Enhancing Kefir with Raspberry Pomace: Storage-Dependent Changes in Quality and Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kefir Production
2.2. Physicochemical Characterization
2.3. Microbiological Analysis
2.4. Determination of Antioxidant Capacity and Polyphenol Content of Kefir Samples
2.4.1. Extract Preparation
2.4.2. Antioxidant Activity
2.4.3. Total Phenolic Content
2.5. HPLC Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Antioxidative Activity and Polyphenol Content in Kefir Samples
3.3. Enumeration of Viable Bacteria
3.4. HPLC Analysis
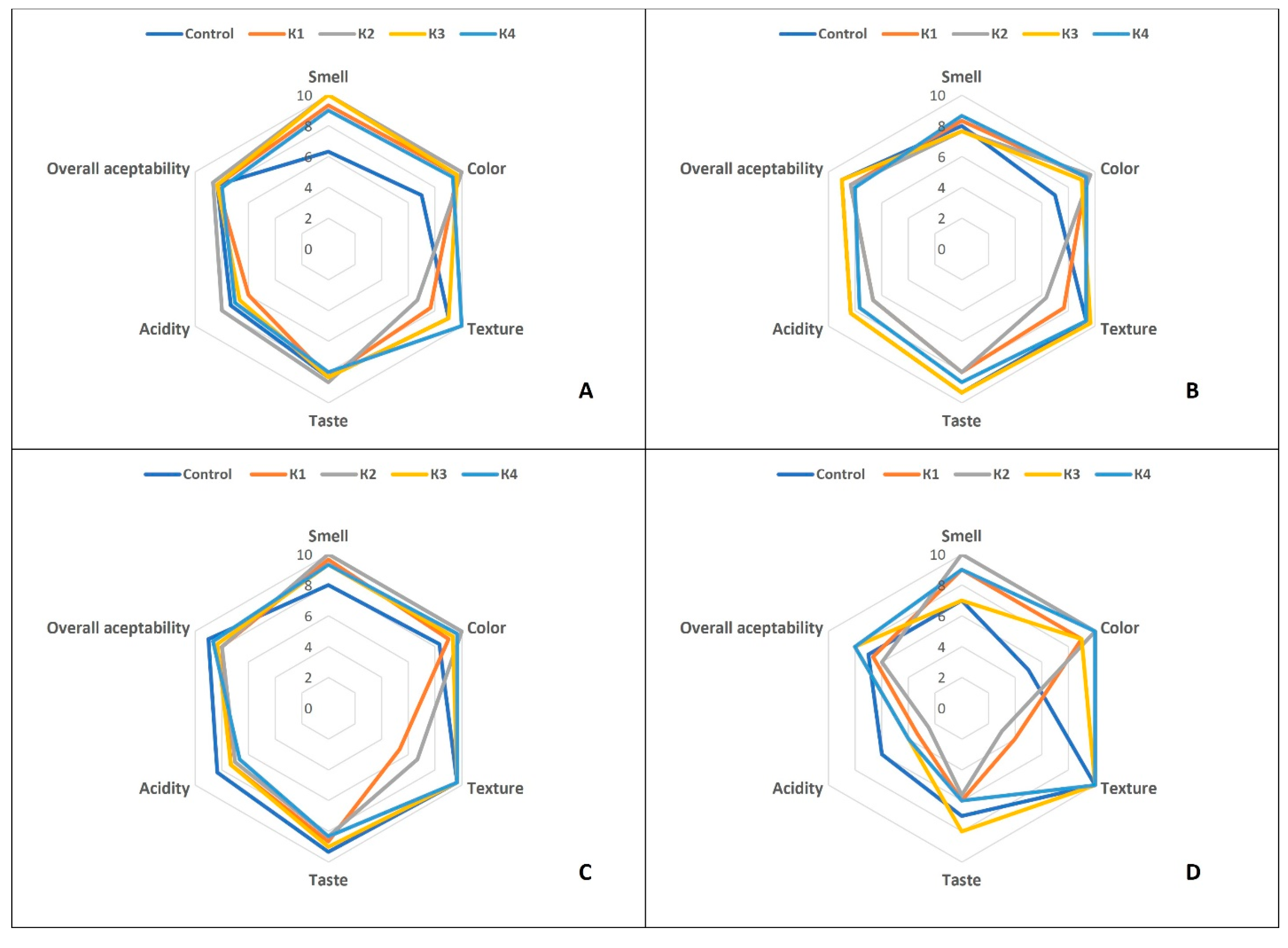
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High Performance Liquid Chromatography |
| LAB | Lactic Acid Bacteria |
| MRS | De Man, Rogosa, and Sharpe Medium |
| SMA | Sabouraud Maltose agar |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
References
- Liu, J.R.; Chen, M.J.; Lin, C.W. Antimutagenic and Antioxidant Properties of Milk-Kefir and Soymilk-Kefir. J. Agric. Food Chem. 2005, 53, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Hussain, M.; Raza, A.; Rasheed, A.; Asghar, A.; Asif Shah, M. Nutritional and Functional Properties of Kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A Comparison of Milk Kefir and Water Kefir: Physical, Chemical, Microbiological and Functional Properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, Physicochemical, and Sensory Characteristics of Kefir during Storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Yilmaz, L.; Özcan Yilsay, T.; Akpinar Bayizit, A. The Sensory Characteristics of Berry-Flavoured Kefir. Czech J. Food Sci. 2006, 24, 26–32. [Google Scholar] [CrossRef]
- Kabakcı, S.A.; Türkyılmaz, M.; Özkan, M. Changes in the Quality of Kefir Fortified with Anthocyanin-Rich Juices during Storage. Food Chem. 2020, 326, 126977. [Google Scholar] [CrossRef]
- Travičić, V.; Šovljanski, O.; Tomić, A.; Perović, M.; Milošević, M.; Ćetković, N.; Antov, M. Augmenting Functional and Sensorial Quality Attributes of Kefir through Fortification with Encapsulated Blackberry Juice. Foods 2023, 12, 4163. [Google Scholar] [CrossRef]
- Aiello, F.; Restuccia, D.; Spizzirri, U.G.; Carullo, G.; Leporini, M.; Loizzo, M.R. Improving Kefir Bioactive Properties by Functional Enrichment with Plant and Agro-Food Waste Extracts. Fermentation 2020, 6, 83. [Google Scholar] [CrossRef]
- Vimercati, W.C.; da Silva Araújo, C.; Macedo, L.L.; Fonseca, H.C.; Guimarães, J.S.; de Abreu, L.R.; Pinto, S.M. Physicochemical, Rheological, Microbiological and Sensory Properties of Newly Developed Coffee Flavored Kefir. LWT 2020, 123, 109069. [Google Scholar] [CrossRef]
- Raczkowska, E.; Serek, P. Health-Promoting Properties and the Use of Fruit Pomace in the Food Industry—A Review. Nutrients 2024, 16, 2757. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry Pomace—A Review of Processing and Chemical Analysis of Its Polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Gunenc, A.; Fang, S.; Hosseinian, F. Raspberry and Strawberry Addition Improves Probiotic Viability in Yogurt and Possess Antioxidant Activity. J. Food Res. 2015, 4, 47. [Google Scholar] [CrossRef]
- Pecyna, A.; Krzywicka, M.; Blicharz-Kania, A.; Buczaj, A.; Kobus, Z.; Zdybel, B.; Domin, M.; Siłuch, D. Impact of Incorporating Two Types of Dried Raspberry Pomace into Gluten-Free Bread on Its Nutritional and Antioxidant Characteristics. Appl. Sci. 2024, 14, 1561. [Google Scholar] [CrossRef]
- McDougall, N.R.; Beames, R.M. Composition of Raspberry Pomace and Its Nutritive Value for Monogastric Animals. Anim. Feed. Sci. Technol. 1994, 45, 139–148. [Google Scholar] [CrossRef]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of Raspberry and Blueberry Pomace through the Formulation of Value-Added Gluten-Free Cookies. J. Food Sci. Technol. 2015, 53, 1140. [Google Scholar] [CrossRef]
- Szymanowska, U.; Karaś, M.; Jakubczyk, A.; Kocki, J.; Szymanowski, R.; Kapusta, I.T. Raspberry Pomace as a Good Additive to Apple Freeze-Dried Fruit Bars: Biological Properties and Sensory Evaluation. Molecules 2024, 29, 5690. [Google Scholar] [CrossRef]
- AOAC: Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Washington, VA, USA, 1990.
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of Antioxidant Capacity of Cow and Ewe Milk Kefirs. J. Dairy Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef]
- Malićanin, M.; Karabegović, I.; Đorđević, N.; Mančić, S.; Stojanović, S.S.; Brković, D.; Danilović, B. Influence of the Extraction Method on the Biological Potential of Solidago virgaurea L. Essential Oil and Hydrolates. Plants 2024, 13, 2187. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Leite, D.C.A.; Del Aguila, E.M.; Alvares, T.S.; Peixoto, R.S.; Miguel, M.A.L.; Silva, J.T.; Paschoalin, V.M.F. Microbiological and Chemical Characteristics of Brazilian Kefir during Fermentation and Storage Processes. J. Dairy. Sci. 2013, 96, 4149–4159. [Google Scholar] [CrossRef]
- Mančić, S.; Danilović, B.; Malićanin, M.; Stojanović, S.S.; Nikolić, N.; Lazić, M.; Karabegović, I. Fermentative Potential of Native Yeast Candida Famata for Prokupac Grape Must Fermentation. Agriculture 2021, 11, 358. [Google Scholar] [CrossRef]
- Karabegović, I.; Stamenković-Stojanović, S.; Lazić, M.; Đorđević, N.; Danilović, B. Antimicrobial Activity and Overall Sensory Acceptance of Fermented Goat Whey Beverage: Process Conditions Optimization Using Response Surface Approach. Adv. Technol. 2022, 11, 26–35. [Google Scholar] [CrossRef]
- Różyło, R.; Amarowicz, R.; Janiak, M.A.; Domin, M.; Gawłowski, S.; Kulig, R.; Łysiak, G.; Rząd, K.; Matwijczuk, A. Micronized Powder of Raspberry Pomace as a Source of Bioactive Compounds. Molecules 2023, 28, 4871. [Google Scholar] [CrossRef] [PubMed]
- Legarová, V.; Kouřimská, L. Sensory Quality Evaluation of Whey-Based Beverages. Mljekarstvo 2010, 60, 280–287. [Google Scholar]
- Avila-Reyes, S.V.; Márquez-Morales, C.E.; Moreno-León, G.R.; Jiménez-Aparicio, A.R.; Arenas-Ocampo, M.L.; Solorza-Feria, J.; García-Armenta, E.; Villalobos-Espinosa, J.C. Comparative Analysis of Fermentation Conditions on the Increase of Biomass and Morphology of Milk Kefir Grains. Appl. Sci. 2022, 12, 2459. [Google Scholar] [CrossRef]
- Atalar, I. Functional Kefir Production from High Pressure Homogenized Hazelnut Milk. LWT 2019, 107, 256–263. [Google Scholar] [CrossRef]
- Otles, S.; Cagindi, O. Kefir: A Probiotic Dairy-Composition, Nutritional and Therapeutic Aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar] [CrossRef]
- Putri, Y.D.; Setiani, N.A.; Warya, S. The Effect of Temperature, Incubation and Storage Time on Lactic Acid Content, PH and Viscosity of Goat Milk Kefir. Curr. Res. Biosci. Biotechnol. 2020, 2, 101–104. [Google Scholar] [CrossRef]
- M’Hir, S.; Mejri, A.; Atrous, H.; Ayed, L. Optimization of Parameters Using Response Surface Methodology to Develop a Novel Kefir-like Functional Beverage from Cheese Whey Enriched with Myrtle Juice. J. Chem. 2021, 2021, 10–21. [Google Scholar] [CrossRef]
- Aroua, M.; Ben Haj Koubaier, H.; Bouacida, S.; Ben Saïd, S.; Mahouachi, M.; Salimei, E. Chemical, Physicochemical, Microbiological, Bioactive, and Sensory Characteristics of Cow and Donkey Milk Kefir during Storage. Beverages 2023, 9, 2. [Google Scholar] [CrossRef]
- Bauza-Kaszewska, J.; Zary-Sikorska, E.; Gugolek, A.; Ligocka, A.; Kosmala, M.; Karlińska, E.; Fotschki, B.; Juśkiewicz, J. Synergistic Antimicrobial Effect of Raspberry (Rubus Idaeus L., Rosaceae) Preparations and Probiotic Bacteria on Enteric Pathogens. Pol. J. Food Nutr. Sci. 2021, 71, 51–59. [Google Scholar] [CrossRef]
- Delgado-Fernández, P.; Corzo, N.; Lizasoain, S.; Olano, A.; Moreno, F.J. Fermentative Properties of Starter Culture during Manufacture of Kefir with New Prebiotics Derived from Lactulose. Int. Dairy. J. 2019, 93, 22–29. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of Antioxidant Activity and Physicochemical Properties of Yogurt Enriched with Concentrated Strawberry Pulp Obtained by Block Freeze Concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Hwang, E.S. Quality Characteristics and Antioxidant Activity of Yogurt Supplemented with Aronia (Aronia melanocarpa) Juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef]
- Baniasadi, M.; Azizkhani, M.; Erik Joakim Saris, P.; Tooryan, F. Comparative Antioxidant Potential of Kefir and Yogurt of Bovine and Non-Bovine Origins. J. Food Sci. Technol. 2022, 59, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Buzás, H.; Kapcsándi, V.; Lakatos, E.; Daróczi, F.; Székelyhidi, R. Antioxidant and Polyphenol Content of Different Milk and Dairy Products. J. King Saud. Univ. Sci. 2023, 35, 102839. [Google Scholar] [CrossRef]
- Sabokbar, N.; Khodaiyan, F. Total Phenolic Content and Antioxidant Activities of Pomegranate Juice and Whey Based Novel Beverage Fermented by Kefir Grains. J. Food Sci. Technol. 2016, 53, 739–747. [Google Scholar] [CrossRef]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and Characterization of Kefir Made from Cow and Buffalo Milk, Using Kefir Grain and Starter Culture. J. Dairy. Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]
- Kim, D.H.; Chon, J.W.; Kim, H.; Kim, H.S.; Choi, D.; Hwang, D.G.; Seo, K.H. Detection and Enumeration of Lactic Acid Bacteria, Acetic Acid Bacteria and Yeast in Kefir Grain and Milk Using Quantitative Real-Time PCR. J. Food Saf. 2015, 35, 102–107. [Google Scholar] [CrossRef]
- Danilović, B.; Đorđević, N.; Savić, D. Microbiological and Chemical Changes during Two-Phase Fermentation of Kefir. Adv. Technol. 2019, 8, 5–9. [Google Scholar] [CrossRef]
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in Carbohydrate Profile in Kefir Fermentation. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- Da Costa, M.P.; Frasao, B.D.S.; Lima, B.R.C.D.C.; Rodrigues, B.L.; Junior, C.A.C. Simultaneous Analysis of Carbohydrates and Organic Acids by HPLC-DAD-RI for Monitoring Goat’s Milk Yogurts Fermentation. Talanta 2016, 152, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, J.A.; Johansson, M.; Hansson, H.; Abrahamson, A.; Byberg, L.; Smedman, A.; Lindmark-Månsson, H.; Lundh, Å. Lactose, Glucose and Galactose Content in Milk, Fermented Milk and Lactose-Free Milk Products. Int. Dairy. J. 2017, 73, 151–154. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Granato, D.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Haminiuk, C.W.I. Modelling the Extraction of Phenolic Compounds and in Vitro Antioxidant Activity of Mixtures of Green, White and Black Teas (Camellia sinensis L. Kuntze). J. Food Sci. Technol. 2015, 52, 6966–6977. [Google Scholar] [CrossRef]
- Naibaho, J.; Butula, N.; Jonuzi, E.; Korzeniowska, M.; Laaksonen, O.; Föste, M.; Kütt, M.L.; Yang, B. Potential of Brewers’ Spent Grain in Yogurt Fermentation and Evaluation of Its Impact in Rheological Behaviour, Consistency, Microstructural Properties and Acidity Profile during the Refrigerated Storage. Food Hydrocoll. 2022, 125, 107412. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and Chemical Properties of Norwegian Kefir during Storage. Int. Dairy. J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Turker, G.; Kizilkaya, B.; Arifoglu, N. Determination of Organic Acid Composition and Free Radical Scavenging Capacity of Kefir. Asian J. Chem. 2014, 26, 2443–2446. [Google Scholar] [CrossRef]
- Alirezalu, K.; Inácio, R.S.; Hesari, J.; Remize, F.; Nemati, Z.; Saraiva, J.A.; Barba, F.J.; Sant’Ana, A.S.; Lorenzo, J.M. Nutritional, Chemical, Syneresis, Sensory Properties, and Shelf Life of Iranian Traditional Yoghurts during Storage. LWT 2019, 114, 108417. [Google Scholar] [CrossRef]



| Storage Duration, Days | |||||||
|---|---|---|---|---|---|---|---|
| Analysis | Sample | Inoculation | 0 | 3 | 6 | 10 | 14 |
| pH | Control | 6.09 d ± 0.01 | 4.92 c ± 0.02 | 4.86 e ± 0.01 | 4.79 d ± 0.01 | 4.66 d ± 0.01 | 4.54 e ± 0.01 |
| K1 | 5.72 c ± 0.00 | 4.46 b ± 0.02 | 4.30 b ± 0.01 | 4.21 b ± 0.01 | 4.19 c ± 0.01 | 4.09 b ± 0.01 | |
| K2 | 5.48 b ± 0.00 | 4.42 a ± 0.01 | 4.21 a ± 0.02 | 4.16 a ± 0.01 | 4.05 a ± 0.01 | 3.99 a ± 0.01 | |
| K3 | 5.72 c ± 0.02 | 4.43 a ± 0.01 | 4.36 d ± 0.01 | 4.24 c ± 0.01 | 4.21 c ± 0.02 | 4.13 d ± 0.01 | |
| K4 | 5.44 a ± 0.02 | 4.44 ab ± 0.01 | 4.33 c ± 0.02 | 4.23 c ± 0.01 | 4.16 b ± 0.01 | 4.11 c ± 0.01 | |
| Titratable acidity °SH | Control | 6.0 a ± 0.030 | 20.0 c ± 0.045 | 17.0 b ± 0.035 | 15.8 a ± 0.020 | 16.0 a ± 0.040 | 15.0 a ± 0.025 |
| K1 | 9.2 b ± 0.030 | 13.2 a ± 0.025 | 20.0 d ± 0.050 | 20.8 d ± 0.035 | 21.0 c ± 0.020 | 21.8 c ± 0.045 | |
| K2 | 12.0 d ± 0.050 | 15.2 b ± 0.030 | 16.0 a ± 0.024 | 24.6 e ± 0.045 | 25.0 d ± 0.035 | 27.8 e ± 0.050 | |
| K3 | 9.4 c ± 0.028 | 13.2 a ± 0.020 | 19.4 c ± 0.040 | 17.0 b ± 0.030 | 19.6 b ± 0.045 | 20.6 b ± 0.025 | |
| K4 | 12.0 d ± 0.020 | 20.4 d ± 0.035 | 24.0 e ± 0.050 | 20.6 c ± 0.030 | 26.0 e ± 0.045 | 25.4 d ± 0.025 | |
| Compound | Day | Control | K1 | K2 | K3 | K4 |
|---|---|---|---|---|---|---|
| Lactose | Inoculation | 50.24 a ± 2.04 | 49.9 a ± 1.64 | 48.19 a ± 1.88 | 49.79 a ± 1.71 | 49.23 a ± 1.96 |
| 0 | 41.33 a ± 1.79 | 40.87 a ± 1.47 | 40.19 a ± 1.63 | 40.79 a ± 1.39 | 40.23 a ± 1.55 | |
| 6 | 20.55 a ± 1.63 | 20.63 a ± 1.71 | 21.03 a ± 1.55 | 19.33 a ± 1.95 | 20.13 a ± 1.80 | |
| 10 | 13.88 a ± 1.28 | 12.92 a ± 1.04 | 14.11 a ± 2.12 | 11.99 a ± 1.63 | 14.55 a ± 1.29 | |
| 14 | 10.11 a ± 1.55 | 9.15 a ± 1.30 | 9.88 a ± 1.79 | 10.35 a ± 1.46 | 11.23 a ± 1.63 | |
| Glucose | Inoculation | 10.22 a ± 0.73 | 15.36 bc ± 0.69 | 16.52 c ± 0.74 | 14.01 b ± 0.65 | 15.49 bc ± 0.69 |
| 0 | 15.16 a ± 0.65 | 17.27 b ± 0.73 | 18.59 b ± 0.66 | 16.33 ab ± 0.69 | 15.55 a ± 0.65 | |
| 6 | 10.18 a ± 0.77 | 12.40 b ± 0.65 | 15.64 c ± 0.77 | 12.31 b ± 0.73 | 12.30 b ± 0.73 | |
| 10 | 10.25 a ± 0.69 | 12.38 bc ± 0.74 | 13.61 c ± 0.69 | 11.36 ab ± 0.65 | 12.27 bc ± 0.69 | |
| 14 | 11.22 ab ± 0.65 | 13.46 c ± 0.79 | 12.60 bc ± 0.73 | 10.33 a ± 0.69 | 11.37 ab ± 0.65 | |
| Fructose | Inoculation | 0.09 a ± 0.014 | 0.36 b ± 0.032 | 0.50 c ± 0.041 | 0.38 b ± 0.031 | 0.47 c ± 0.028 |
| 0 | 0.13 a ± 0.009 | 0.58 c ± 0.021 | 0.63 d ± 0.033 | 0.47 b ± 0.026 | 0.53 bc ± 0.037 | |
| 6 | 0.14 a ± 0.008 | 0.74 c ± 0.040 | 0.77 c ± 0.038 | 0.51 b ± 0.019 | 0.56 b ± 0.009 | |
| 10 | 0.16 a ± 0.009 | 0.85 d ± 0.033 | 0.94 e ± 0.051 | 0.52 b ± 0.017 | 0.58 c ± 0.023 | |
| 14 | 0.17 a ± 0.006 | 0.95 c ± 0.028 | 1.02 d ± 0.042 | 0.55 b ± 0.025 | 0.60 b ± 0.025 | |
| Etanol | Inoculation | nd | nd | nd | nd | nd |
| 0 | 1.27 b ± 0.02 | 1.67 d ± 0.02 | 1.91 e ± 0.03 | 1.00 a ± 0.02 | 1.47 c ± 0.03 | |
| 6 | 1.10 a ± 0.02 | 1.81 c ± 0.03 | 2.14 d ± 0.02 | 1.15 a ± 0.01 | 1.70 b ± 0.02 | |
| 10 | 1.21 a ± 0.03 | 1.88 c ± 0.02 | 2.43 d ± 0.04 | 1.61 b ± 0.02 | 1.84 c ± 0.03 | |
| 14 | 1.62 a ± 0.02 | 1.99 d ± 0.02 | 2.45 e ± 0.03 | 1.70 b ± 0.02 | 1.83 c ± 0.02 | |
| Citric acid | Inoculation | nd | nd | nd | nd | nd |
| 0 | 0.19 a ± 0.016 | 0.33 b ± 0.024 | 0.69 e ± 0.032 | 0.43 c ± 0.012 | 0.61 d ± 0.020 | |
| 6 | 0.19 a ± 0.017 | 0.35 b ± 0.021 | 0.62 d ± 0.020 | 0.54 c ± 0.016 | 0.60 d ± 0.024 | |
| 10 | 0.21 a ± 0.012 | 1.28 e ± 0.032 | 1.00 d ± 0.024 | 0.59 b ± 0.017 | 0.66 c ± 0.021 | |
| 14 | 0.33 a ± 0.016 | 1.42 d ± 0.028 | 1.40 d ± 0.021 | 0.55 b ± 0.013 | 0.77 c ± 0.019 | |
| Malic acid | Inoculation | nd | nd | nd | nd | nd |
| 0 | 0.01 a ± 0.002 | 0.01 ac ± 0.004 | 0.03 c ± 0.001 | 0.02 b ± 0.008 | 0.03 c ± 0.002 | |
| 6 | 0.02 a ± 0.002 | 0.03 b ± 0.001 | 0.08 d ± 0.003 | 0.03 b ± 0.001 | 0.04 c ± 0.002 | |
| 10 | 0.01 a ± 0.001 | 0.04 c ± 0.001 | 0.07 d ± 0.002 | 0.03 b ± 0.002 | 0.03 b ± 0.001 | |
| 14 | 0.01 a ± 0.001 | 0.05 d ± 0.002 | 0.06 e ± 0.002 | 0.03 b ± 0.001 | 0.04 c ± 0.003 | |
| Lactic acid | Inoculation | 1.11 c ± 0.05 | 0.92 b ± 0.02 | 1.24 d ± 0.04 | 0.84 b ± 0.03 | 0.74 a ± 0.02 |
| 0 | 7.18 a ± 0.12 | 7.09 a ± 0.09 | 8.71 b ± 0.16 | 7.00 a ± 0.08 | 7.06 a ± 0.08 | |
| 6 | 7.39 a ± 0.14 | 7.46 a ± 0.13 | 8.94 c ± 0.17 | 7.78 b ± 0.11 | 7.66 ab ± 0.11 | |
| 10 | 7.38 a ± 0.13 | 7.68 b ± 0.12 | 8.85 c ± 0.17 | 7.55 ab ± 0.09 | 7.53 ab ± 0.08 | |
| 14 | 7.15 a ± 0.13 | 8.07 c ± 0.15 | 8.82 d ± 0.18 | 7.99 bc ± 0.14 | 7.70 b ± 0.14 | |
| Acetic acid | Inoculation | nd | nd | nd | nd | nd |
| 0 | 0.77 a ± 0.012 | 0.81 b ± 0.016 | 0.82 bc ± 0.014 | 0.85 c ± 0.012 | 0.88 c ± 0.020 | |
| 6 | 0.76 a ± 0.011 | 0.90 b ± 0.014 | 0.94 c ± 0.016 | 0.89 b ± 0.016 | 0.91 bc ± 0.016 | |
| 10 | 0.80 a ± 0.011 | 1.14 e ± 0.024 | 1.10 d ± 0.017 | 0.95 b ± 0.014 | 0.99 c ± 0.016 | |
| 14 | 0.99 a ± 0.016 | 1.15 c ± 0.018 | 1.27 d ± 0.014 | 1.04 b ± 0.016 | 1.01 ab ± 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamenković Stojanović, S.; Živković, L.; Stanojević, J.; Danilović, B.; Mančić, S.; Karabegović, I. Enhancing Kefir with Raspberry Pomace: Storage-Dependent Changes in Quality and Stability. Fermentation 2025, 11, 265. https://doi.org/10.3390/fermentation11050265
Stamenković Stojanović S, Živković L, Stanojević J, Danilović B, Mančić S, Karabegović I. Enhancing Kefir with Raspberry Pomace: Storage-Dependent Changes in Quality and Stability. Fermentation. 2025; 11(5):265. https://doi.org/10.3390/fermentation11050265
Chicago/Turabian StyleStamenković Stojanović, Sandra, Ljubica Živković, Jelena Stanojević, Bojana Danilović, Stojan Mančić, and Ivana Karabegović. 2025. "Enhancing Kefir with Raspberry Pomace: Storage-Dependent Changes in Quality and Stability" Fermentation 11, no. 5: 265. https://doi.org/10.3390/fermentation11050265
APA StyleStamenković Stojanović, S., Živković, L., Stanojević, J., Danilović, B., Mančić, S., & Karabegović, I. (2025). Enhancing Kefir with Raspberry Pomace: Storage-Dependent Changes in Quality and Stability. Fermentation, 11(5), 265. https://doi.org/10.3390/fermentation11050265









