Improving Nutrition Facts of Cassava and Soybean Residue Through Solid-State Fermentation by Pleurotus ostreatus Mycelium: A Pathway to Safety Animal Feed Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Sources and Baterial Strains
2.2. Preparation of P. ostreatus Colonies in the Liquid Medium
2.3. Solid-State Fermentation of Cassava and Soybean Residue with P. ostreatus Mycelium
2.4. Extraction and Quantification of PS
2.5. Assessment of the Impact of PS on the Growth of Gut Probiotics
2.6. Calculation of Prebiotic Index
2.7. Assessment of Mycelial Biomass Quality
2.8. Data Analysis
3. Results and Discussion
3.1. Impact of the Cultivation Substrate on the Growth of P. ostreatus Mycelium
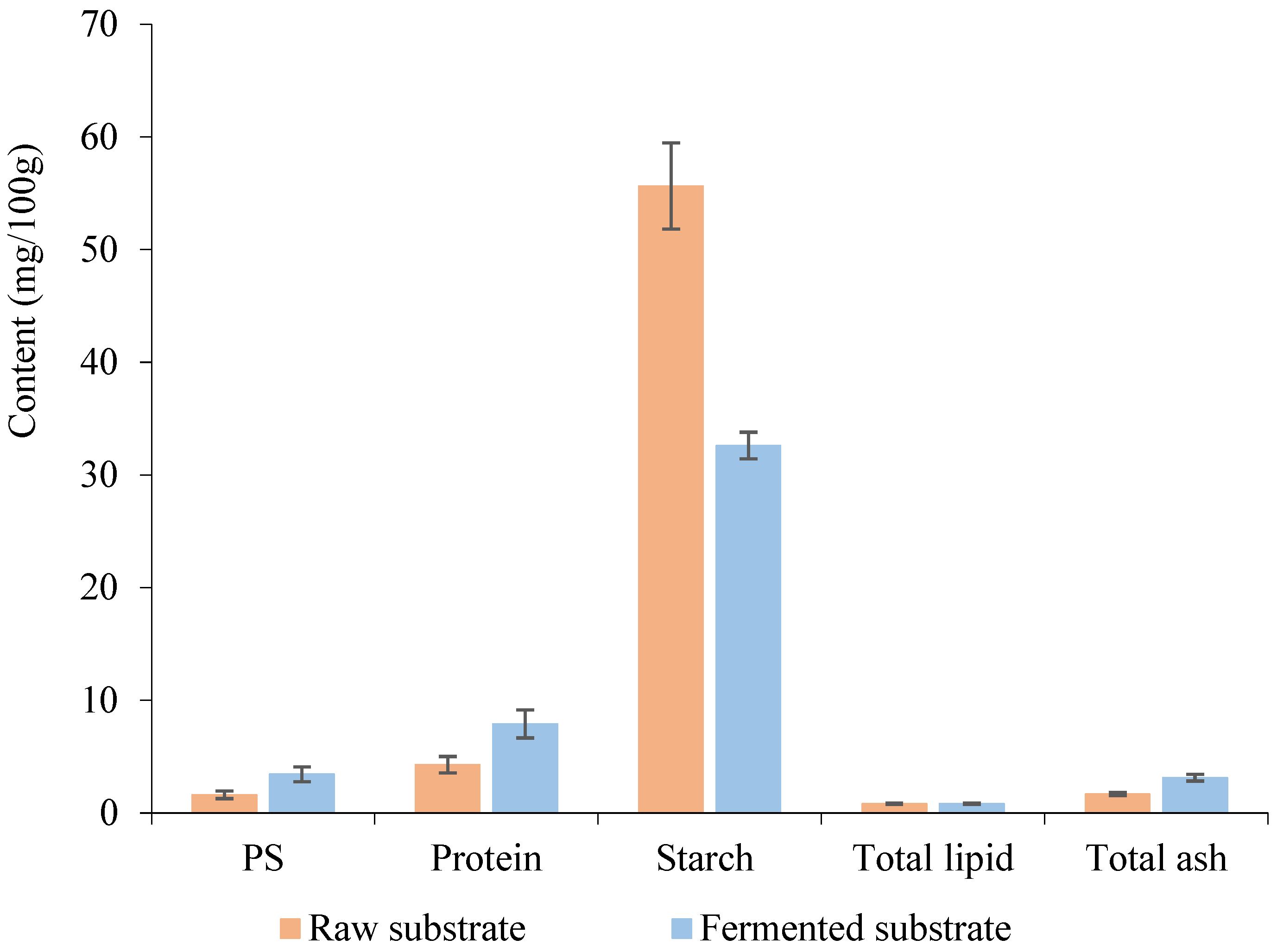
3.2. Nutrients Profile of Fermented Substrate After SSF by P. ostreatus Mycelium
3.3. Prebiotic Activity of PS After Extraction
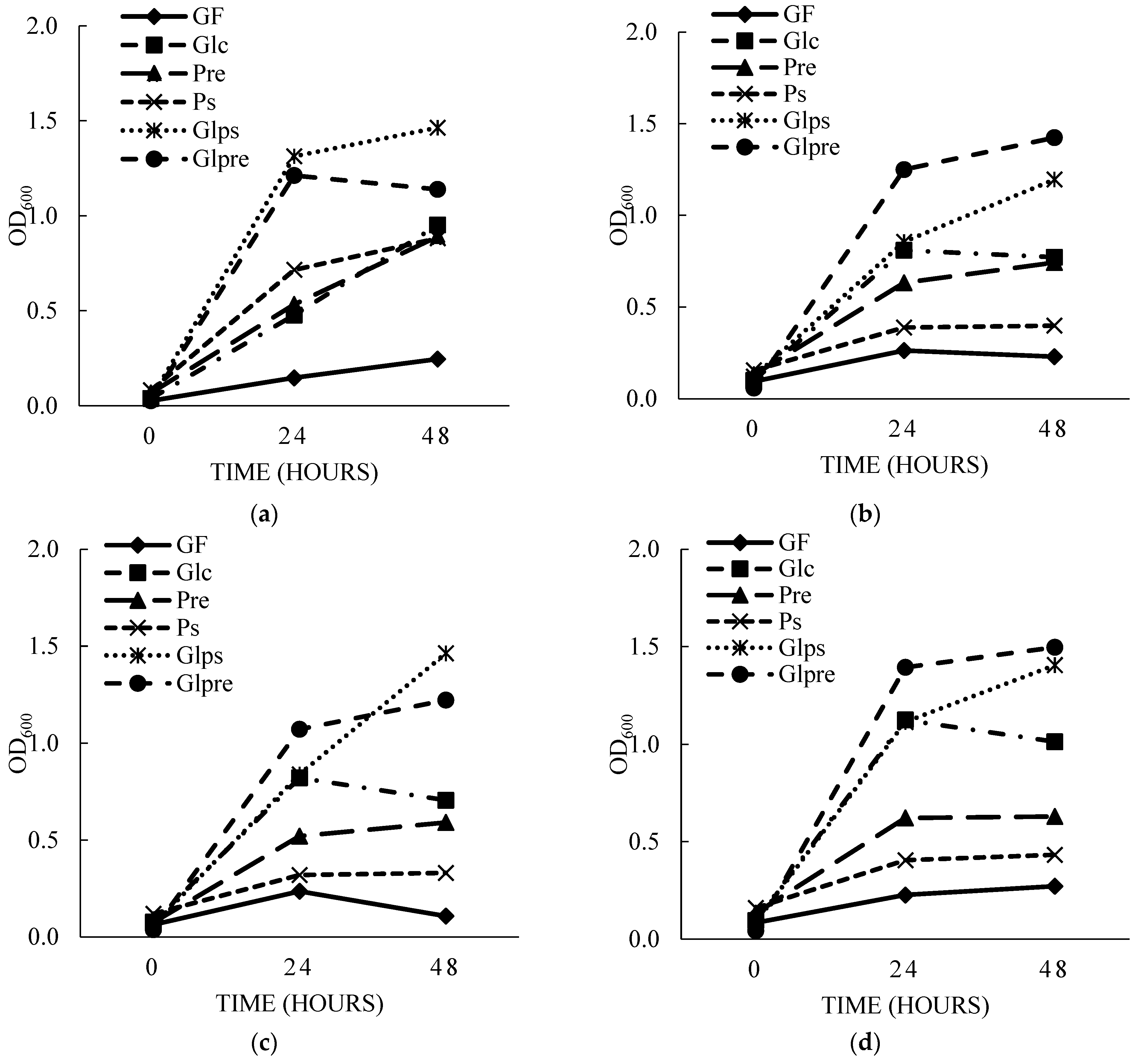
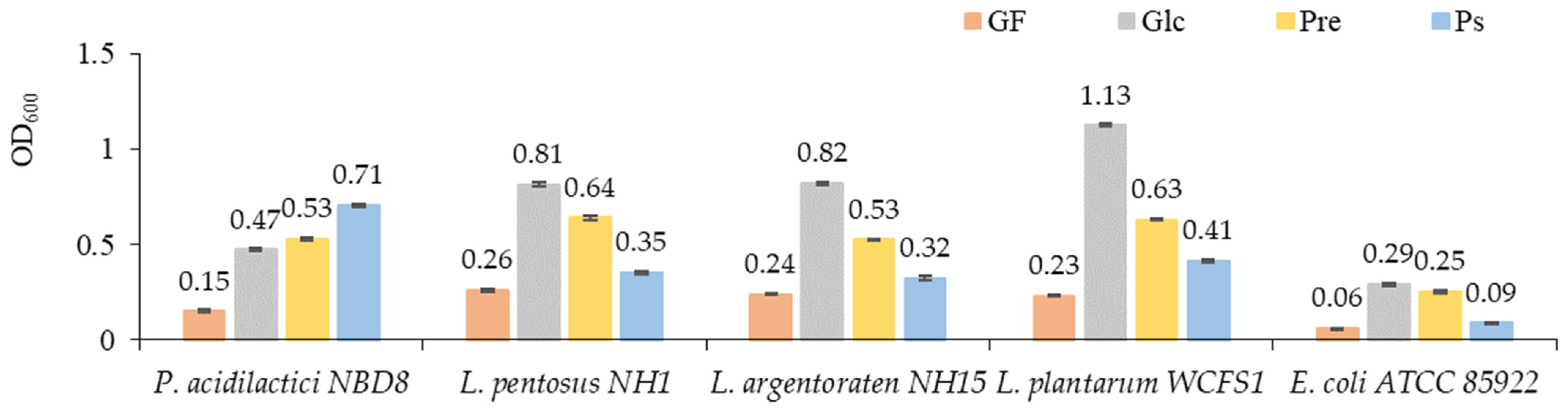
3.3.1. The Impact of PS on the Growth of Beneficial Gut Probiotics
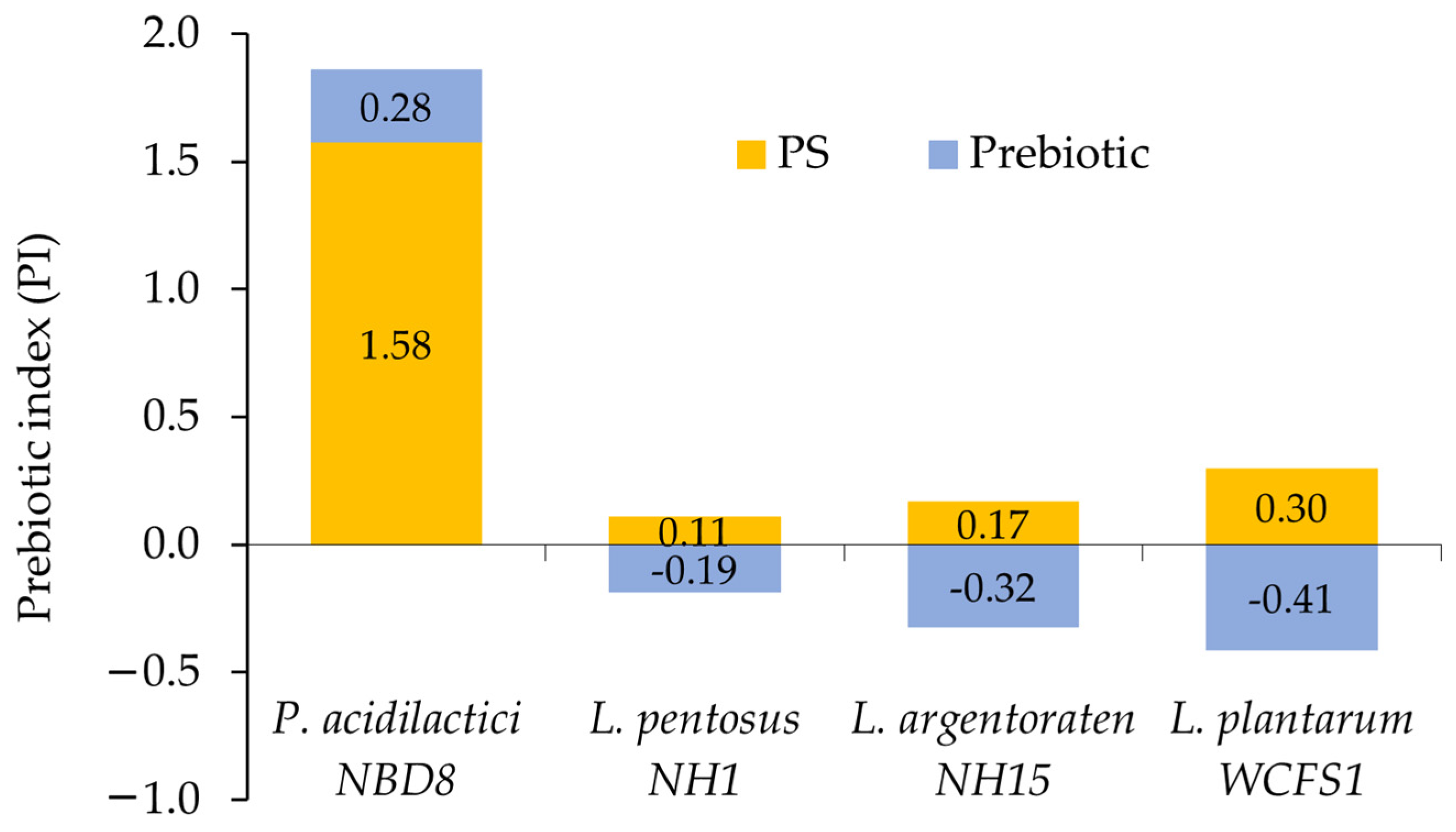
3.3.2. Prebiotic Index (PI)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvanitoyannis, I.S.; Kassaveti, A.; Ladas, D. Chapter 6. Food Waste Treatment Methodologies. In Waste Management for the Food Industries; Arvanitoyannis, I.S., Ed.; Academic Press: Amsterdam, The Netherlands, 2008; pp. 345–410. ISBN 978-0-12-373654-3. [Google Scholar]
- Parmar, A.B.; Patel, V.; Usadadia, S.; Rathwa, S.; Prajapati, D. A Solid State Fermentation, Its Role in Animal Nutrition: A Review. Int. J. Chem. Stud. 2019, 7, 4626–4633. [Google Scholar]
- Mitchell, D.A.; de Lima Luz, L.F.; Krieger, N.; Berovič, M. 2.25—Bioreactors for Solid-State Fermentation. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon Press: Oxford, UK, 2011; pp. 347–360. ISBN 978-0-08-088504-9. [Google Scholar]
- Feng, X.; Ng, K.; Ajlouni, S.; Zhang, P.; Fang, Z. Effect of Solid-State Fermentation on Plant- Sourced Proteins: A Review Effect of Solid-State Fermentation on Plant-Sourced Proteins: A Review. Food Rev. Int. 2023, 40, 2580–2617. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-State Fermentation for Enhancing the Nutraceutical Content of Agrifood by-Products: Recent Advances and Its Industrial Feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in Research on Solid-State Fermented Feed and Its Utilization: The Pioneer of Private Customization for Intestinal Microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-State Fermentation Technology and Innovation for the Production of Agricultural and Animal Feed Bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Rothmann, C.; Rothmann, L.; Viljoen, B.; Cason, E.D. Application of Solid-State Fermentation Using Mushrooms for the Production of Animal Feed. J. Basic. Microbiol. 2023, 63, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Farinas, C.S. Developments in Solid-State Fermentation for the Production of Biomass-Degrading Enzymes for the Bioenergy Sector. Renew. Sustain. Energy Rev. 2015, 52, 179–188. [Google Scholar] [CrossRef]
- Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production. Fuels 2022, 3, 730–752. [Google Scholar] [CrossRef]
- Khot, M.B. Solid-State Fermentation: An Alternative Approach to Produce Fungal Lipids as Biodiesel Feedstock BT. In Status and Future Challenges for Non-Conventional Energy Sources Volume 2; Joshi, S.J., Sen, R., Sharma, A., Salam, P.A., Eds.; Springer Nature: Singapore, 2022; pp. 123–137. ISBN 978-981-16-4509-9. [Google Scholar]
- Karimi, F.; Mazaheri, D.; Saei Moghaddam, M.; Mataei Moghaddam, A.; Sanati, A.L.; Orooji, Y. Solid-State Fermentation as an Alternative Technology for Cost-Effective Production of Bioethanol as Useful Renewable Energy: A Review. Biomass Convers. Biorefin 2021. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; De Jesús Cázares-Marinero, J. Biosurfactants: The Green Generation of Speciality Chemicals and Potential Production Using Solid-State Fermentation (SSF) Technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Faria, D.J.; Carvalho, A.P.; Conte-Junior, C.A. Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications. Fermentation 2023, 9, 920. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A Review on Valorization of Spent Coffee Grounds (SCG) towards Biopolymers and Biocatalysts Production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid State Fermentation (SSF): Diversity of Applications to Valorize Waste and Biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Couto, S.R.; Gundín, M.; Lorenzo, M.; Sanromán, M.Á. Screening of Supports and Inducers for Laccase Production by Trametes Versicolor in Semi-Solid-State Conditions. Process Biochem. 2002, 38, 249–255. [Google Scholar] [CrossRef]
- Tsutsui, T.; Hayashi, N.; Maizumi, H.; Huff, J.; Barrett, J.C. Benzene-, Catechol-, Hydroquinone- and Phenol-Induced Cell Transformation, Gene Mutations, Chromosome Aberrations, Aneuploidy, Sister Chromatid Exchanges and Unscheduled DNA Synthesis in Syrian Hamster Embryo Cells. Mutat. Res. 1997, 373, 113–123. [Google Scholar] [CrossRef]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.S.; Hofrichter, M.; Rogalski, J. Biodegradation of Lignin by White Rot Fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Son, N.H.; Lan, B.T.P.; Minh, N.D. The Current Status of Agricultural Wastes and Residuals Management and Recycling in Vietnam. In Practice and Benefits of Circular Agriculture in Waste Reducing and Recycling; Food and Fertilizer Technology Center: Taipei, Taiwan, 2021. [Google Scholar]
- Razavizadeh, S.; Alencikiene, G.; Salaseviciene, A.; Vaiciulyte-Funk, L.; Ertbjerg, P.; Zabulione, A. Impact of Fermentation of Okara on Physicochemical, Techno-Functional, and Sensory Properties of Meat Analogues. Eur. Food Res. Technol. 2021, 247, 2379–2389. [Google Scholar] [CrossRef]
- Sengupta, S.; Chakraborty, M.; Bhowal, J.; Bhattacharya, D. Study on the Effects of Drying Process on the Composition and Quality of Wet Okara. Int. J. Sci. Environ. Technol. 2012, 1, 319–330. [Google Scholar]
- Toda, K.; Chiba, K.; Ono, T. Effect of Components Extracted from Okara on the Physicochemical Properties of Soymilk and Tofu Texture. J. Food Sci. 2007, 72, C108–C113. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, M.; Lu, F. Composition, Nutrition, and Utilization of Okara (Soybean Residue). Food Rev. Int. 2012, 28, 231–252. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yu, O.-K.; Byun, M.-S.; Cha, Y.-S. Okara, a Soybean by-Product, Prevents High Fat Diet-Induced Obesity and Improves Serum Lipid Profiles in C57BL/6J Mice. Food Sci. Biotechnol. 2016, 25, 607–613. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Shah, Y.A.; Islam, F.; Hussain, M.; Akram, N.; Shah, M.A. Valorization and Food Applications of Okara (Soybean Residue): A Concurrent Review. Food Sci. Nutr. 2023, 11, 3631–3640. [Google Scholar] [CrossRef]
- Yimin, C.; Napasirth, V.; Napasirth, P.; Sulinthone, T.; Phommachanh, K. Microbial Population, Chemical Composition and Silage Fermentation of Cassava Residues. Anim. Sci. J. 2015, 86, 842–848. [Google Scholar] [CrossRef]
- Fanelli, N.S.; Torres-Mendoza, L.J.; Abelilla, J.J.; Stein, H.H. Chemical Composition of Cassava-Based Feed Ingredients from South-East Asia. Anim. Biosci. 2023, 36, 908–919. [Google Scholar] [CrossRef]
- Okrathok, S.; Thumanu, K.; Pukkung, C.; Molee, W.; Khempaka, S. Extraction of Dietary Fibers from Cassava Pulp and Cassava Distiller’s Dried Grains and Assessment of Their Components Using Fourier Transform Infrared Spectroscopy to Determine Their Further Use as a Functional Feed in Animal Diets. Anim. Biosci. 2022, 35, 1048–1058. [Google Scholar] [CrossRef]
- Dang, P.K.; Huong, D.T. Protein Enrichment of Cassava Residues by Simultaneous Saccharification and Fermentation. Vietnam. J. Agric. Sci. 2018, 16, 207–214. [Google Scholar]
- Zhu, J.; Tan, W.K.; Song, X.; Gao, Z.; Wen, Y.; Ong, C.N.; Loh, C.S.; Swarup, S.; Li, J. Converting Okara to Superabsorbent Hydrogels as Soil Supplements for Enhancing the Growth of Choy Sum (Brassica sp.) under Water-Limited Conditions. ACS Sustain. Chem. Eng. 2020, 8, 9425–9433. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Oktaviani, N.; Sarwono, K.A.; Utama, G.I. Bioconversion Rice Bran and Cassava Peel into Yeasts Cell Walls Mannoprotein as Environmental Friendly Antioxidant. E3S Web Conf. 2021, 249, 3004. [Google Scholar] [CrossRef]
- Suriyapha, C.; Supapong, C.; So, S.; Wanapat, M.; Cherdthong, A. Bioconversion of Agro-Industrial Residues as a Protein Source Supplementation for Multiparous Holstein Thai Crossbreed Cows. PLoS ONE 2022, 17, e0273916. [Google Scholar] [CrossRef]
- Verardi, A.; Sangiorgio, P.; Blasi, A.; Lopresto, C.G.; Calabrò, V. Bioconversion of Crop Residues Using Alternative Fermentation-Based Approaches. Front. Biosci. 2023, 15, 17. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Adnane, I.; Taoumi, H.; Elouahabi, K.; Lahrech, K.; Oulmekki, A. Valorization of Crop Residues and Animal Wastes: Anaerobic Co-Digestion Technology. Heliyon 2024, 10, e26440. [Google Scholar] [CrossRef] [PubMed]
- Cruz, I.A.; Santos Andrade, L.R.; Bharagava, R.N.; Nadda, A.K.; Bilal, M.; Figueiredo, R.T.; Romanholo Ferreira, L.F. Valorization of Cassava Residues for Biogas Production in Brazil Based on the Circular Economy: An Updated and Comprehensive Review. Clean. Eng. Technol. 2021, 4, 100196. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Sankaran, M. Pleurotus ostreatus: An Oyster Mushroom with Nutritional and Medicinal Properties. J. Biochem. Technol. 2014, 5, 718–726. [Google Scholar]
- Murphy, E.J.; Rezoagli, E.; Pogue, R.; Simonassi-Paiva, B.; Abidin, I.I.Z.; Fehrenbach, G.W.; O’neil, E.; Major, I.; Laffey, J.G.; Rowan, N. Immunomodulatory Activity of β-Glucan Polysaccharides Isolated from Different Species of Mushroom—A Potential Treatment for Inflammatory Lung Conditions. Sci. Total Environ. 2022, 809, 152177. [Google Scholar] [CrossRef]
- Yang, B.-K.; Gu, Y.-A.; Jeong, Y.-T.; Song, C.-H. Anti-Complementary Activities of Exo- and Endo-Biopolymer Produced by Submerged Mycelial Culture of Eight Different Mushrooms. Mycobiology 2007, 35, 145–149. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Phirom-On, K.; Apiraksakorn, J. Development of Cellulose-Based Prebiotic Fiber from Banana Peel by Enzymatic Hydrolysis. Food Biosci. 2021, 41, 101083. [Google Scholar] [CrossRef]
- Elbing, K.L.; Brent, R. Recipes and Tools for Culture of Escherichia coli. Curr. Protoc. Mol. Biol. 2019, 125, e83. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.L.; Parkhurst, A.; Hutkins, R.W. Effect of Processing Conditions on the Prebiotic Activity of Commercial Prebiotics. Int. Dairy J. 2008, 18, 287–293. [Google Scholar] [CrossRef]
- ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. International Standard Organization: Geneva, Switzerland, 2009.
- ISO 6492:1999; Animal Feeding Stuffs—Determination of Fat Content. International Standard Organization: Geneva, Switzerland, 1999.
- ISO 5984:2022; Animal Feeding Stuffs—Determination of Crude Ash. International Standard Organization: Geneva, Switzerland, 2022.
- Nair, M.P.; Padmaja, G.; Sankarakutty, S.; Sheriff, T. Bioconversion of Cellulo-Starch Waste from Cassava Starch Industries for Ethanol Production: Pretreatment Techniques and Improved Enzyme Systems. Ind. Biotechnol. 2012, 8, 300–308. [Google Scholar] [CrossRef]
- O’Toole, D.K. Characteristics and Use of Okara, the Soybean Residue from Soy Milk Production—A Review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Adenipekun, C.O.; Jonathan, G. Nutritional Requirements of Pleurotus florida (Mont.) Singer, A Nigerian Mushroom. Pak. J. Nutr. 2006, 5, 597–600. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Li, F.; Liu, P. Active Polysaccharides from Lentinula edodes and Pleurotus ostreatus by Addition of Corn Straw and Xylosma Sawdust through Solid-State Fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef]
- Heidari, F.; Øverland, M.; Hansen, J.Ø.; Mydland, L.T.; Urriola, P.E.; Chen, C.; Shurson, G.C.; Hu, B. Solid-State Fermentation of Pleurotus ostreatus to Improve the Nutritional Profile of Mechanically-Fractionated Canola Meal. Biochem. Eng. J. 2022, 187, 108591. [Google Scholar] [CrossRef]
- Tolera, K.D.; Abera, S. Nutritional Quality of Oyster Mushroom (Pleurotus ostreatus) as Affected by Osmotic Pretreatments and Drying Methods. Food Sci. Nutr. 2017, 5, 989–996. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Brennan, M.; Brennan, C. An Insight into the Mechanism of Interactions between Mushroom Polysaccharides and Starch. Curr. Opin. Food Sci. 2021, 37, 17–25. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of Probiotics, Prebiotics, and Prebiotic-like Components in Common Functional Foods. Compr. Rev. Food Sci. Food safety 2020, 19, 1908–1933. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, F.C.; De Barros Ranke, F.F.; De Oliva-Neto, P. Evaluation of Xylooligosaccharides and Fructooligosaccharides on Digestive Enzymes Hydrolysis and as a Nutrient for Different Probiotics and Salmonella typhimurium. LWT 2020, 118, 108761. [Google Scholar] [CrossRef]
- Hang, N.T.B.; Cuong, D.C.; Nhat, D.M.; Thang, D.B. Prebiotic Properties of Polysaccharides Isolated from Cordyceps Militaris Mycelia. Vietnam. Trade Ind. Rev. 2023, 4, 413–420. [Google Scholar]
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a Potential Source of Prebiotics: A Review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from Fruit Bodies of Cultivated Mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and Potential Prebiotic Activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.L.; Hutkins, R.W. Functional Activity of Commercial Prebiotics. Int. Dairy. J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T. Functional and Comparative Genomic Analyses of an Operon Involved in Fructooligosaccharide Utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef]

| Treatments 1 | Cassava Residue (%) | Soybean Residue (%) |
|---|---|---|
| CT1 (n = 15) | 100 | 0 |
| CT2 (n = 15) | 90 | 10 |
| CT3 (n = 15) | 80 | 20 |
| CT4 (n = 15) | 70 | 30 |
| CT5 (n = 15) | 60 | 40 |
| Treatments | Culture Time (Days) | Characteristics of Mycelium | ||||
|---|---|---|---|---|---|---|
| 1st | 3rd | 5th | 7th | 9th | ||
| CT1 (n = 15) | 1.77 ± 0.15 a | 19.37 ± 1.32 a | 51.8 ± 3.93 a | 110.62 ± 3.45 a | 120 ± 0 a | Fine, low density |
| CT2 (n = 15) | 0.91 ± 0.05 b | 10.26 ± 1.42 c | 33.99 ± 2.04 b | 83.18 ± 3.59 b | 120 ± 0 a | Fine, evenly white, low density |
| CT3 (n = 15) | 1.73 ± 0.17 a | 13.32 ± 1.04 b | 34.56 ± 2.41 b | 82.51 ± 3.66 b | 120 ± 0 a | Thick, evenly white, high density |
| CT4 (n = 15) | 0.97 ± 0.03 b | 6.47 ± 0.81 d | 24.01 ± 1.68 c | 60.06 ± 2.99 c | 108.07 ± 3.85 b | Thick, fluffy, evenly white, high density |
| CT5 (n = 15) | 0.91 ± 0.03 b | 2.69 ± 0.53 e | 11.56 ± 1.29 d | 41.61 ± 2.35 d | 84.29 ± 3.52 c | Thick, fluffy, high density |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, N.T.B.; Doan, C.C. Improving Nutrition Facts of Cassava and Soybean Residue Through Solid-State Fermentation by Pleurotus ostreatus Mycelium: A Pathway to Safety Animal Feed Production. Fermentation 2025, 11, 271. https://doi.org/10.3390/fermentation11050271
Hang NTB, Doan CC. Improving Nutrition Facts of Cassava and Soybean Residue Through Solid-State Fermentation by Pleurotus ostreatus Mycelium: A Pathway to Safety Animal Feed Production. Fermentation. 2025; 11(5):271. https://doi.org/10.3390/fermentation11050271
Chicago/Turabian StyleHang, Nguyen Thi Bich, and Chi Cuong Doan. 2025. "Improving Nutrition Facts of Cassava and Soybean Residue Through Solid-State Fermentation by Pleurotus ostreatus Mycelium: A Pathway to Safety Animal Feed Production" Fermentation 11, no. 5: 271. https://doi.org/10.3390/fermentation11050271
APA StyleHang, N. T. B., & Doan, C. C. (2025). Improving Nutrition Facts of Cassava and Soybean Residue Through Solid-State Fermentation by Pleurotus ostreatus Mycelium: A Pathway to Safety Animal Feed Production. Fermentation, 11(5), 271. https://doi.org/10.3390/fermentation11050271






