Isolation and Functional Characterization of Yeasts from Fermented Plant Based Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Fermentation and Isolation of Yeast Strains
2.3. Molecular Identification
2.4. Evaluation of Probiotic Properties of Yeast Isolates
2.4.1. Tolerance to Different Incubation Conditions
2.4.2. Evaluation of Cell Surface Properties
2.4.3. Antibiotic/Antifungal Agent Susceptibility Assay
2.4.4. Antimicrobial Activity and Characterization
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Yeast Isolates
3.2. Probiotic Properties of Yeast Isolates
3.2.1. Tolerance to Different Incubation Conditions
3.2.2. Cell Surface Properties
3.2.3. Antibiotic/Antifungal Agent Susceptibility Assay
3.2.4. Antimicrobial Activity
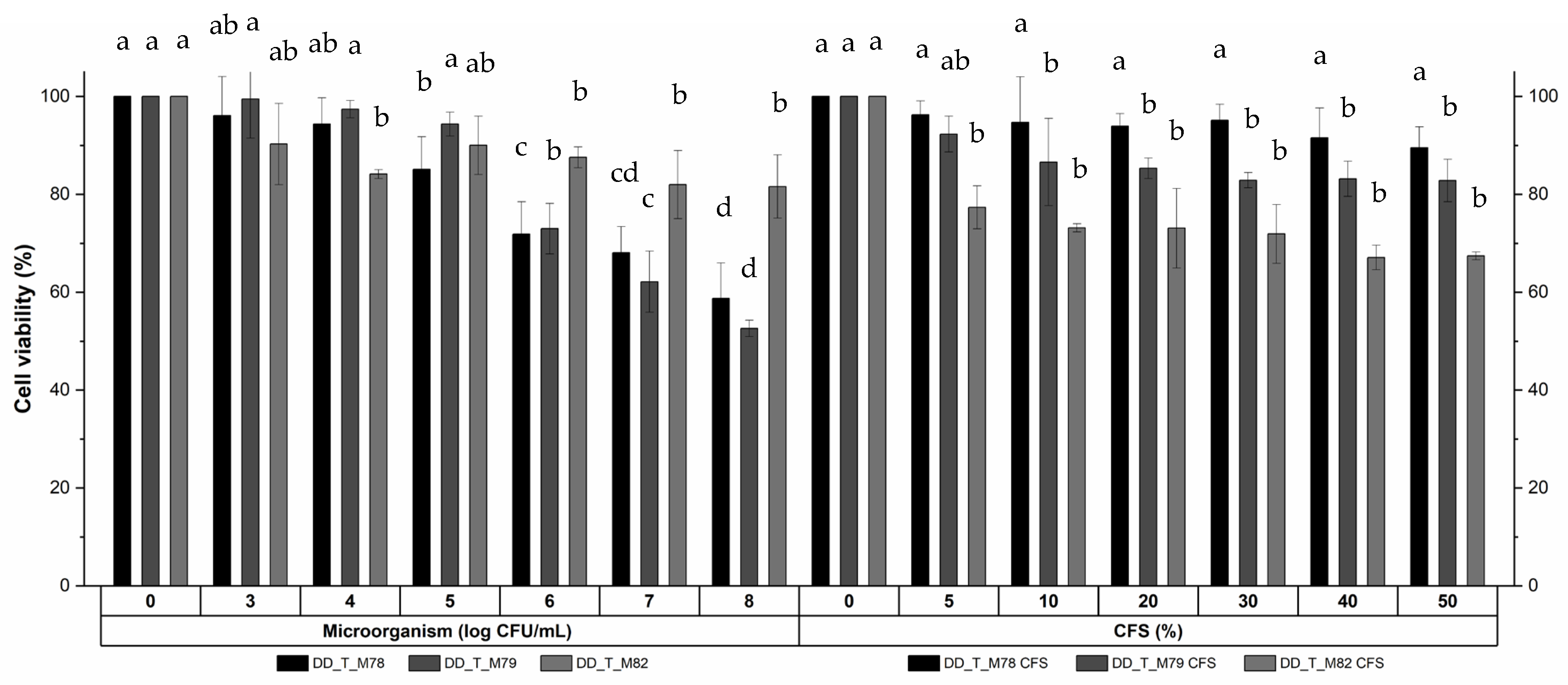
3.2.5. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO food and nutrition paper; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Menezes, A.G.T.; Ramos, C.L.; Cenzi, G.; Melo, D.S.; Dias, D.R.; Schwan, R.F. Probiotic Potential, Antioxidant Activity, and Phytase Production of Indigenous Yeasts Isolated from Indigenous Fermented Foods. Probiotics Antimicrob. Proteins 2020, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Alian Samakkhah, S.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-Promoting Properties of Saccharomyces cerevisiae var. boulardii as a Probiotic; Characteristics, Isolation, and Applications in Dairy Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Ramakrishnan, S.R.; Prabhu, P.R.; Antony, U. Evaluation of Antimicrobial Activity and Probiotic Properties of Wild-Strain Pichia kudriavzevii Isolated from Frozen idli Batter. Yeast 2016, 33, 385–401. [Google Scholar] [CrossRef]
- Merchán, A.V.; Benito, M.J.; Galván, A.I.; Ruiz-Moyano Seco de Herrera, S. Identification and Selection of Yeast with Functional Properties for Future Application in Soft Paste Cheese. LWT 2020, 124, 109173. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A.I. Development of Cereal-Based Functional Food Using Cereal-Mix Substrate Fermented with Probiotic Strain—Pichia kudriavzevii OG32. Food Sci. Nutr. 2015, 3, 486–494. [Google Scholar] [CrossRef]
- Binetti, A.; Carrasco, M.; Reinheimer, J.; Suárez, V. Yeasts from Autochthonal Cheese Starters: Technological and Functional Properties. J. Appl. Microbiol. 2013, 115, 434–444. [Google Scholar] [CrossRef]
- Gotcheva, V.; Hristozova, E.; Hristozova, T.; Guo, M.; Roshkova, Z.; Angelov, A. Assessment of Potential Probiotic Properties of Lactic Acid Bacteria and Yeast Strains. Food Biotechnol. 2002, 16, 211–225. [Google Scholar] [CrossRef]
- Simões, L.A.; Cristina de Souza, A.; Ferreira, I.; Melo, D.S.; Lopes, L.A.A.; Magnani, M.; Schwan, R.F.; Dias, D.R. Probiotic Properties of Yeasts Isolated from Brazilian Fermented Table Olives. J. Appl. Microbiol. 2021, 131, 1983–1997. [Google Scholar] [CrossRef]
- Usal, M.; Özgölet, M.; Arici, M.; Törnük, F. Enzymatic and Antimicrobial Activities of Lactic Acid Bacteria and Yeasts Isolated from Boza, a Traditional Fermented Grain Based Beverage. Food Biosci. 2024, 61, 104681. [Google Scholar] [CrossRef]
- Altay, F.; Karbancıoglu-Güler, F.; Daskaya-Dikmen, C.; Heperkan, D. A Review on Traditional Turkish Fermented Non-Alcoholic Beverages: Microbiota, Fermentation Process and Quality Characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef]
- Guney, D.; Başdoğan, M.G.B.; Sengun, I. Probiotic Characterisation of Lactic Acid Bacteria Isolated from Pickles and Their Potential Application as Presumptive Probiotic Starter Culture in Cucumber Pickles. J. Food Meas. Charact. 2025, 19, 2077–2097. [Google Scholar] [CrossRef]
- Akmal, U.; Ghori, I.; Elasbali, A.M.; Alharbi, B.; Farid, A.; Alamri, A.S.; Muzammal, M.; Asdaq, S.M.B.; Naiel, M.A.E.; Ghazanfar, S. Probiotic and Antioxidant Potential of the Lactobacillus spp. Isolated from Artisanal Fermented Pickles. Fermentation 2022, 8, 328. [Google Scholar] [CrossRef]
- Yilmaz, D.; Sagiroglu, H.C. Development of Measurement System for Grain Loss of Some Chickpea Varieties. Measurement 2015, 66, 73–79. [Google Scholar] [CrossRef]
- Yalim, S.; Özdemır, Y. Effects of Preparation Procedures on Ascorbic Acid Retention in Pickled Hot Peppers. Int. J. Food Sci. Nutr. 2003, 54, 291–296. [Google Scholar] [CrossRef]
- Kumari, V.B.C.; Huligere, S.; MK, J.; Goh, K.W.; Desai, S.M.; HL, K.; Ramu, R. Characterization of Lactobacillus spp. as Probiotic and Antidiabetic Potential Isolated from Boza, Traditional Fermented Beverage in Turkey. Int. J. Microbiol. 2024, 2024, 2148676. [Google Scholar] [CrossRef]
- Ahmada Kh, A.; Abdo, A.; Khan, S.; Aleryani, H.; Mi, S.; Wang, X. Advancing Pickling Techniques to Enhance Bioactive Compounds and Probiotic Content in Pickled Vegetables. Food Rev. Int. 2025, 1–27. [Google Scholar] [CrossRef]
- Adak, M.S.; Kayan, N.; Gunes, A.; Inal, A.; Alpaslan, M.; Cicek, N.; Guzelordu, T. Effect of Harvest Timing on Yield and Mineral Nutritional Value of Kabuli Type Chickpea Seeds. J. Plant Nutr. 2007, 30, 1397–1407. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Ramos Monge, I.M.; Fernández-González, M.; Poveda Colado, J.M.; Arévalo-Villena, M. Safety Evaluation of Yeasts With Probiotic Potential. Front. Nutr. 2021, 8, 659328. [Google Scholar] [CrossRef]
- Gürkan Özlü, B.; Terzi, Y.; Uyar, E.; Shatila, F.; Yalçın, H.T. Characterization and Determination of the Potential Probiotic Yeasts Isolated from Dairy Products. Biologia 2022, 77, 1471–1480. [Google Scholar] [CrossRef]
- Pundir, R.K.; Kaur, S.R.N.; Kaur, A. Probiotic Potential of Lactic Acid Bacteria Isolated from Food Samples: An in Vitro Study. J. Appl. Pharm. Sci. 2013, 3, 85–93. [Google Scholar] [CrossRef]
- Topçu, K.C.; Kaya, M.; Kaban, G. Probiotic Properties of Lactic Acid Bacteria Strains Isolated from Pastırma. LWT 2020, 134, 110216. [Google Scholar] [CrossRef]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and Growth Characterization of a Novel Strain of Saccharomyces boulardii Isolated From Soya Paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing; Twenty Third Informational Supplement. Clin. Lab. Stand. Inst. 2013, 31, 100–121. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Goktas, H.; Dikmen, H.; Demirbas, F.; Sagdic, O.; Dertli, E. Characterisation of Probiotic Properties of Yeast Strains Isolated from Kefir Samples. Int. J. Dairy Technol. 2021, 74, 715–722. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T.; et al. In Vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Baruzzi, F.; Borcakli, M.; Morea, M. Molecular and Phenotypic Characterization of Pichia Fermentans Strains Found among Boza Yeasts. Food Res. Int. 2012, 48, 755–762. [Google Scholar] [CrossRef]
- Pongcharoen, P. The Ability of Pichia kudriavzevii to Tolerate Multiple Stresses Makes It Promising for Developing Improved Bioethanol Production Processes. Lett. Appl. Microbiol. 2022, 75, 36–44. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Piraine, R.E.A.; Retzlaf, G.M.; Gonçalves, V.S.; Cunha, R.C.; Conrad, N.L.; Bochman, M.L.; Leite, F.P.L. Brewing and Probiotic Potential Activity of Wild Yeasts Hanseniaspora uvarum PIT001, Pichia kluyveri LAR001 and Candida intermedia ORQ001. Eur. Food Res. Technol. 2023, 249, 133–148. [Google Scholar] [CrossRef]
- Motey, G.A.; Johansen, P.G.; Owusu-Kwarteng, J.; Ofori, L.A.; Obiri-Danso, K.; Siegumfeldt, H.; Larsen, N.; Jespersen, L. Probiotic Potential of Saccharomyces cerevisiae and Kluyveromyces marxianus Isolated from West African Spontaneously Fermented Cereal and Milk Products. Yeast 2020, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Romero-Luna, H.E.; García, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Key Stress Response Mechanisms of Probiotics During Their Journey Through the Digestive System: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2025, 17, 138–158. [Google Scholar] [CrossRef]
- Zoumpourtikoudi, V.; Pyrgelis, N.; Chatzigrigoriou, M.; Tasakis, R.N.; Touraki, M. Interactions among Yeast and Probiotic Bacteria Enhance Probiotic Properties and Metabolism Offering Augmented Protection to Artemia Franciscana against Vibrio Anguillarum. Microb. Pathog. 2018, 125, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lata, P.; Kumari, R.; Sharma, K.B.; Rangra, S. Savitri In Vitro Evaluation of Probiotic Potential and Enzymatic Profiling of Pichia kudriavzevii Y33 Isolated from Traditional Home-Made Mango Pickle. J. Genet. Eng. Biotechnol. 2022, 20, 132. [Google Scholar] [CrossRef]
- Helmy, E.A.; Soliman, S.A.; Abdel-Ghany, T.M.; Ganash, M. Evaluation of Potentially Probiotic Attributes of Certain Dairy Yeast Isolated from Buffalo Sweetened Karish Cheese. Heliyon 2019, 5, e01649. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.; Kim, J.-I.; Lee, H.-Y.; Moon, G.-S.; Kang, C.-H. Improvements in Human Keratinocytes and Antimicrobial Effect Mediated by Cell-Free Supernatants Derived from Probiotics. Fermentation 2022, 8, 332. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Sornsenee, P.; Romyasamit, C. Lacticaseibacillus spp.; Probiotic Candidates from Palmyra Palm Sugar Possesses Antimicrobial and Anti-Biofilm Activities against Methicillin-Resistant Staphylococcus aureus. Vet. World 2022, 15, 299–308. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, Characterization, and Assessment of Lactic Acid Bacteria toward Their Selection as Poultry Probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Rahmani, B.; Alimadadi, N.; Attaran, B.; Nasr, S. Yeasts from Iranian Traditional Milk Kefir Samples: Isolation, Molecular Identification and Their Potential Probiotic Properties. Lett. Appl. Microbiol. 2022, 75, 1264–1274. [Google Scholar] [CrossRef]
- Abouloifa, H.; Rokni, Y.; Hasnaoui, I.; Bellaouchi, R.; Gaamouche, S.; Ghabbour, N.; Karboune, S.; Ben Salah, R.; Brasca, M.; D’hallewin, G.; et al. Characterization of Antimicrobial Compounds Obtained from the Potential Probiotic Lactiplantibacillus Plantarum S61 and Their Application as a Biopreservative Agent. Braz. J. Microbiol. 2022, 53, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Poloni, V.L.; Bainotti, M.B.; Vergara, L.D.; Escobar, F.; Montenegro, M.; Cavaglieri, L. Influence of Technological Procedures on Viability, Probiotic and Anti-Mycotoxin Properties of Saccharomyces boulardii RC009, and Biological Safety Studies. Curr. Res. Food Sci. 2021, 4, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chandel, D.; Shukla, G. Antigenotoxicity and Cytotoxic Potentials of Metabiotics Extracted from Isolated Probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 Human Colon Cancer Cells. Nutr. Cancer 2020, 72, 110–119. [Google Scholar] [CrossRef]


| Microorganism | Control | pH 2.0 | 1% Bile Salt | |||
|---|---|---|---|---|---|---|
| Initial | 3 h | Initial | 3 h | Initial | 3 h | |
| DD_B_M85 | 7.78 ± 0.01 b,B | 8.28 ± 0.06 b,A | 7.68 ± 0.08 a,B | 7.84 ± 0.13 a,B | 7.25 ± 0.04 e,A | 7.36 ± 0.08 e,A |
| DD_T_M80 | 7.63 ± 0.04 c,B | 8.07 ± 0.08 c,A | 7.54 ± 0.07 a,b,B | 7.64 ± 0.11 a,b,B | 7.24 ± 0.04 e,B | 7.64 ± 0.03 d,A |
| DD_T_M78 | 7.16 ± 0.04 e,A | 7.35 ± 0.02 e,A | 7.26 ± 0.06 c,d,A | 7.18 ± 0.08 c,d,A | 7.62 ± 0.05 c,B | 7.99 ± 0.08 b,c,A |
| DD_T_M79 | 7.17 ± 0.01 e,B | 7.60 ± 0.04 d,A | 7.16 ± 0.04 d,B | 7.06 ± 0.06 d,B | 7.40 ± 0.03 d,B | 7.82 ± 0.03 b,c,A |
| DD_T_M82 | 7.49 ± 0.04 c,d,B | 7.68 ± 0.06 d,A | 7.39 ± 0.03 b,c,B | 7.40 ± 0.01 b,c,B | 7.82 ± 0.03 b,B | 8.09 ± 0.08 b,A |
| DD_B_M88 | 7.40 ± 0.05 d,B | 7.77 ± 0.04 d,A | 7.35 ± 0.04 b,c,d,B | 7.40 ± 0.03 b,c,B | 7.34 ± 0.02 d,e,B | 7.69 ± 0.03 d,A |
| DD_NB_M90 | 8.52 ± 0.03 a,B | 8.70 ± 0.03 a,A | 6.02 ± 0.06 e,C | 5.62 ± 0.02 e,D | 8.47 ± 0.04 a,B | 8.59 ± 0.05 a,A,B |
| Analysis | Microorganisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DD_T_M78 | DD_T_M79 | DD_T_M80 | DD_T_M82 | DD_B_M85 | DD_B_M88 | DD_NB_M90 | |||
| Hydrophobicity (%) | 34.31 ± 1.07 c | 29.74 ± 0.42 d | 38.59 ± 0.84 b | 35.40 ± 0.73 c | 34.74 ± 0.86 c | 63.07 ± 0.14 a | 16.88 ± 0.33 e | ||
| Auto-aggregation (%) | 2 h | 90.79 ± 0.69 a,B | 93.89 ± 1.12 a,A | 76.40 ± 1.89 b,C | 79.40 ± 1.85 b,B | 67.69 ± 1.13 c,C | 91.13 ± 1.39 a,B | 7.94 ± 1.25 d,B | |
| 4 h | 93.97 ± 0.58 a,A | 95.37 ± 0.51 a,A | 86.05 ± 1.01 b,B | 93.84 ± 1.10 a,A | 72.42 ± 0.93 c,B | 94.94 ± 0.22 a,A | 8.58 ± 0.64 d,B | ||
| 24 h | 94.25 ± 0.27 a,A | 95.31 ± 1.14 a,A | 95.23 ± 0.78 a,A | 94.47 ± 0.59 a,A | 91.77 ± 0.53 a,A | 94.75 ± 0.57 a,A,B | 53.29 ± 3.38 b,A | ||
| Co-aggregation (%) | E. coli | 2 h | 77.54 ± 0.67 b,B | 83.25 ± 1.60 a,B | 51.25 ± 0.83 c,C | 31.65 ± 1.84 e,B | 42.84 ± 1.00 d,C | 82.96 ± 1.10 a,A | 0.77 ± 0.37 f,C |
| 4 h | 88.46 ± 0.68 a,b,A | 89.76 ± 0.95 a,A | 84.53 ± 0.04 b,B | 89.64 ± 0.63 a,A | 67.44 ± 0.93 c,B | 88.53 ± 1.97 a,b,A | 7.60 ± 0.80 d,B | ||
| 24 h | 88.78 ± 0.44 a,A | 88.18 ± 0.69 a,A | 89.44 ± 0.41 a,A | 88.88 ± 0.30 a,A | 89.22 ± 1.05 a,A | 88.77 ± 0.91 a,A | 52.12 ± 1.03 b,A | ||
| S. aureus | 2 h | 86.55 ± 0.61 a,B | 80.76 ± 0.83 c,B | 76.95 ± 0.08 d,C | 44.12 ± 0.69 f,B | 48.84 ± 0.87 e,C | 83.88 ± 0.14 b,B | 8.58 ± 0.61 g,C | |
| 4 h | 88.02 ± 0.10 a,A,B | 88.46 ± 0.95 a,A | 84.02 ± 0.90 b,B | 90.73 ± 1.07 a,A | 75.36 ± 0.62 c,B | 88.01 ± 1.17 a,A | 13.20 ± 0.35 d,B | ||
| 24 h | 89.01 ± 0.40 a,A | 88.90 ± 0.72 a,A | 88.60 ± 1.60 a,A | 87.64 ± 1.05 a,A | 88.09 ± 0.87 a,A | 88.18 ± 0.06 a,A | 57.45 ± 0.77 b,A | ||
| C. albicans | 2 h | 78.61 ± 1.34 b,B | 87.49 ± 0.83 a,A | 50.92 ± 0.21 c,C | 89.64 ± 0.75 a,A | 43.17 ± 0.13 d,C | 86.94 ± 1.34 a,A | 8.60 ± 0.68 e,C | |
| 4 h | 89.40 ± 2.00 a,A | 91.77 ± 2.06 a,A | 81.04 ± 0.42 b,B | 91.17 ± 1.05 a,A | 67.61 ± 1.48 c,B | 89.91 ± 1.13 a,A | 13.47 ± 1.42 d,B | ||
| 24 h | 89.75 ± 0.62 a,A | 88.97 ± 0.42 a,A | 90.44 ± 0.88 a,A | 89.57 ± 1.58 a,A | 89.43 ± 1.70 a,A | 89.45 ± 0.62 a,A | 56.99 ± 0.09 b,A | ||
| Agents | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| DD_T_M78 | DD_T_M79 | DD_T_M80 | DD_T_M82 | DD_B_M85 | DD_B_M88 | DD_NB_M90 | |
| Antibiotics | |||||||
| Ampicillin (10 µg) | - * | - | 7.25 ± 0.05 | - | 9.17 ± 0.24 | - | 7.73 ± 0.17 |
| Gentamycin (10 µg) | - | - | - | - | 6.12 ± 0.08 | - | 9.30 ± 0.05 |
| Kanamycin (30 µg) | - | - | 7.97 ± 0.12 | - | 6.17 ± 0.29 | - | 12.17 ± 0.29 |
| Streptomycin (10 µg) | - | - | 9.77 ± 0.21 | - | 6.83 ± 0.15 | - | 7.27 ± 0.25 |
| Vancomycin (30 µg) | - | - | 6.17 ± 0.24 | - | 6.60 ± 0.17 | - | 10.33 ± 0.15 |
| Antifungals | |||||||
| Amphotericin B (20 µg) | 6.25 ± 0.25 | 7.38 ± 1.19 | 11.75 ± 1.09 | 6.00 ± 0.00 | 9.00 ± 0.00 | 6.50 ± 0.00 | 6.75 ± 0.25 |
| Nystatin (100 µg) | 10.50 ± 0.87 | 18.00 ± 0.71 | 14.75 ± 0.43 | 20.25 ± 0.43 | 20.75 ± 0.83 | 19.25 ± 2.28 | 18.38 ± 0.41 |
| Ketoconazole (10 µg) | 9.00 ± 0.71 | 7.75 ± 1.25 | 13.50 ± 0.50 | 6.38 ± 0.41 | 6.00 ± 0.00 | 6.50 ± 0.50 | 8.00 ± 1.00 |
| Itraconazole (10 µg) | 10.75 ± 0.83 | 12.25 ± 0.83 | 10.17 ± 0.62 | 10.75 ± 0.43 | 7.50 ± 0.87 | 13.75 ± 0.83 | 10.00 ± 0.00 |
| Fluconazole (25 µg) | 13.50 ± 1.50 | 14.25 ± 0.83 | 14.38 ± 0.41 | 10.75 ± 1.09 | 6.00 ± 0.00 | 7.00 ± 0.00 | 6.00 ± 0.00 |
| Analysis | Microorganisms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DD_T_M78 | DD_T_M79 | DD_T_M80 | DD_T_M82 | DD_B_M85 | DD_B_M88 | DD_NB_M90 | |||
| Inhibition (%) | E. coli | 24 h | 22.72 ± 1.47 c.B | 31.13 ± 1.42 b,B | 98.72 ± 0.28 a,A | 24.64 ± 1.43 c,B | 34.06 ± 1.63 b,B | 7.02 ± 0.05 d,C | -1 |
| 48 h | 18.69 ± 1.46 c.B | 27.11 ± 0.83 b,C | 92.82 ± 0.50 a,B | 19.44 ± 0.93 c,C | 25.10 ± 1.45 b,C | 5.54 ± 0.28 d,C | - | ||
| S. aureus | 24 h | 95.48 ± 1.60 a.b.A | 84.67 ± 0.47 c,A | 97.72 ± 0.69 a,A | 94.34 ± 0.47 b,A | 96.93 ± 0.68 a,b,A | 39.53 ± 0.64 d,A | - | |
| 48 h | 96.35 ± 0.96 a.A | 85.87 ± 0.76 b,A | 97.31 ± 0.60 a,A | 97.34 ± 0.80 a,A | 97.59 ± 0.86 a,A | 31.01 ± 1.38 c,B | - | ||
| CFS Characterization | Protein (µg BSA/mL) | 48.97 ± 1.42 a | 14.81 ± 1.04 b | 13.14 ± 1.04 b,c | 15.64 ± 1.42 b | 10.08 ± 0.68 c,d | 9.81 ± 0.79 d | 11.19 ± 0.39 c,d | |
| DPPH (% inhibition) | 34.02 ± 0.82 b | 30.33 ± 0.94 c | 44.36 ± 1.62 a | 23.81 ± 0.94 d | 19.92 ± 1.55 e | 32.08 ± 1.97 b,c | 14.29 ± 0.61 f | ||
| Lactic acid (g/L) | 0.121 ± 0.002 c | 0.116 ± 0.003 c | ND2 | 0.144 ± 0.004 b,c | ND | 0.197 ± 0.006 a | 0.154 ± 0.014 b | ||
| Citric acid (g/L) | 0.127 ± 0.001 c | 0.105 ± 0.011 c | 0.067 ± 0.005 d | 0.187 ± 0.006 b | 0.120 ± 0.001 c | 0.293 ± 0.002 a | 0.281 ± 0.005 a | ||
| Tartaric acid (g/L) | 0.035 ± 0.000 a | 0.018 ± 0.001 b | ND | 0.018 ± 0.003 b | 0.012 ± 0.001 b | 0.020 ± 0.001 b | ND | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devecioglu, D.; Kuscu, A.; Karbancioglu-Guler, F. Isolation and Functional Characterization of Yeasts from Fermented Plant Based Products. Fermentation 2025, 11, 305. https://doi.org/10.3390/fermentation11060305
Devecioglu D, Kuscu A, Karbancioglu-Guler F. Isolation and Functional Characterization of Yeasts from Fermented Plant Based Products. Fermentation. 2025; 11(6):305. https://doi.org/10.3390/fermentation11060305
Chicago/Turabian StyleDevecioglu, Dilara, Anı Kuscu, and Funda Karbancioglu-Guler. 2025. "Isolation and Functional Characterization of Yeasts from Fermented Plant Based Products" Fermentation 11, no. 6: 305. https://doi.org/10.3390/fermentation11060305
APA StyleDevecioglu, D., Kuscu, A., & Karbancioglu-Guler, F. (2025). Isolation and Functional Characterization of Yeasts from Fermented Plant Based Products. Fermentation, 11(6), 305. https://doi.org/10.3390/fermentation11060305







