 34, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Kosalková, K.; García-Estrada, C.; Ullán, R.V.; Martín, J.F. A Moderate Amplification of the mecB Gene Encoding Cystathionine-γ-Lyase Stimulates Cephalosporin Biosynthesis in Acremonium chrysogenum. J. Ind. Microbiol. Biotechnol. 2001, 27, 252–258. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Xie, L.P.; Zhu, B.Q.; Hu, Y.J. Comparative Expression Profiling of Genes Involved in Primary Metabolism in High-Yield and Wild-Type Strains of Acremonium chrysogenum. Antonie Van Leeuwenhoek 2016, 109, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Gutiérrez, S.; Fernández, F.J.; Marcos, A.T.; Martín, J.F. Exogenous Methionine Increases Levels of mRNAs Transcribed from pcbAB, pcbC, and cefEF Genes, Encoding Enzymes of the Cephalosporin Biosynthetic Pathway, in Acremonium chrysogenum. J. Bacteriol. 1994, 176, 985–991. [Google Scholar] [CrossRef]
- Demain, A.L.; Zhang, J. Cephalosporin C Production by Cephalosporium acremonium: The Methionine Story. Crit. Rev. Biotechnol. 1998, 18, 283–294. [Google Scholar] [CrossRef]
- Seiler, S.; Justa-Schuch, D. Conserved Components, But Distinct Mechanisms for the Placement and Assembly of the Cell Division Machinery in Unicellular and Filamentous Ascomycetes. Mol. Microbiol. 2010, 78, 1058–1076. [Google Scholar] [CrossRef]
- Harris, S.D. Septum Formation in Aspergillus nidulans. Curr. Opin. Microbiol. 2002, 4, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yang, J.; An, Y.; Liu, G. Disruption of a Glutathione Reductase Encoding Gene in Acremonium chrysogenum Leads to Reduction of its Growth, Cephalosporin Production and Antioxidative Ability which is Recovered by Exogenous Methionine. Fungal Genet. Biol. 2012, 49, 114–122. [Google Scholar] [CrossRef]
- Nagy, M.A.; Emri, T.; Fekete, E.; Sándor, E.; Springael, J.Y.; Penninckx, M.J.; Pócsi, I. Glutathione Metabolism of Acremonium chrysogenum in Relation to Cephalosporin C Production: Is γ-glutamyltransferase in the Center? Folia Microbiol. 2003, 48, 149–155. [Google Scholar] [CrossRef]
- Cohen, G.; Argaman, A.; Schreiber, R.; Mislovati, M.; Aharonowitz, Y. The Thioredoxin System of Penicillium chrysogenum and its Possible Role in Penicillin Biosysnthesis. J. Bacteriol. 1994, 176, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Long, L.K.; An, Y.; Yang, J.; Xu, X.; Hu, C.; Liu, G. The Thioredoxin Reductase-encoding Gene ActrxR1 is Involved in the Cephalosporin C Production of Acremonium chrysogenum in Methionine-supplemented Medium. Appl. Microbiol. Biotechnol. 2013, 97, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Narasaiah, K.W.; Sashidhar, R.B.; Subramanyam, C. Biochemical Analysis of Oxidative Stress in the Production of Aflatoxin and its Precursor Intermediates. Mycopathologia 2006, 162, 179–189. [Google Scholar] [CrossRef] [PubMed]

| Variable | Standardized Effects | Contribution | p-Value | |
|---|---|---|---|---|
| Model | 0.0251 | significant | ||
| Maltose | 0.8991 | 22.29 | 0.0056 | |
| Glucose | 0.7617 | 16.00 | 0.0090 | |
| Lactic acid | 0.4688 | 6.06 | 0.0335 | |
| Phytic acid | −0.8093 | 18.06 | 0.0076 | |
| KH2PO4 | −0.0722 | 0.14 | 0.6056 | |
| (NH4)2SO4 | 0.2677 | 1.98 | 0.1227 | |
| DL-Methionine | 0.8533 | 20.08 | 0.0065 | |
| L-Aspartic acid | 0.3682 | 3.74 | 0.0608 | |
| L-Threonine | −0.0734 | 0.15 | 0.5998 | |
| L-Serine | 0.2143 | 1.27 | 0.1863 | |
| L-Tyrosine | 0.1796 | 0.89 | 0.2480 | |
| Biotin | 0.1813 | 0.91 | 0.2444 | |
| MgSO4·7H2O | 0.3189 | 2.80 | 0.0847 | |
| CaCO3 | −0.2195 | 1.33 | 0.1788 | |
| Soybean oil | −0.1384 | 0.53 | 0.3508 | |
| Trace element | −0.2999 | 2.48 | 0.0968 |
| Coefficient Estimate | F-Value | p-Value | ||
|---|---|---|---|---|
| Model | 47.87 | <0.0001 | significant | |
| A-Maltose | 0.1374 | 50.12 | 0.0002 | |
| B-Phytic acid | 0.0469 | 5.84 | 0.0464 | |
| C-DL-Methionine | 0.0459 | 5.60 | 0.0499 | |
| AB | −0.0017 | 0.0040 | 0.9514 | |
| AC | −0.0731 | 7.10 | 0.0323 | |
| BC | 0.0136 | 0.25 | 0.6342 | |
| A2 | −0.3945 | 217.55 | <0.0001 | |
| B2 | −0.2349 | 77.11 | <0.0001 | |
| C2 | −0.1587 | 35.21 | 0.0006 | |
| Lack of Fit | 3.96 | 0.1085 | not significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, Z.; Hong, W.; Feng, T.; Tian, X.; Chu, J. Application of Transcriptome Analysis for the Exploration of the Mechanism of Methionine Promoting the Synthesis of Cephalosporin C in Acremonium chrysogenum by Employing a Chemically Defined Medium. Fermentation 2025, 11, 325. https://doi.org/10.3390/fermentation11060325
Li Y, Chen Z, Hong W, Feng T, Tian X, Chu J. Application of Transcriptome Analysis for the Exploration of the Mechanism of Methionine Promoting the Synthesis of Cephalosporin C in Acremonium chrysogenum by Employing a Chemically Defined Medium. Fermentation. 2025; 11(6):325. https://doi.org/10.3390/fermentation11060325
Chicago/Turabian StyleLi, Yifan, Zhen Chen, Wei Hong, Tao Feng, Xiwei Tian, and Ju Chu. 2025. "Application of Transcriptome Analysis for the Exploration of the Mechanism of Methionine Promoting the Synthesis of Cephalosporin C in Acremonium chrysogenum by Employing a Chemically Defined Medium" Fermentation 11, no. 6: 325. https://doi.org/10.3390/fermentation11060325
APA StyleLi, Y., Chen, Z., Hong, W., Feng, T., Tian, X., & Chu, J. (2025). Application of Transcriptome Analysis for the Exploration of the Mechanism of Methionine Promoting the Synthesis of Cephalosporin C in Acremonium chrysogenum by Employing a Chemically Defined Medium. Fermentation, 11(6), 325. https://doi.org/10.3390/fermentation11060325





