Insights into Physiochemical and Biological Characteristics of Pig Manure During Anaerobic Digestion and Sheep Manure During Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Analytical Methods
2.3. DNA Extraction, PCR Amplification, and Sequencing Analysis
2.4. Data Analyses
3. Results and Discussion
3.1. Physiochemical Properties of Animal Manures
3.1.1. Pig Manures
3.1.2. Sheep Manures
3.2. Variation in Physiochemical Properties of Animal Manures During Anaerobic Digestion and Composting
3.2.1. Pig Manure During Anaerobic Digestion
3.2.2. Sheep Manure During Composting
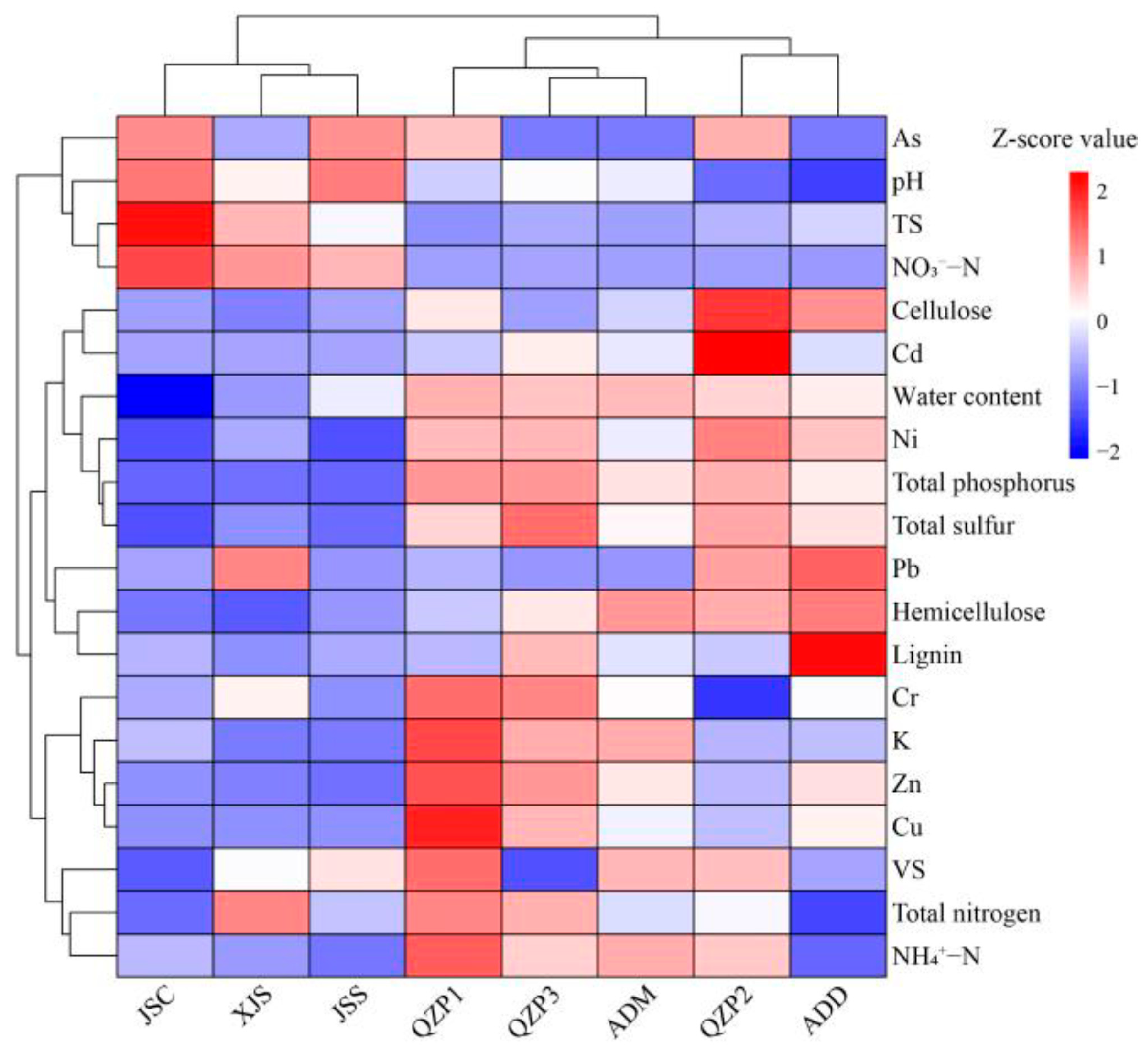
3.3. Cluster Analysis of Physiochemical Properties of Animal Manures and Their Treatment Products
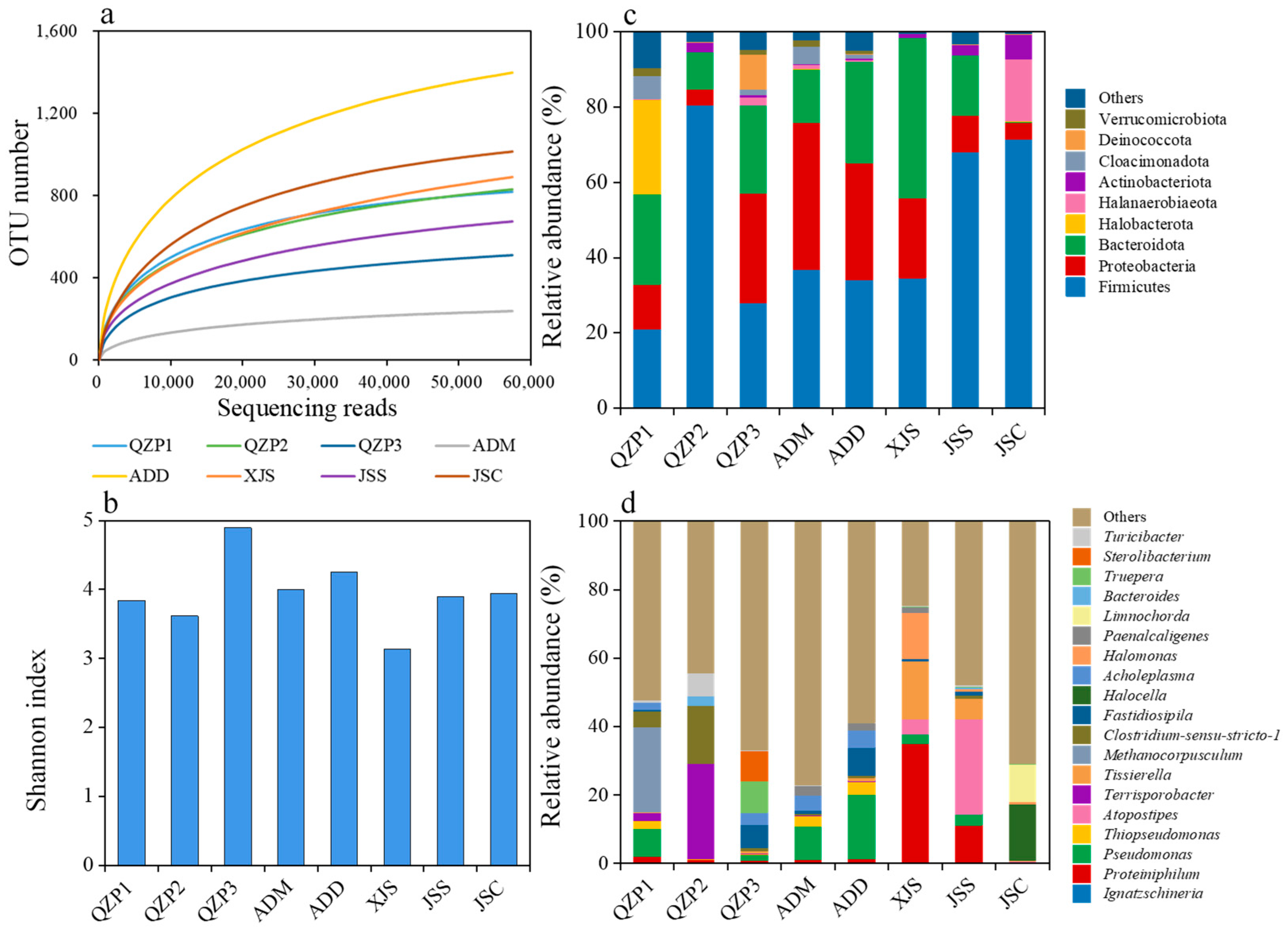
3.4. Microbial Community Structure in Animal Manures and Their Treatment Products
3.5. Potential Pathogenic Bacteria in the Animal Manures and Their Treatment Products
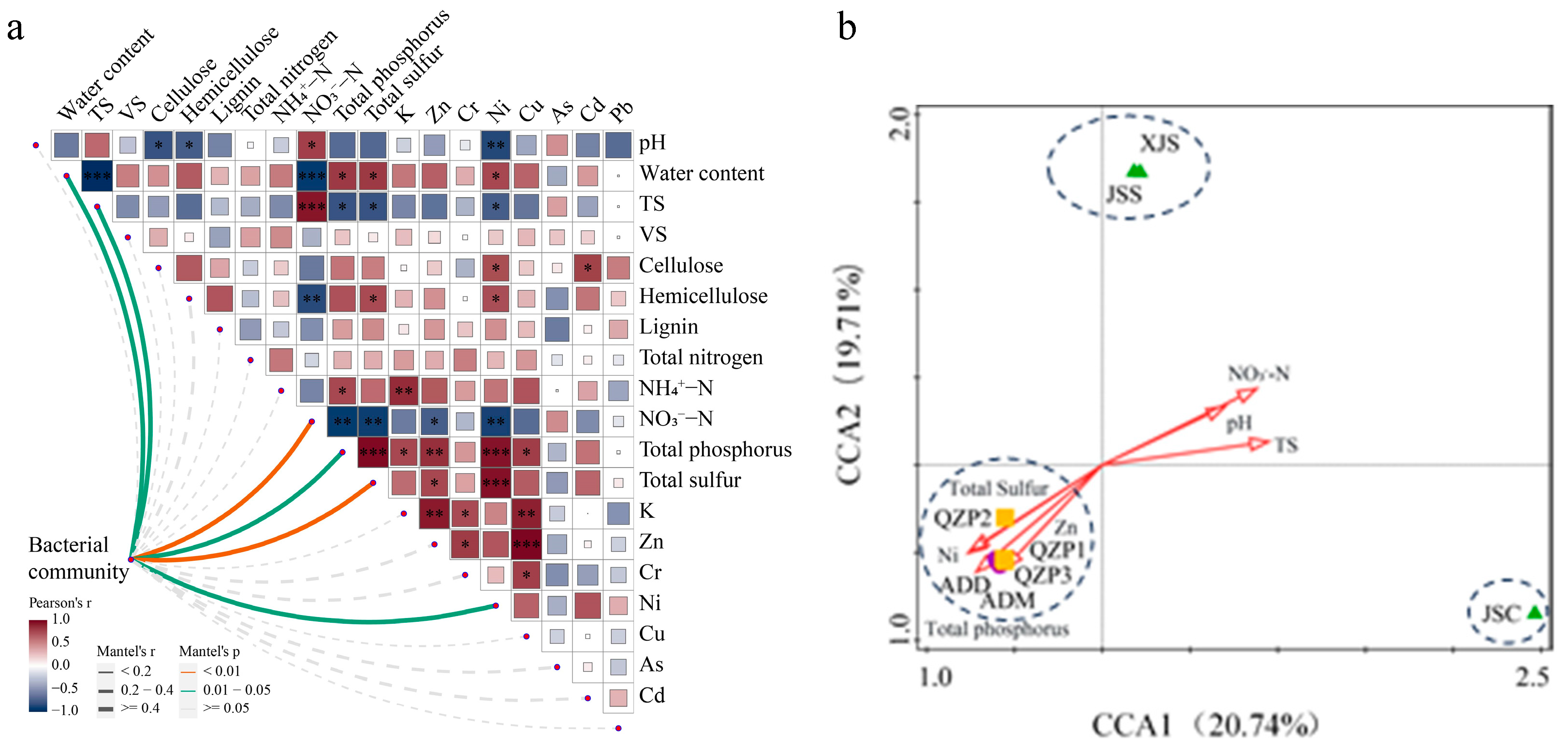
3.6. Relationship Between Physiochemical Properties and Microbial Communities in Animal Manures and Their Treatment Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China (NBSC). China Statistical Yearbook 2024; China Statistical Press: Beijing, China, 2024. [Google Scholar]
- Rotz, A.; Stout, R.; Leytem, A.; Feyereisen, G.; Waldrip, H.; Thoma, G.; Holly, M.; Bjorneberg, D.; Baker, J.; Vadas, P.; et al. Environmental assessment of United States dairy farms. J. Clean. Prod. 2021, 315, 128153. [Google Scholar] [CrossRef]
- Zhou, S.; Su, S.; Meng, L.; Liu, X.; Zhang, H.; Bi, X. Potentially toxic trace element pollution in long-term fertilized agricultural soils in China: A meta-analysis. Sci. Total Environ. 2021, 789, 147967. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.; Wei, J.; Tong, Y.; Yu, G.; Shen, Q.; Chen, Q. Improving manure nutrient management towards sustainable agricultural intensification in China. Agric. Ecosyst. Environ. 2015, 209, 34–46. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, Z.G.; Huang, Z.T.; Ma, C.J.; Liu, G.; Huhe, T.; Chen, Y.S.; Xie, J.; Chen, Y. Evaluating the nutrient and pollutant flows of the Chinese livestock manure management system from 1949 to 2050. Resour. Conserv. Recycl. 2025, 215, 108092. [Google Scholar] [CrossRef]
- Zhang, L.L.; Reaihan, E.; Mahmoud, M.A.L.I.; Lin, H.J.; Zhang, S.; Jin, S.Q.; Zhu, Z.P.; Hu, J.J.; Yao, Y.Q.; Sun, Y.; et al. Livestock and poultry manure management from the perspective of carbon neutrality in China. Front. Agric. Sci. Eng. 2023, 10, 341–362. [Google Scholar]
- Qi, J.; Yang, H.; Wang, X.; Zhu, H.; Wang, Z.; Zhao, C.; Li, B.; Liu, Z. State-of-the-art on animal manure pollution control and resource utilization. J. Environ. Chem. Eng. 2023, 11, 110462. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, D.; Wu, Q.; Wang, H.; Li, S.; Liu, F.; Xiao, Z. Review on fate and bioavailability of heavy metals during anaerobic digestion and composting of animal manure. Waste Manag. 2022, 150, 75–89. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K.; et al. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Kadam, R.; Jo, S.; Lee, J.; Khanthong, K.; Jang, H.; Park, J.G. A review on the anaerobic co-digestion of livestock manures in the context of sustainable waste management. Energies 2024, 17, 546. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Kumar Khanal, S. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.M.; Mohd Ghazi, T.I.; Omar, R. Anaerobic digestion technology in livestock manure treatment for biogas production: A review. Eng. Life Sci. 2012, 12, 258–269. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, X.; He, C.; Chen, C.L.; Bai, J.; Ren, N.; Wang, J.Y. Transformation of dissolved organic matters in swine, cow and chicken manures during composting. Bioresour. Technol. 2014, 168, 222–228. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sust. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Duan, X.; Chen, Y. Integrated metagenomic and metaproteomic analyses unravel ammonia toxicity to active methanogens and syntrophs, enzyme synthesis, and key enzymes in anaerobic digestion. Environ. Sci. Technol. 2021, 55, 14817–14827. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, W.; Dong, Q.; Wu, D.; Yang, P.; Peng, Y.; Li, L.; Peng, X. Integrated multi-omics analyses reveal the key microbial phylotypes affecting anaerobic digestion performance under ammonia stress. Water Res. 2022, 213, 118152. [Google Scholar] [CrossRef]
- Huang, Z.; Niu, Q.; Nie, W.; Li, X.; Yang, C. Effects of heavy metals and antibiotics on performances and mechanisms of anaerobic digestion. Bioresour. Technol. 2022, 361, 127683. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, H.; Song, D.; Chen, H.; Lin, X.; Wang, Y.; Ji, L. Distribution of antibiotic, heavy metals and antibiotic resistance genes in livestock and poultry feces from different scale of farms in Ningxia, China. J. Hazard. Mater. 2022, 440, 129719. [Google Scholar] [CrossRef]
- NY884-2021; Microbial Organic Fertilizers. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- Bao, S.D. Soil Agro-Chemistry Analysis; China Agriculture Press: Beijing, Cina, 2000. [Google Scholar]
- Redin, M.; Guénon, R.; Recous, S.; Schmatz, R.; de Freitas, L.L.; Aita, C.; Giacomini, S.J. Carbon mineralization in soil of roots from twenty crop species, as affected by their chemical composition and botanical family. Plant Soil 2014, 378, 205–214. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, S.; Fang, K.; Qian, Z.; Chen, J. Determination of cadmium(II), cobalt(II), nickel(II), lead(II), zinc(II), and copper(II) in water samples using dual-cloud point extraction and inductively coupled plasma emission spectrometry. J. Hazard. Mater. 2012, 239–240, 206–212. [Google Scholar] [CrossRef]
- He, R.; Yao, X.Z.; Chen, M.; Ma, R.C.; Li, H.J.; Wang, C.; Ding, S.H. Conversion of sulfur compounds and microbial community in anaerobic treatment of fish and pork waste. Waste Manag. 2018, 76, 383–393. [Google Scholar] [CrossRef]
- Marszałek, M.; Kowalski, Z.; Makara, A. The possibility of contamination of water-soil environment as a result of the use of pig slurry. Ecol. Chem. Eng. 2019, 26, 313–330. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, L.; Ye, Q.; Wang, Q.; Liao, D.; Chang, X.; Xi, S.; He, R. Chemical and microbiological characterization of pig manures and digestates. Environ. Technol. 2023, 44, 1916–1925. [Google Scholar] [CrossRef]
- Hoyos-Sebá, J.J.; Arias, N.P.; Salcedo-Mendoza, J.; Aristizábal-Marulanda, V. Animal manure in the context of renewable energy and value-added products: A review. Chem. Eng. Process. 2024, 196, 109660. [Google Scholar] [CrossRef]
- Zhu, Y.; Merbold, L.; Leitner, S.; Pelster, D.E.; Okoma, S.A.; Ngetich, F.; Onyango, A.A.; Pellikka, P.; Butterbach-Bahl, K. The effects of climate on decomposition of cattle, sheep and goat manure in Kenyan tropical pastures. Plant Soil 2020, 451, 325–343. [Google Scholar] [CrossRef]
- Jin, H.; Chang, Z. Distribution of heavy metal contents and chemical fractions in anaerobically digested manure slurry. Appl. Biochem. Biotech. 2011, 164, 268–282. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, W.; Westerholm, M.; Huang, G.; Taherzadeh, M.J.; Dong, R. Microbiological and technological insights on anaerobic digestion of animal manure: A review. Fermentation 2023, 9, 436. [Google Scholar] [CrossRef]
- Basak, B.; Kumar, R.; Tanpure, R.S.; Mishra, A.; Tripathy, S.K.; Chakrabortty, S.; Roh, H.S.; Yadav, K.K.; Chung, W.; Jeon, B.H. Roles of engineered lignocellulolytic microbiota in bioaugmenting lignocellulose biomethanation. Renew. Sust. Energy Rev. 2025, 207, 114913. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Ye, M.; Zha, X.; He, R. Effects of Fe-modified digestate hydrochar at different hydrothermal temperatures on anaerobic digestion of swine manure. Bioresour. Technol. 2024, 395, 130393. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Huang, J.; Du, Z.; Zhouyang, S.; Wang, Y.; Zheng, Y.; Li, Q.; Shen, X. The influence of variables on the bioavailability of heavy metals during the anaerobic digestion of swine manure. Ecotoxicol. Environ. Saf. 2020, 195, 110457. [Google Scholar] [CrossRef]
- Chen, Y.X.; Huang, X.D.; Han, Z.Y.; Huang, X.; Hu, B.; Shi, D.Z.; Wu, W.X. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 2010, 78, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wang, X.; Liu, Q.; Li, T.; Chen, X.; Chai, L.; Liu, D.; Shen, Q. Evolution of various fractions during the windrow composting of chicken manure with rice chaff. J. Environ. Manag. 2018, 207, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Huhe; Jiang, C.; Wu, Y.; Cheng, Y. Bacterial and fungal communities and contribution of physicochemical factors during cattle farm waste composting. MicrobiologyOpen 2017, 6, e00518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Z.; Ma, S.C.; Wang, S.P.; Wang, T.T.; Sun, Z.Y.; Tang, Y.Q.; Deng, Y.; Kida, K. A comparative study of composting the solid fraction of dairy manure with or without bulking material: Performance and microbial community dynamics. Bioresour. Technol. 2018, 247, 443–452. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Li, M.; Li, Q. A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J. Environ. Manag. 2019, 243, 240–249. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, X.; Li, M.; Zhu, Q.; Li, G.; Ma, C.; Li, Q.; Meng, J.; Liu, Y.; Li, Q. Impacts of red mud on lignin depolymerization and humic substance formation mediated by laccase-producing bacterial community during composting. J. Hazard. Mater. 2021, 410, 124557. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Y.; Luo, Y.L.; Pan, Y.R.; Liu, J.; Butler, D. Alkaline environments benefit microbial K-strategists to efficiently utilize protein substrate and promote valorization of protein waste into short-chain fatty acids. Chem. Eng. J. 2021, 404, 127147. [Google Scholar] [CrossRef]
- Tan, W.; Huang, C.; Chen, C.; Liang, B.; Wang, A. Bioaugmentation of activated sludge with elemental sulfur producing strain Thiopseudomonas denitrificans X2 against nitrate shock load. Bioresour. Technol. 2016, 220, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, X.; Tang, X.; Jiang, L.; He, R. Uncovering anaerobic oxidation of methane and active microorganisms in landfills by using stable isotope probing. Environ. Res. 2025, 271, 121139. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Zhou, H.; Shen, Y.; Meng, H.; Li, R.; Zhou, J.; Cheng, H.; Zhang, X.; Ding, J.; Wang, J.; et al. Effect of aeration rates on enzymatic activity and bacterial community succession during cattle manure composting. Bioresour. Technol. 2020, 304, 122928. [Google Scholar] [CrossRef]
- Wang, F.; Xie, L.; Gao, W.; Wu, D.; Chen, X.; Wei, Z. The role of microbiota during chicken manure and pig manure co-composting. Bioresour. Technol. 2023, 384, 129360. [Google Scholar] [CrossRef] [PubMed]
- Lahr, R.H.; Goetsch, H.E.; Haig, S.J.; Noe-Hays, A.; Love, N.G.; Aga, D.S.; Bott, C.B.; Foxman, B.; Jimenez, J.; Luo, T.; et al. Urine bacterial community convergence through fertilizer production: Storage, pasteurization, and struvite precipitation. Environ. Sci. Technol. 2016, 50, 11619–11626. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Yamamoto, Y.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Tangtaweewipat, S.; Umetsu, K. The survival of pathogenic bacteria and plant growth promoting bacteria during mesophilic anaerobic digestion in full-scale biogas plants. Anim. Sci. J. 2019, 90, 297–303. [Google Scholar] [CrossRef]
- Zhan, J.; Han, Y.; Xu, S.; Wang, X.; Guo, X. Succession and change of potential pathogens in the co-composting of rural sewage sludge and food waste. Waste Manag. 2022, 149, 248–258. [Google Scholar] [CrossRef]
- Oliva, A.; Onana, V.E.; Garner, R.E.; Kraemer, S.A.; Fradette, M.; Walsh, D.A.; Huot, Y. Geospatial analysis reveals a hotspot of fecal bacteria in Canadian prairie lakes linked to agricultural non-point sources. Water Res. 2023, 231, 119596. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Sugawa, Y.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Kawamoto, K.; Ihara, I.; Umetsu, K. Comparative fertilizer properties of digestates from mesophilic and thermophilic anaerobic digestion of dairy manure: Focusing on plant growth promoting bacteria (PGPB) and environmental risk. J. Mater. Cycles Waste 2018, 20, 1448–1457. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Chen, H.; Duan, Y.; Liu, T.; Awasthi, S.K.; Wang, Q.; Pandey, A.; Zhang, Z. An assessment of the persistence of pathogenic bacteria removal in chicken manure compost employing clay as additive via meta-genomic analysis. J Hazard. Mater. 2019, 366, 184–191. [Google Scholar] [CrossRef]
- Ravindran, B.; Awasthi, M.K.; Karmegam, N.; Chang, S.W.; Chaudhary, D.K.; Selvam, A.; Nguyen, D.D.; Rahman Milon, A.; Munuswamy-Ramanujam, G. Co-composting of food waste and swine manure augmenting biochar and salts: Nutrient dynamics, gaseous emissions and microbial activity. Bioresour. Technol. 2022, 344, 126300. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; Gómez, X.; Morán, A.; García-González, M.C. Anaerobic co-digestion of livestock and vegetable processing wastes: Fibre degradation and digestate stability. Waste Manag. 2013, 33, 1332–1338. [Google Scholar] [CrossRef]
- Liu, X.P.; Bi, Q.F.; Qiu, L.L.; Li, K.J.; Yang, X.R.; Lin, X.Y. Increased risk of phosphorus and metal leaching from paddy soils after excessive manure application: Insights from a mesocosm study. Sci. Total Environ. 2019, 666, 778–785. [Google Scholar] [CrossRef] [PubMed]


| Indexes | Anaerobic Digestion | Composting | ||||
|---|---|---|---|---|---|---|
| ADM | ADD | Change Rate (%) | JSS | JSC | Change Rate (%) | |
| pH | 7.72 | 6.90 | −10.63 | 8.44 | 8.46 | 0.24 |
| Water content (%) | 90.99 | 83.23 | −8.53 | 77.46 | 39.93 | −48.45 |
| TS (%) | 9.01 | 16.77 | 86.08 | 22.54 | 60.07 | 166.49 |
| VS (%) | 78.57 | 72.94 | −7.16 | 77.05 | 70.38 | −8.66 |
| Cellulose (%) | 8.73 | 13.96 | 59.97 | 7.15 | 7.02 | −1.85 |
| Hemicellulose (%) | 26.70 | 28.54 | 6.90 | 11.96 | 9.56 | −20.11 |
| Lignin (%) | 6.30 | 17.13 | 171.81 | 3.95 | 4.28 | 8.42 |
| Total nitrogen (g/Kg) | 17.02 | 13.94 | −18.06 | 16.46 | 14.63 | −11.11 |
| NH4+-N (g/Kg) | 6.59 | 3.41 | −48.27 | 3.60 | 4.55 | 26.32 |
| NO3−-N (mg/Kg) | 0.03 | 0.02 | −24.28 | 489.54 | 0.79 | 61.82 |
| Total phosphorus (g/Kg) | 54.60 | 51.60 | −5.50 | 2.89 | 5.63 | 20.57 |
| Total sulfur (g/Kg) | 6.50 | 6.93 | 6.65 | 4.67 | 2.21 | −23.61 |
| K (g/Kg) | 25.81 | 48.38 | −46.66 | 15.62 | 25.23 | 61.53 |
| Zn (mg/Kg) | 1212.30 | 1131.80 | 7.11 | 131.13 | 322.07 | 145.60 |
| Cr (mg/Kg) | 12.42 | 12.51 | −0.71 | 9.13 | 9.87 | 8.11 |
| Ni (mg/Kg) | 13.14 | 10.01 | 31.27 | 3.95 | 3.95 | 0.12 |
| Cu (mg/Kg) | 126.52 | 100.44 | 25.97 | — | — | / b |
| As (mg/Kg) | — a | — | / | 6.51 | 6.74 | 3.55 |
| Cd (mg/Kg) | 0.52 | 0.62 | −16.05 | — | — | / |
| Pb (mg/Kg) | 8.85 | — | / | — | 0.48 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Feng, B.; Zha, X.; Chu, Y.; Zhang, X.; He, R. Insights into Physiochemical and Biological Characteristics of Pig Manure During Anaerobic Digestion and Sheep Manure During Composting. Fermentation 2025, 11, 307. https://doi.org/10.3390/fermentation11060307
Li F, Feng B, Zha X, Chu Y, Zhang X, He R. Insights into Physiochemical and Biological Characteristics of Pig Manure During Anaerobic Digestion and Sheep Manure During Composting. Fermentation. 2025; 11(6):307. https://doi.org/10.3390/fermentation11060307
Chicago/Turabian StyleLi, Feixing, Bo Feng, Xianghao Zha, Yixuan Chu, Xin Zhang, and Ruo He. 2025. "Insights into Physiochemical and Biological Characteristics of Pig Manure During Anaerobic Digestion and Sheep Manure During Composting" Fermentation 11, no. 6: 307. https://doi.org/10.3390/fermentation11060307
APA StyleLi, F., Feng, B., Zha, X., Chu, Y., Zhang, X., & He, R. (2025). Insights into Physiochemical and Biological Characteristics of Pig Manure During Anaerobic Digestion and Sheep Manure During Composting. Fermentation, 11(6), 307. https://doi.org/10.3390/fermentation11060307






