Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Analytical Methods
2.3. Experimental Setup
2.3.1. Biochemical Methane Potential (BMP) Test: Optimum Mixing Ratio
2.3.2. Biochemical Methane Potential (BMP) Test: Optimum Mixing Rate
2.4. Kinetic Study
3. Results and Discussion
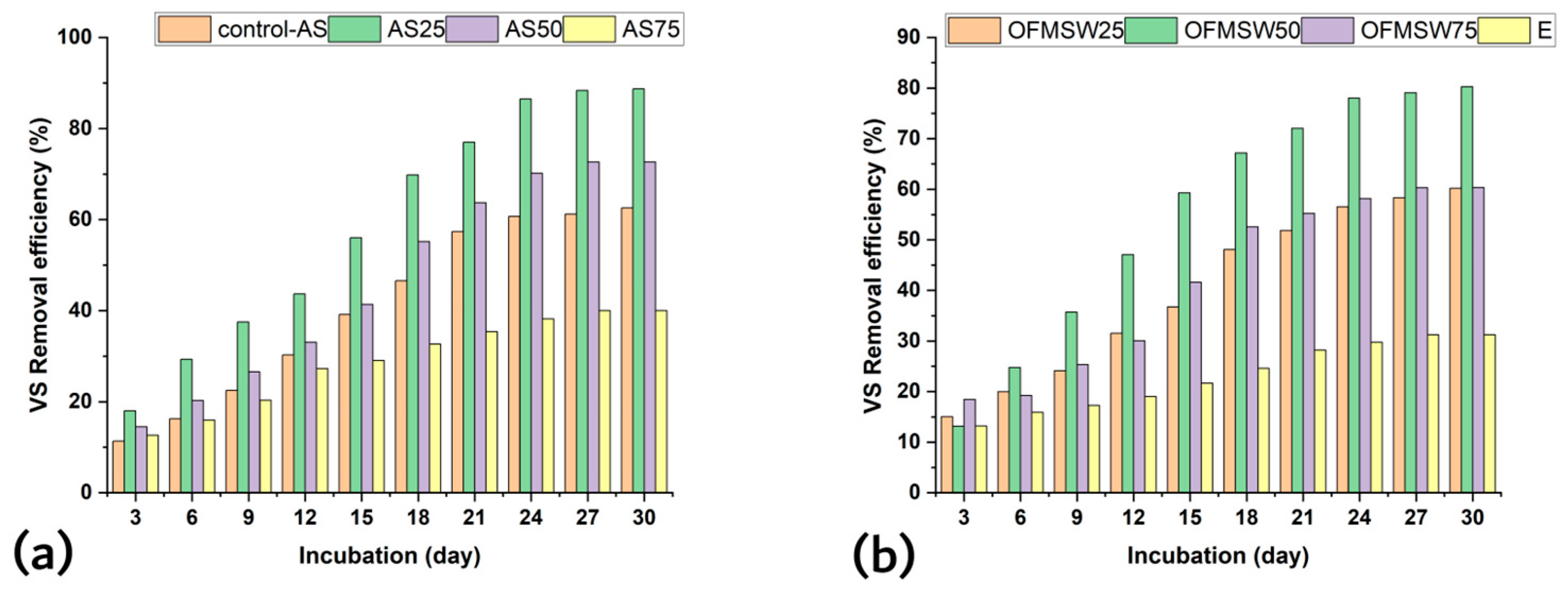
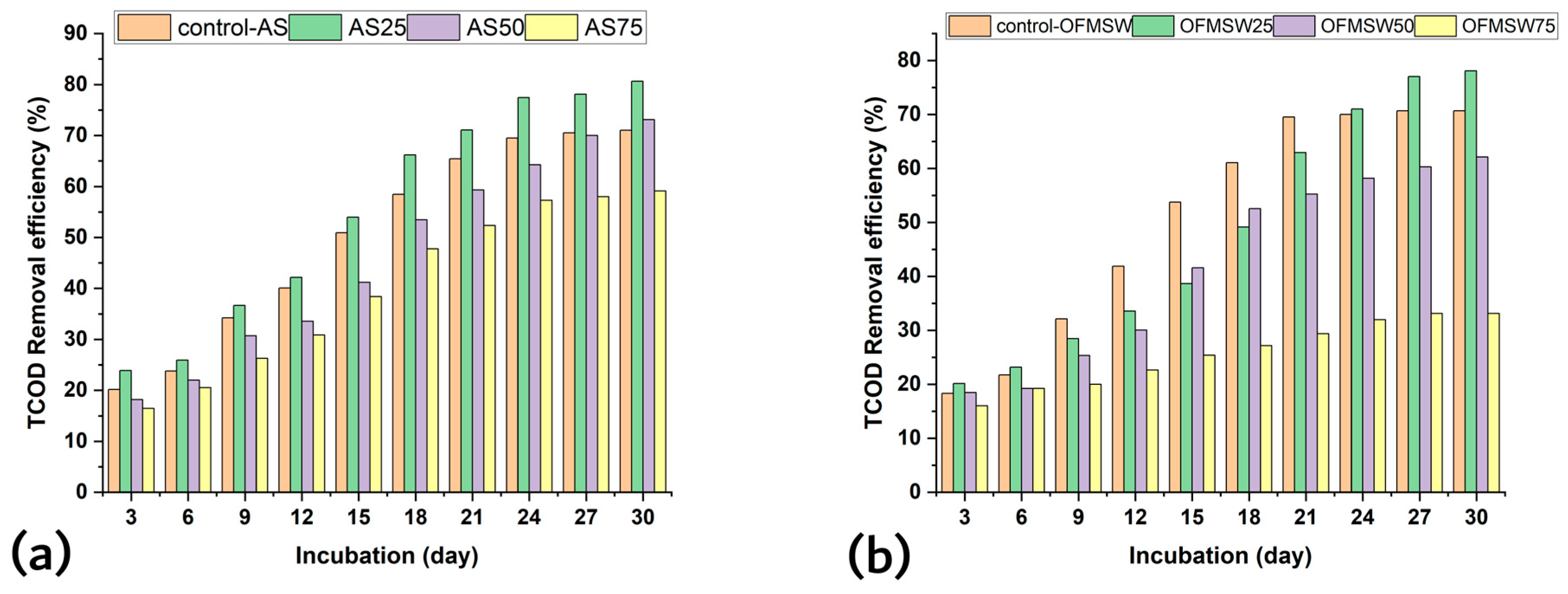
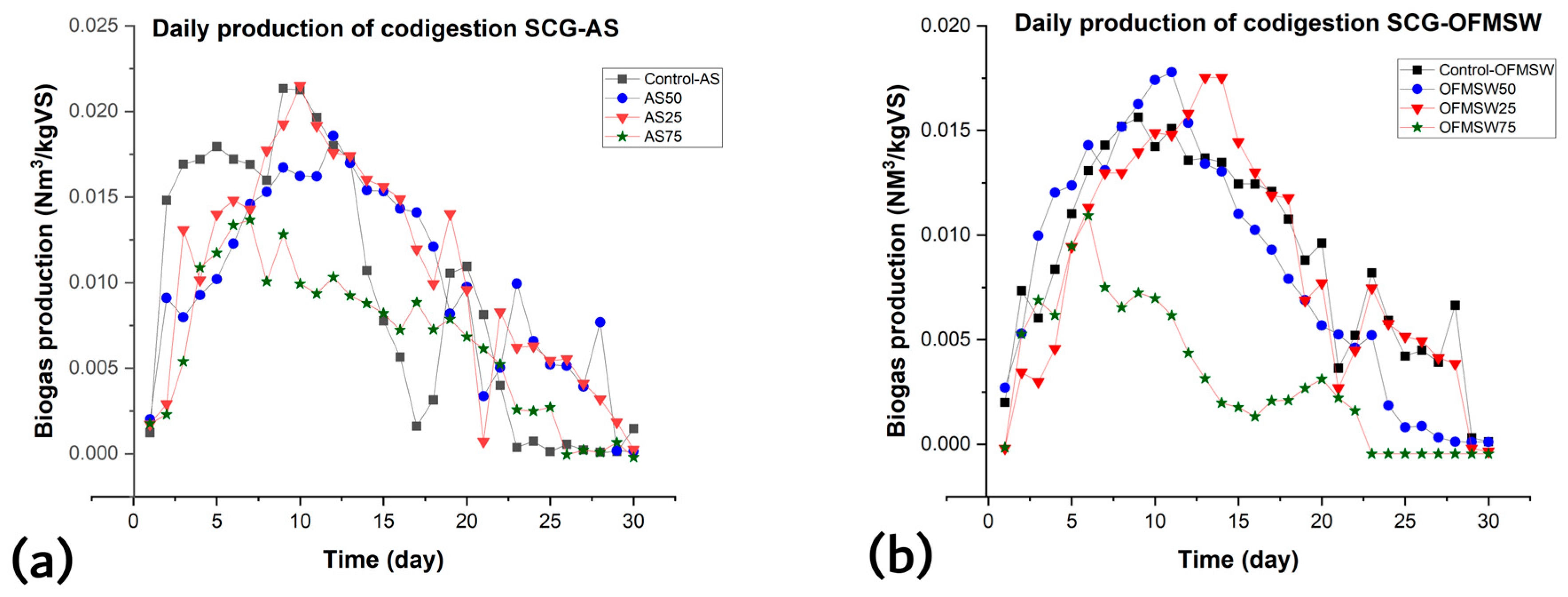
3.1. Experimental Performance of BMP Test: Effect of SCGs as Co-Substrates
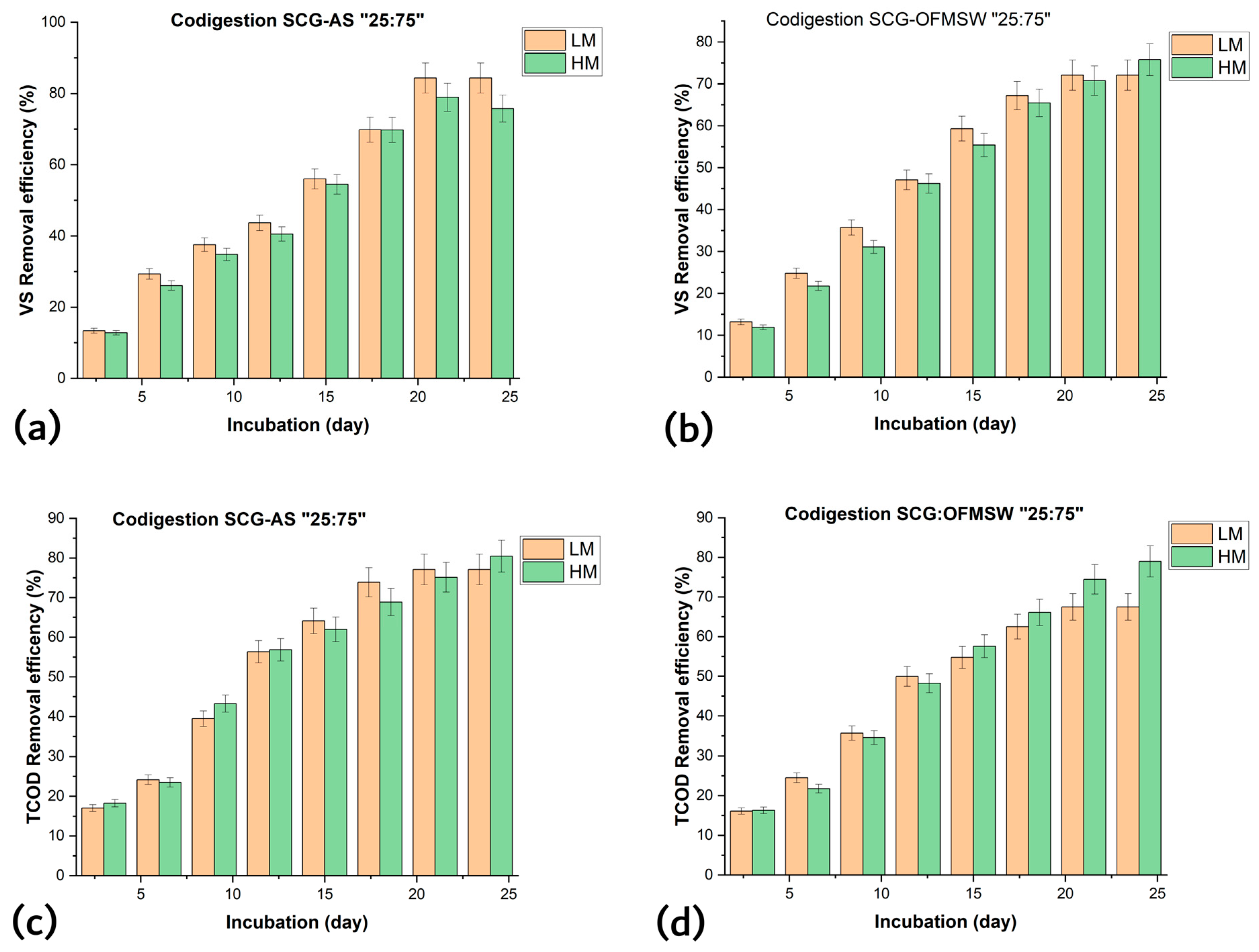
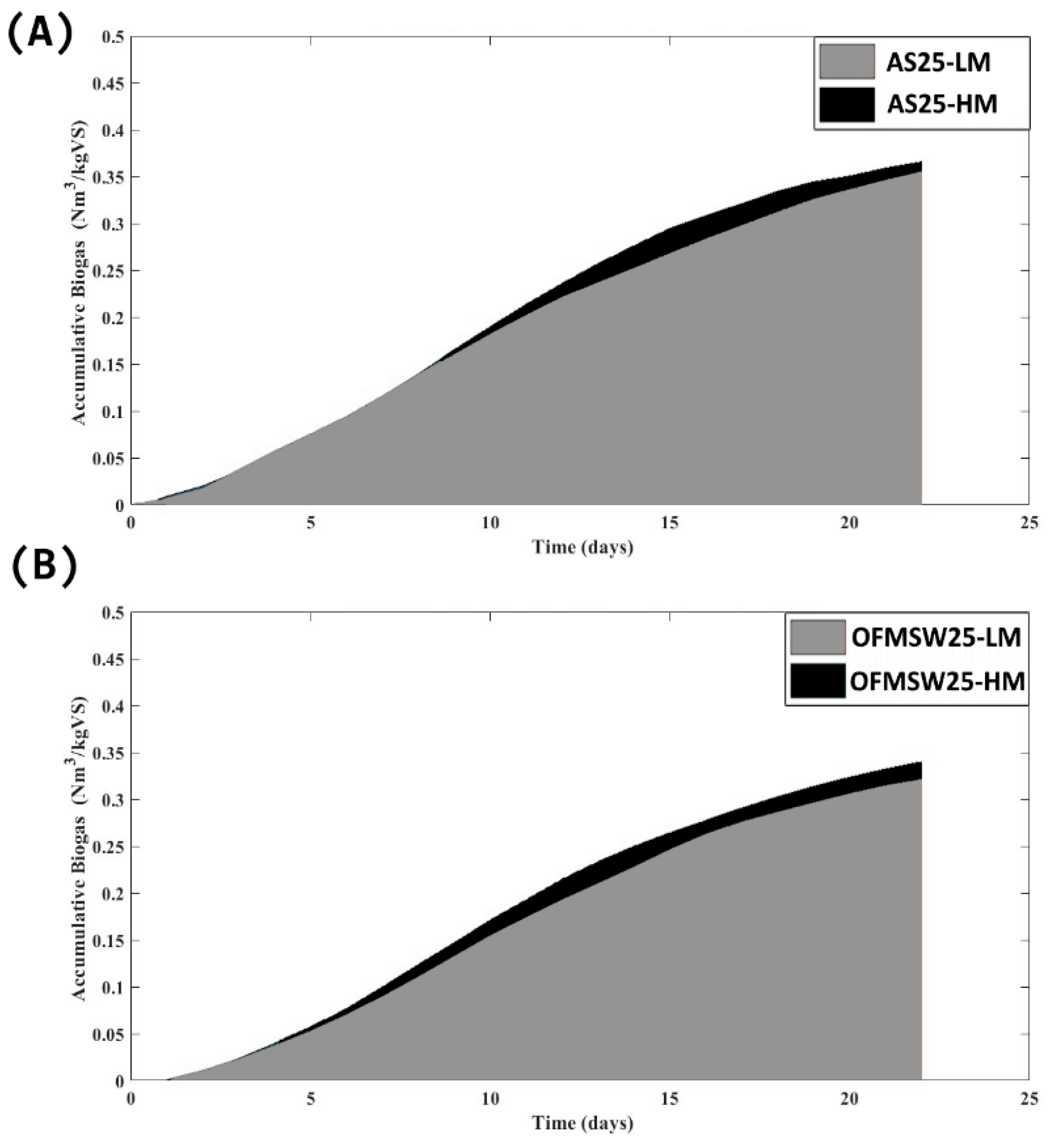
3.2. Effect of Mixing Mode on Co-Digestion Potential
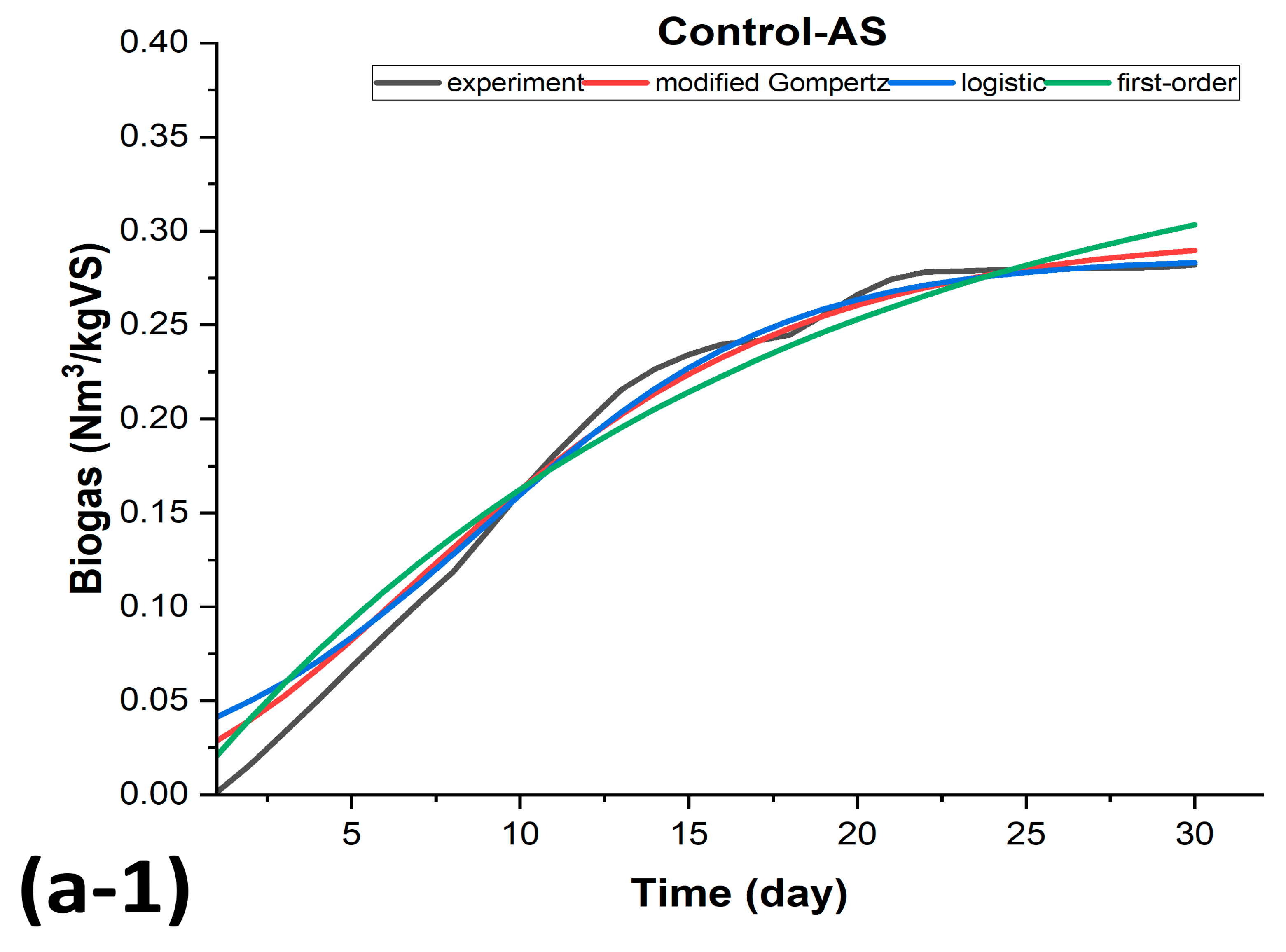
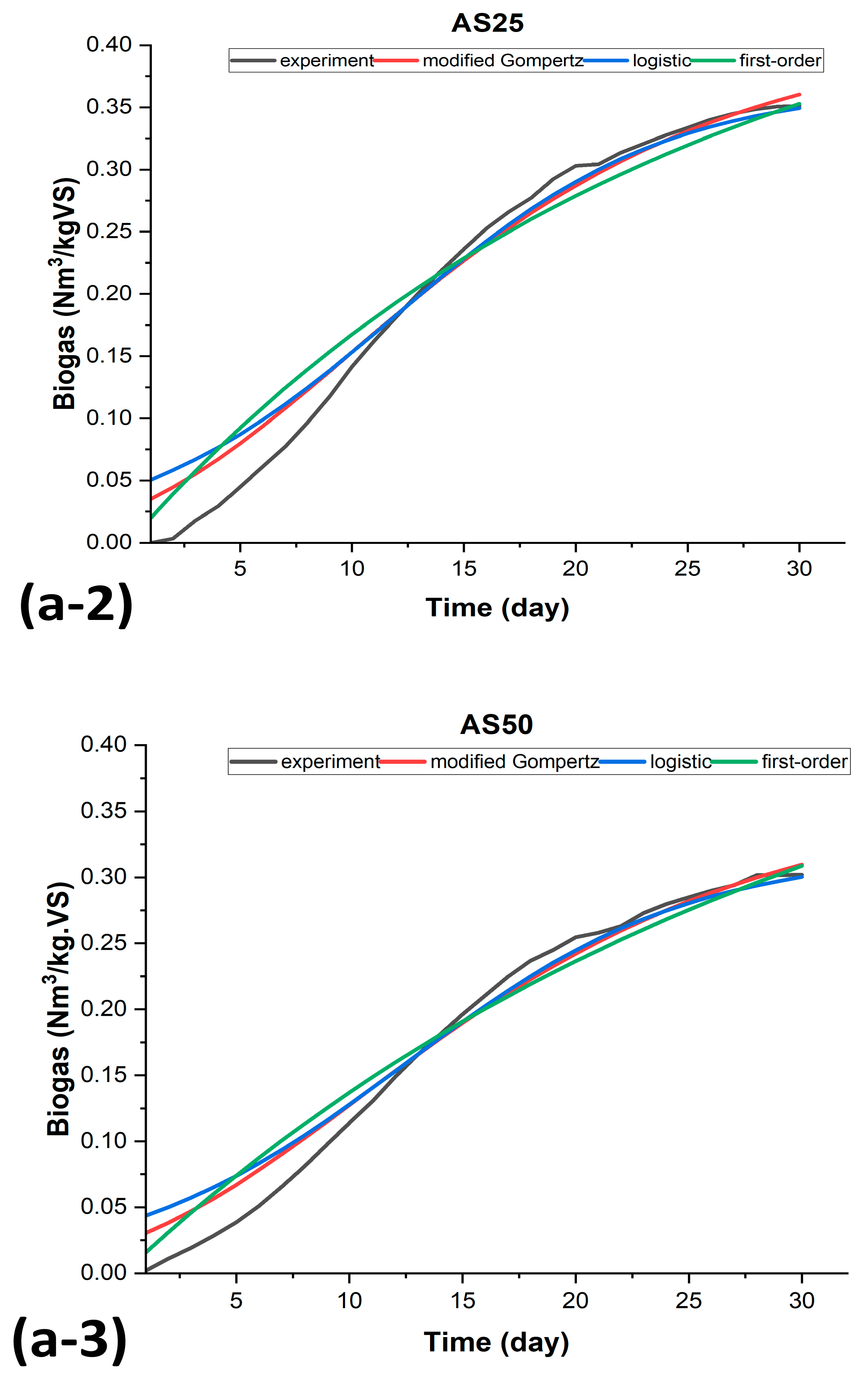
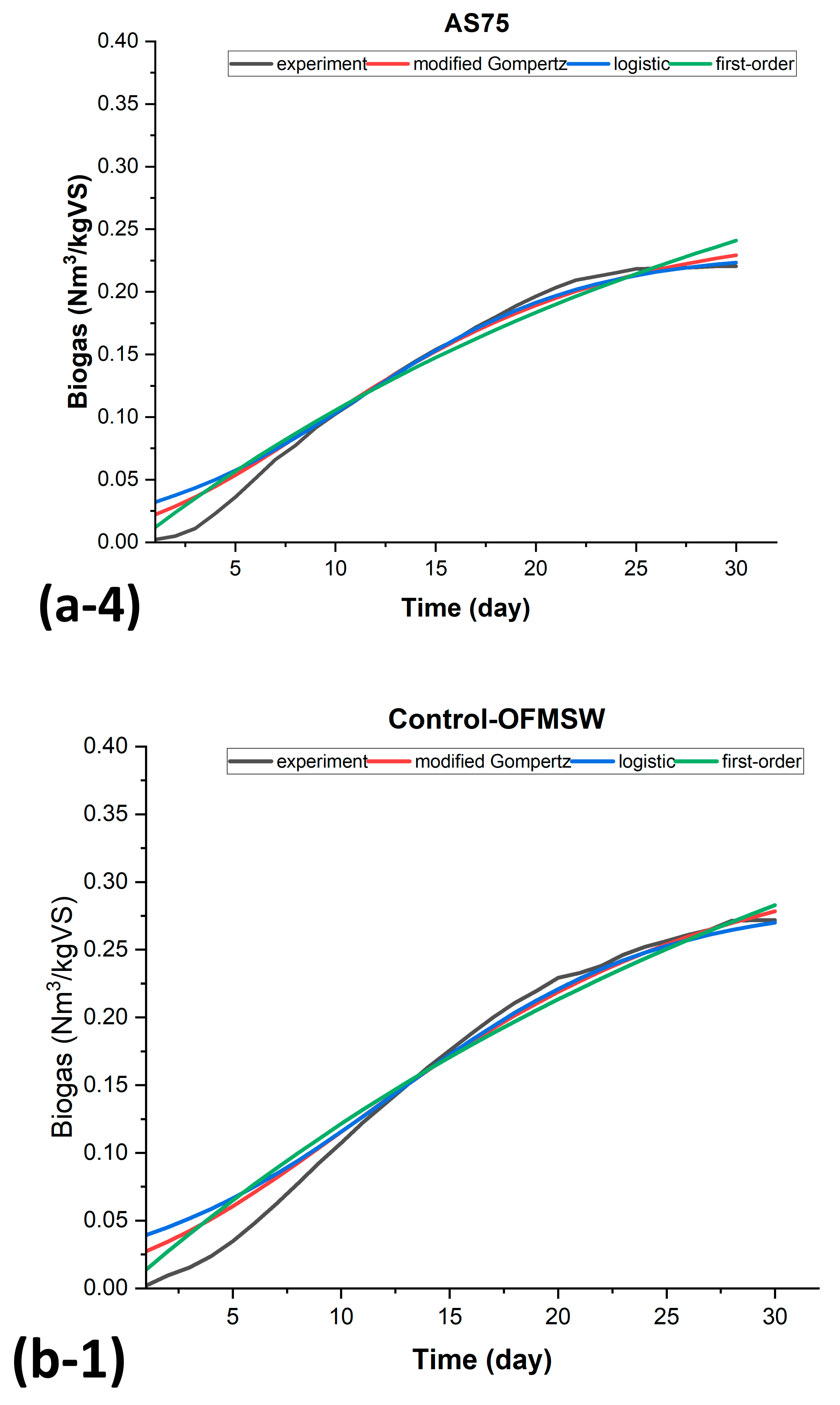
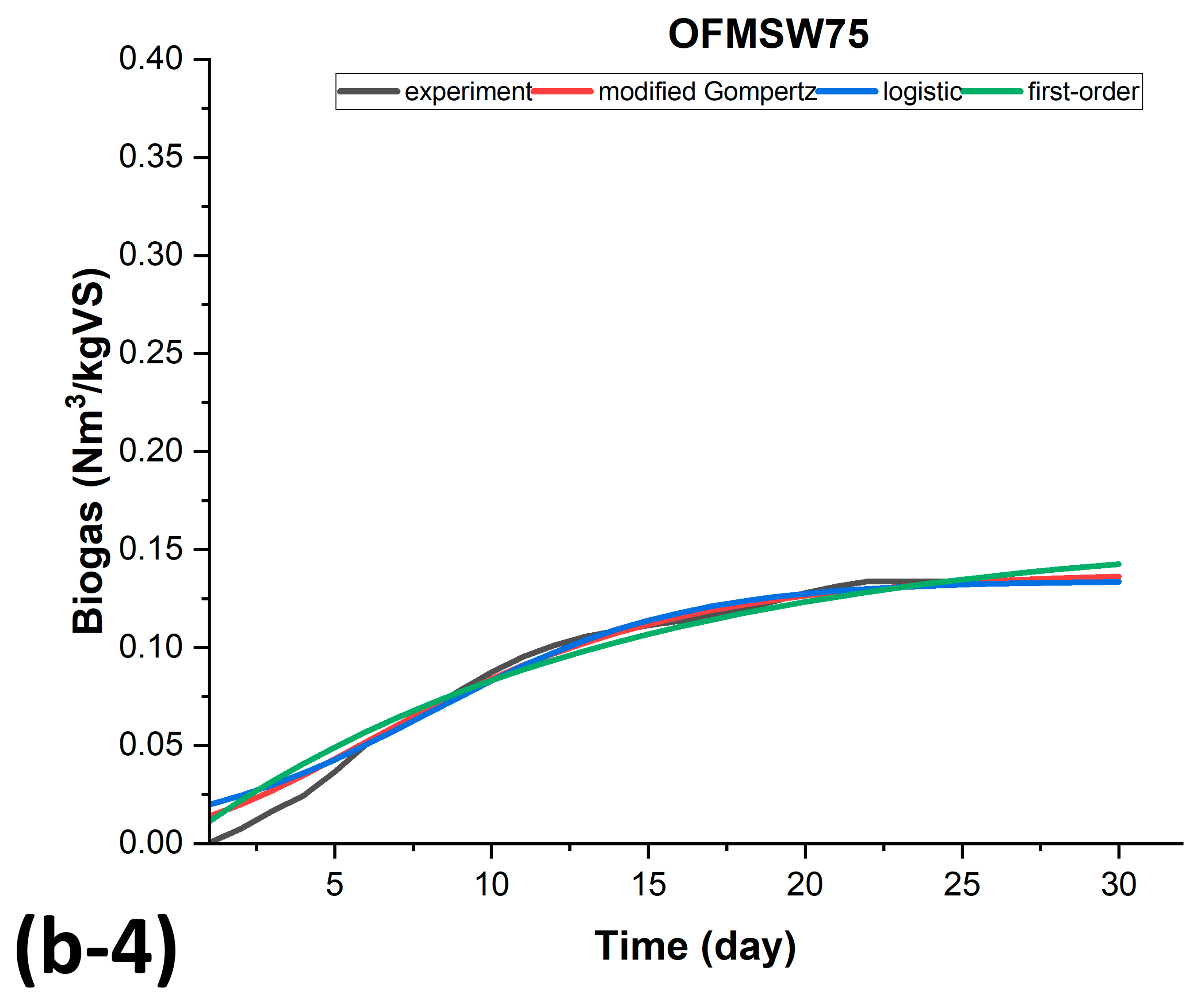
3.3. Kinetic Modeling of Biogas Production for BMP-MM Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Jordan. Food Balance Sheet. Available online: http://www.dos.gov.jo/dos_home_e/main/agriculture/food_budget/index.htm (accessed on 9 December 2019).
- Al-Dalain, S.Y.; Haddad, M.A.; Parisi, S.; Al-Tarawneh, M.A.; Qaralleh, H. Determination of Macroelements, Transition Elements, and Anionic Contents of Commercial Roasted Ground Coffee Available in Jordanian Markets. Beverages 2020, 6, 16. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete Utilization of Spent Coffee Grounds To Produce Biodiesel, Bio-Oil, and Biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Silva, M.A.; Nebra, S.A.; Machado Silva, M.J.; Sanchez, C.G. The use of biomass residues in the brazilian soluble coffee industry. Biomass Bioenergy 1998, 14, 457–467. [Google Scholar] [CrossRef]
- Chang, S. Overview of Mushroom Cultivation and Utilization as Functional Foods. In Mushrooms as Functional Foods; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–33. [Google Scholar] [CrossRef]
- Qiao, W.; Takayanagi, K.; Niu, Q.; Shofie, M.; Li, Y.Y. Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour. Technol. 2013, 149, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of Untreated and Treated Sewage Sludge with the Organic Fraction of Municipal Solid Wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Zboon, K.K.; Al Rabadi, S.; Gul, E.; Al-Samrraie, L.A.; Ali, R.; Al-Tabbal, J.A. Impact of Iron oxide nanoparticles on sustainable production of biogas through anaerobic co-digestion of chicken waste and wastewater. Front. Chem. Eng. 2022, 4, 974546. [Google Scholar] [CrossRef]
- Bkoor, A.; Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Samrraie, L.A.; Al Bsoul, A.; Damseh, R.A.; Khasawneh, A.; Dessouky, Y.; Tonbol, K.; Ali, B.M.; et al. The Effects of Nanoparticles- Zerovalent Iron on Sustainable Biomethane Production through Co-Digestion of Olive Mill Wastewater and Chicken Manure. Fermentation 2023, 9, 183. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Essa, A.H. Anaerobic Co-digestion Mill Wastewater—Activated Sludge Effect of Aerobic Pretreatment on the Performance of OMW Anaerobic Digestion. Waste Biomass Valorization 2020, 11, 4781–4788. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Samrraie, L.A.; Al Issa, H.A.; Qasem, I.; Hussien, A.A.; Al-Zboon, K.K.; Damseh, R.A.; Gul, E. Prediction and Optimization of Biogas Production from OMW Digestion Using Fenton Pre-Treatment Process with Particle Swarm Optimization. Int. J. Des. Nat. Ecodyn. 2022, 17, 157–168. [Google Scholar] [CrossRef]
- Boulanger, A.; Pinet, E.; Bouix, M.; Bouchez, T.; Mansour, A.A. Théodore Bouchez, Alicia A. Mansour, Effect of inoculum to substrate ratio (I/S) on municipal solid waste anaerobic degradation kinetics and potential. Waste Manag. 2012, 32, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Usack, J.G.; Angenent, L.T. Comparing the inhibitory thresholds of dairy manure co-digesters after prolonged acclimation periods: Part 1–Performance and operating limits. Water Res. 2015, 87, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Montes, J.A.; Rico, J.L.; Rico, C. Influence of headspace pressure on methane production in Biochemical Methane Potential (BMP) tests. Waste Manag. 2016, 48, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- Kampioti, A.; Komilis, D. Anaerobic co-digestion of coffee waste with other organic substrates: A mixture experimental design. Chemosphere 2022, 297, 134124. [Google Scholar] [CrossRef]
- Lahcen, S.A.L.; Ibn Ahmed, S.; Lahboubi, N.; El Bari, H. Energetic Potential of Coffee Waste from Anaerobic Digestion and Combustion. Ecol. Eng. Environ. Technol. 2023, 24, 138–145. [Google Scholar] [CrossRef]
- Gil-Gómez, J.A.; Florez-Pardo, L.M.; Leguizamón-Vargas, Y.C. Valorization of coffee by-products in the industry, a vision towards circular economy. Discov. Appl. Sci. 2024, 6, 1–16. [Google Scholar] [CrossRef]
- Garcia-Freites, S.; Röder, M.; Thornley, P. Environmental trade-offs associated with bioenergy from agri-residues in sub-tropical regions: A case study of the Colombian coffee sector. Biomass Bioenergy 2020, 140, 105581. [Google Scholar] [CrossRef]
- Campos, R.C.; Pinto, V.R.A.; Melo, L.F.; da Rocha, S.J.S.S.; Coimbra, J.S. New sustainable perspectives for “Coffee Wastewater” and other by-products: A critical review. Futur. Foods 2021, 4, 100058. [Google Scholar] [CrossRef]
- Fonoll, X.; Astals, S.; Dosta, J.; Mata-Alvarez, J. Anaerobic co-digestion of sewage sludge and fruit wastes: Evaluation of the transitory states when the co-substrate is changed. Chem. Eng. J. 2015, 262, 1268–1274. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; Al-Sameraie, L.; Al Bsoul, A.; Khasawneh, A.; Al-Tabbal, J. Correlation between kinetic parameters, reactor performance, and biogas and methane potential of co-digestion and mono-digestion of active sludge and olive mill wastewater. Int. J. Low-Carbon Technol. 2024, 19, 1501–1515. [Google Scholar] [CrossRef]
- Sossa, J.P.R. Evaluación de La Producción de Biogás Por Medio de La Biodigestión Anaerobia Semicontinua Utilizando Residuos Del Beneficiado Del Café Como Substrato. Ph.D. Thesis, Universidad de Costa Rica, San Pedro, Costa Rica, 2015. Available online: https://repositorio.sibdi.ucr.ac.cr/items/95f25706-4af8-4378-9fd8-bc50877be362/full (accessed on 18 May 2025).
- Chala, B.; Oechsner, H.; Latif, S.; Müller, J. Biogas Potential of Coffee Processing Waste in Ethiopia. Sustainability 2018, 10, 2678. [Google Scholar] [CrossRef]
- Selvamurugan, M.; Doraisamy, P.; Maheswari, M. An integrated treatment system for coffee processing wastewater using anaerobic and aerobic process. Ecol. Eng. 2010, 36, 1686–1690. [Google Scholar] [CrossRef]
- Hernández, M.A.; Rodríguez Susa, M.; Andres, Y. Use of coffee mucilage as a new substrate for hydrogen production in anaerobic co-digestion with swine manure. Bioresour. Technol. 2014, 168, 112–118. [Google Scholar] [CrossRef]
- Abouelenien, F.; Namba, Y.; Kosseva, M.R.; Nishio, N.; Nakashimada, Y. Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic co-digestion of spent coffee grounds with different waste feedstocks for biogas production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, W.; Wang, X.; Takayanagi, K.; Shofie, M.; Li, Y.-Y. Kinetic characterization of thermophilic and mesophilic anaerobic digestion for coffee grounds and waste activated sludge. Waste Manag. 2015, 36, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Oechsner, H.; Müller, J. Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage. Appl. Sci. 2019, 9, 412. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Budiyono; Sumardiono, S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Budiyono; Widiasa, I.N.; Johari, S.; Sunarso. The Kinetic of Biogas Production Rate from Cattle Manure in Batch Mode. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2010, 4, 75–80. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and biogas production potential of municipal solid wastes pretreated with a rotary drum reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Atelge, M.R.; Atabani, A.E.; Abut, S.; Kaya, M.; Eskicioglu, C.; Semaan, G.; Lee, C.; Yildiz, Y.; Unalan, S.; Mohanasundaram, R.; et al. Anaerobic co-digestion of oil-extracted spent coffee grounds with various wastes: Experimental and kinetic modeling studies. Bioresour. Technol. 2021, 322, 124470. [Google Scholar] [CrossRef]
- Sharma, K.D.; Jain, S. Effect of different inoculum and substrate inoculum ratios on biogas yield for anaerobic digestion of organic fraction of solid waste. Glob. NEST J. 2023, 25, 15–24. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, Y.; Ma, X.; Guan, W.; Gao, M.; Li, Y.-Y.; Wang, Q.; Wu, C. Effect of zero-valent iron addition on the biogas fermentation of food waste after anaerobic preservation. J. Environ. Chem. Eng. 2021, 9, 106013. [Google Scholar] [CrossRef]
- UNI 5667–13; Water quality—Sampling—Part 13: Guidance on sampling of sludges. Ente Nazionale Italiano di Unificazione (UNI): Milano, Italy, 2000.
- Alrawashdeh, K.A.B.; Slopiecka, K.; Alshorman, A.A.; Bartocci, P.; Fantozzi, F. Pyrolytic Degradation of Olive Waste Residue (OWR) by TGA: Thermal Decomposition Behavior and Kinetic Study. J. Energy Power Eng. 2017, 11, 497–510. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B. Anaerobic Co-digestion efficiency under the stress exerted by different heavy metals concentration: An energy nexus analysis. Energy Nexus 2022, 7, 100099. [Google Scholar] [CrossRef]
- ASTM D5373-16; Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in laboratory samples of coal and coke. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Puyuelo, B.; Ponsá, S.; Gea, T.; Sánchez, A. Determining C/N ratios for typical organic wastes using biodegradable fractions. Chemosphere 2011, 85, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Joint Statement of American Water Works Association and American Public Health Association on Procedure For Issuing “Standard Methods for the Examination of Water and Sewage”. J. Am. Water Work. Assoc. 1932, 24, 1609–1612. [CrossRef]
- Alrawashdeh, K.A.B. Improving Anaerobic Co-digestion of Sewage Sludge with Thermal Dried Olive Mill Wastewater. Waste Biomass Valorization 2018, 10, 2213–2219. [Google Scholar] [CrossRef]
- APHA. Standard Methods Committee of the American Public Health Association, American Water Works Association, and Water Environment Federation. 4500-p phosphorus. In Standard Methods For the Examination of Water and Wastewater; Lipps, W.C., Baxter, T.E., Braun-Howland, E., Eds.; APHA Press: Washington, DC, USA, 2018. [Google Scholar]
- Boe, K.; Batstone, D.J.; Angelidaki, I. An innovative online VFA monitoring system for the anerobic process, based on headspace gas chromatography. Biotechnol. Bioeng. 2007, 96, 712–721. [Google Scholar] [CrossRef]
- Kariyama, I.D.; Zhai, X.; Wu, B. Influence of mixing on anaerobic digestion efficiency in stirred tank digesters: A review. Water Res. 2018, 143, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Thorin, E.; Bel Fdhila, R.; Dahlquist, E. Effects of mixing on the result of anaerobic digestion: Review. Renew. Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; A Al-Tabbal, J.; A Al-Samrraie, L.; Al Bsoul, A.; Al Zboon, K.K. Enhancing residue degradation and methane production from active sludge: The role of conductive nanoparticles in anaerobic digestion systems. Int. J. Low-Carbon Technol. 2023, 18, 1307–1317. [Google Scholar] [CrossRef]
- Singh, B.; Kovács, K.L.; Bagi, Z.; Nyári, J.; Szepesi, G.L.; Petrik, M.; Siménfalvi, Z.; Szamosi, Z. Enhancing Efficiency of Anaerobic Digestion by Optimization of Mixing Regimes Using Helical Ribbon Impeller. Fermentation 2021, 7, 251. [Google Scholar] [CrossRef]
- Gnaoui, Y.E.; Sounni, F.; Bakraoui, M.; Karouach, F.; Benlemlih, M.; Barz, M.; El Bari, H. Anaerobic co-digestion assessment of olive mill wastewater and food waste: Effect of mixture ratio on methane production and process stability. J. Environ. Chem. Eng. 2020, 8, 103874. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Donoso-Bravo, A.; Torres-Lozada, P. Digestión anaerobia de residuos de alimentos. Predicción de la producción de metano mediante la comparación de modelos cinéticos. Ing. Y Compet. 2017, 19, 210. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Zhu, J. Development of General Gompertz Models and Their Simplified Two-Parameter Forms Based on Specific Microbial Growth Rate for Microbial Growth, Bio-Products and Substrate Consumption. Adv. Biotechnol. Microbiol. 2017, 4, 555640. [Google Scholar] [CrossRef]
- Lizama, A.C.; Figueiras, C.C.; Gaviria, L.A.; Pedreguera, A.Z.; Espinoza, J.E.R. Nanoferrosonication: A novel strategy for intensifying the methanogenic process in sewage sludge. Bioresour. Technol. 2019, 276, 318–324. [Google Scholar] [CrossRef]
- Pan, X.; Lv, N.; Cai, G.; Zhou, M.; Wang, R.; Li, C.; Ning, J.; Li, J.; Li, Y.; Ye, Z.; et al. Carbon- and metal-based mediators modulate anaerobic methanogenesis and phenol removal: Focusing on stimulatory and inhibitory mechanism. J. Hazard. Mater. 2021, 420, 126615. [Google Scholar] [CrossRef] [PubMed]
- Semaan, G. Optimization of the Inoculum-to-Substrate Ratio in Solid-State Spent Coffee Grounds Anaerobic Digestion Biorefineries Using Anaerobic Sludge for Maximal Bio-CH4 Production; Handle.net. 2020. Available online: https://hdl.handle.net/11250/2683881 (accessed on 18 May 2025).
- Rasit, N.; Idris, A.; Harun, R.; Ghani, W.A.W.A.K. Effects of lipid inhibition on biogas production of anaerobic digestion from oily effluents and sludges: An overview. Sustain. Energy Rev. 2015, 45, 351–358. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Kim, J.; Lee, C. Co-feeding spent coffee grounds in anaerobic food waste digesters: Effects of co-substrate and stabilization strategy. Bioresour. Technol. 2019, 288, 121594. [Google Scholar] [CrossRef] [PubMed]
- Vítěz, T.; Koutný, T.; Šotnar, M.; Chovanec, J. On the Spent Coffee Grounds Biogas Production. Acta Univ. Agric. et Silvic. Mendel. Brun. 2016, 64, 1279–1282. [Google Scholar] [CrossRef]
- Vavilin, V.; Angelidaki, I. Anaerobic degradation of solid material: Importance of initiation centers for methanogenesis, mixing intensity, and 2D distributed model. Biotechnol. Bioeng. 2004, 89, 113–122. [Google Scholar] [CrossRef]
- Dalkılıc, K.; Ugurlu, A. Biogas production from chicken manure at different organic loading rates in a mesophilic-thermopilic two stage anaerobic system. J. Biosci. Bioeng. 2015, 120, 315–322. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Experimental and kinetic study on anaerobic digestion of food waste: The effect of total solids and pH. J. Renew. Sustain. Energy 2015, 7, 063104. [Google Scholar] [CrossRef]
- Elagroudy, S.; Radwan, A.; Banadda, N.; Mostafa, N.G.; Owusu, P.; Janajreh, I. Mathematical models comparison of biogas production from anaerobic digestion of microwave pretreated mixed sludge. Renew. Energy 2020, 155, 1009–1020. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.-Y.; Qiao, W.; Wang, X.; Takayanagi, K. Sulfate addition as an effective method to improve methane fermentation performance and propionate degradation in thermophilic anaerobic co-digestion of coffee grounds, milk and waste activated sludge with AnMBR. Bioresour. Technol. 2015, 185, 308–315. [Google Scholar] [CrossRef]
- Zhong, B.; An, X.; Shen, F.; An, W.; Zhang, Q. Anaerobic Co-digestion of Rice Straw and Pig Manure Pretreated With a Cellulolytic Microflora: Methane Yield Evaluation and Kinetics Analysis. Front. Bioeng. Biotechnol. 2021, 8, 579405. [Google Scholar] [CrossRef]
- Mohammadianroshanfekr, M.; Pazoki, M.; Pejman, M.B.; Ghasemzadeh, R.; Pazoki, A. Kinetic modeling and optimization of biogas production from food waste and cow manure co-digestion. Results Eng. 2024, 24, 103477. [Google Scholar] [CrossRef]
- Ejimofor, M.I.; Ezemagu, I.G.; Menkiti, M.C. Biogas production using coagulation sludge obtained from paint wastewater decontamination: Characterization and anaerobic digestion kinetics. Curr. Res. Green Sustain. Chem. 2020, 3, 100024. [Google Scholar] [CrossRef]


| Parameter | Unit | SCG | OFMSW | AS | Inoculum |
|---|---|---|---|---|---|
| Total solid “TS” | g/L | 13.26 ± 1.20 | 17.84 ± 3.92 | 4.12.1 ± 0.52 | 4.05 ± 0.23 |
| Volatile solid “VS” | g/L | 11.02 ± 0.86 | 13.54 ± 1.38 | 3.150 ± 0.30 | 3.00 ± 0.03 |
| Humidity “U” | % | 86.74 ± 8.90 | 82.16 ± 4.60 | 95.88 ± 4.08 | 96.0 ± 2.59 |
| Fixed carbon “F.C” | g/L | 1.81 ± 1.80 | 2.9 ± 1.03 | 0.02 ± 0.01 | 0.53 ± 0.13 |
| Ash | g/L | 0.43 ± 0.05 | 2.31 ± 0.80 | 0.95 ± 0.23 | 0.52 ± 0.09 |
| TCOD | g/L | 18.93 ± 2.46 | 15.35 ± 3.17 | 16.62 ± 1.60 | 14.5 ± 3.67 |
| SCOD | g/L | 4.78 ± 1.31 | 7.87 ± 0.20 | 3.87 ± 0.29 | 3.80 ± 1.22 |
| Ammonia nitrogen NH3-N | mg/L | 15.2 ± 3.09 | 3.20 ± 1.03 | 46 ± 4.34 | 20.24 ± 4.52 |
| Alkalinity | mg/CaCO3/L | 38.75 ± 4.79 | 1100 ± 16.34 | 230 ± 17.45 | 200 ± 10.58 |
| pH | 7.9 ± 1.22 | 6.06 ± 0.50 | 6.8 ± 0.4 | 7.2 ± 1 | |
| Cu | mg/L | 0.65 ± 0.11 | 0.03 ± 0.01 | 0.32 ± 0.13 | 0.27 ± 0.04 |
| Zn | mg/L | 3.26 ± 0.19 | 0.29 ± 0.01 | 6.38 ± 1.32 | 4.78 ± 1.23 |
| Al | mg/L | 1.54 ± 0.34 | 0.08 ± 0.02 | 76.3 ± 11.17 | 16.09 ± 2.53 |
| C/N | % | 25.18 ± 2.54 | 15.29 ± 1.61 | 13.05 ± 1.27 | 17.33 ± 3.07 |
| H | % | 7.35 ± 2.04 | 6.54 ± 0.33 | 5.481 ± 0.91 | 2.06 ± 0.75 |
| Test | Substrate (g) | VS/TS (%) | U (%) | C/N |
|---|---|---|---|---|
| 25:75 SCG: AS | 22 g SCG | 77.3 | 94.94 | 19.74 |
| 66 g AS | ||||
| 112 g inoculum | ||||
| 50:50 SCG: AS | 37.8 g SCG | 78.3 | 94.23 | 18.22 |
| 37.8 g AS | ||||
| 124.4 g inoculum | ||||
| 75:25 SCG: AS | 48.9 g SCG | 78.84 | 93.73 | 16.71 |
| 16.3 g AS | ||||
| 134.8 g inoculum | ||||
| 25:75 SCG: OFMSW | 10 g SCG | 74.17 | 93.46 | 17.54 |
| 30 g OFMSW | ||||
| 160 g inoculum | ||||
| 50:50 SCG: OFMSW | 21 g SCG | 75.42 | 93.58 | 18.78 |
| 21 g OFMSW | ||||
| 158 g inoculum | ||||
| 75:25 SCG: OFMSW | 33 g SCG | 76.87 | 93.71 | 20.02 |
| 11 g OFMSW | ||||
| 156 g inoculum |
| Control-AS | Control-OFMSW | ||||||
|---|---|---|---|---|---|---|---|
| M.Gempertz | Logistic | First-Order | M.Gempertz | Logistic | First-Order | ||
| SSE | 0.00372324 | 0.00515 | 0.0082598 | SSE | 0.005307 | 0.00834 | 0.0069553 |
| R2 | 0.985301036 | 0.97963 | 0.9673910 | R2 | 0.978764 | 0.96660 | 0.9721720 |
| AdjR2 | 0.984212224 | 0.97890 | 0.9662264 | AdjR2 | 0.977191 | 0.96541 | 0.9711782 |
| RMSE | 0.011743 | 0.01357 | 0.017175 | RMSE | 0.014021 | 0.01726 | 0.015761 |
| AS25 | OFMS25 | ||||||
| M.Gempertz | Logistic | First-Order | M.Gempertz | Logistic | First-Order | ||
| SSE | 0.011617 | 0.01688 | 0.019899 | SSE | 0.016595 | 0.02248 | 0.0257040 |
| R2 | 0.973175 | 0.96101 | 0.954053 | R2 | 0.959086 | 0.94456 | 0.936631 |
| AdjR2 | 0.971188 | 0.95962 | 0.954053 | AdjR2 | 0.957625 | 0.94258 | 0.936631 |
| RMSE | 0.020743 | 0.02455 | 0.026195 | RMSE | 0.024345 | 0.02833 | 0.029772 |
| AS50 | OFMS50 | ||||||
| M.Gempertz | Logistic | First-Order | M.Gempertz | Logistic | First-Order | ||
| SSE | 0.007336 | 0.01104 | 0.011045 | SSE | 0.004913 | 0.00649 | 0.008294 |
| R2 | 0.976479 | 0.96458 | 0.964588 | R2 | 0.977555 | 0.97034 | 0.962112 |
| AdjR2 | 0.974737 | 0.96332 | 0.963323 | AdjR2 | 0.976753 | 0.96928 | 0.960759 |
| RMSE | 0.016483 | 0.01986 | 0.019861 | RMSE | 0.013247 | 0.01522 | 0.017211 |
| AS75 | OFMS75 | ||||||
| M.Gempertz | Logistic | First-Order | M.Gempertz | Logistic | First-Order | ||
| SSE | 0.003117 | 0.00477 | 0.0044705 | SSE | 0.0007297 | 0.00119 | 0.0015854 |
| R2 | 0.981315 | 0.97139 | 0.973203 | R2 | 0.986512 | 0.97792 | 0.970696 |
| AdjR2 | 0.980648 | 0.97037 | 0.972246 | AdjR2 | 0.985513 | 0.97714 | 0.969649 |
| RMSE | 0.010551 | 0.01305 | 0.012636 | RMSE | 0.005199 | 0.00653 | 0.007525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al bkoor Alrawashdeh, K.; Al-Samrraie, L.A.; Damseh, R.A.; Al Bsoul, A.; Gul, E. Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study. Fermentation 2025, 11, 295. https://doi.org/10.3390/fermentation11060295
Al bkoor Alrawashdeh K, Al-Samrraie LA, Damseh RA, Al Bsoul A, Gul E. Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study. Fermentation. 2025; 11(6):295. https://doi.org/10.3390/fermentation11060295
Chicago/Turabian StyleAl bkoor Alrawashdeh, Khalideh, La’aly A. Al-Samrraie, Rebhi A. Damseh, Abeer Al Bsoul, and Eid Gul. 2025. "Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study" Fermentation 11, no. 6: 295. https://doi.org/10.3390/fermentation11060295
APA StyleAl bkoor Alrawashdeh, K., Al-Samrraie, L. A., Damseh, R. A., Al Bsoul, A., & Gul, E. (2025). Improving Biogas Production and Organic Matter Degradation in Anaerobic Co-Digestion Using Spent Coffee Grounds: A Kinetic and Operational Study. Fermentation, 11(6), 295. https://doi.org/10.3390/fermentation11060295






