Potential of Whey Protein-Fortified Blackberry Juice in Transporting and Protecting Lactic Acid Bacteria: A Proteolytic Profile Analysis and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Thermoultrasonic Blackberry Juice
2.3. Fermentation
2.4. Determination of Prebiotic Activity
2.5. Proteolytic Profile Assessment
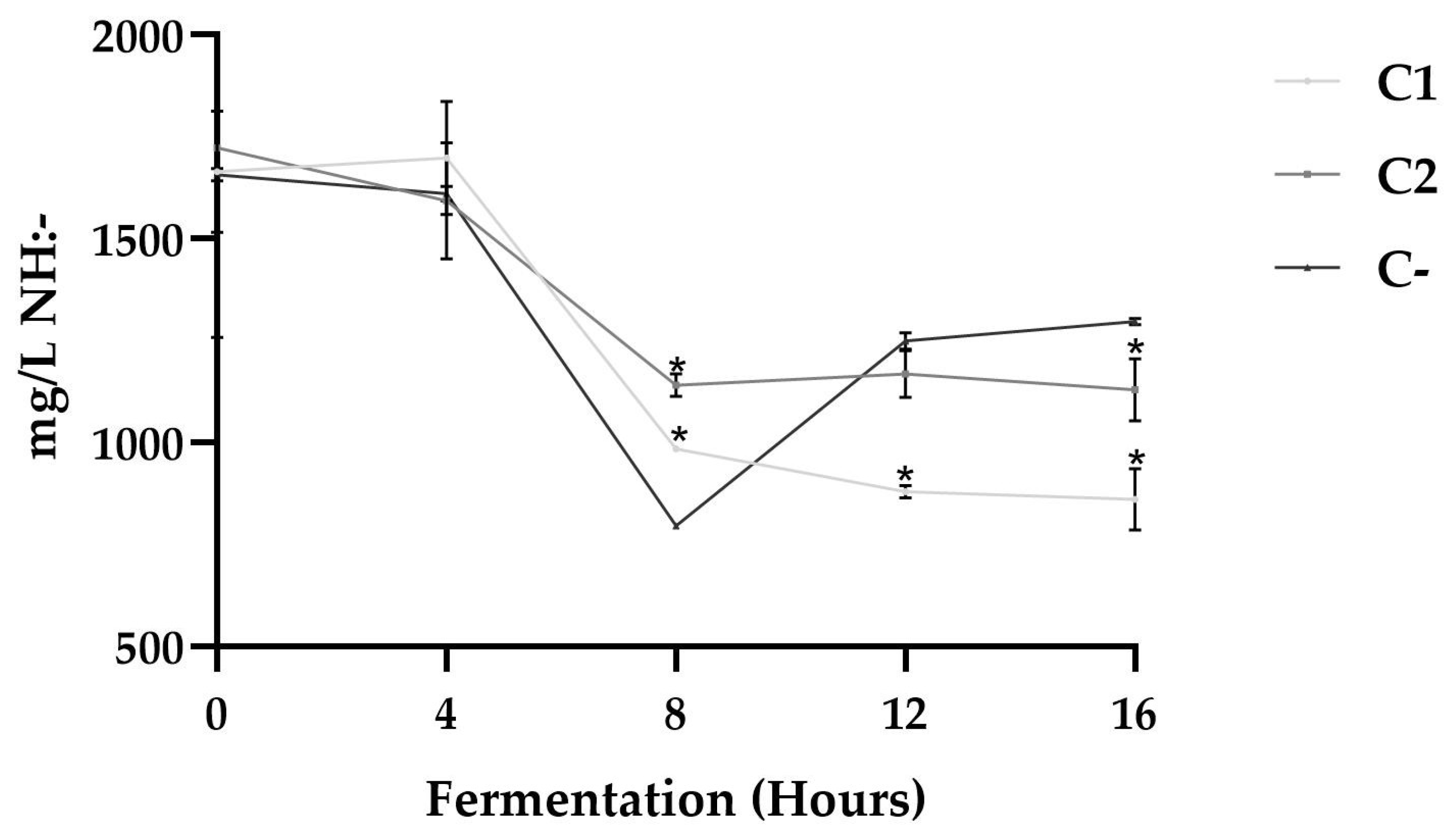
2.5.1. Determination of Free Amino Groups by the TNBS Technique
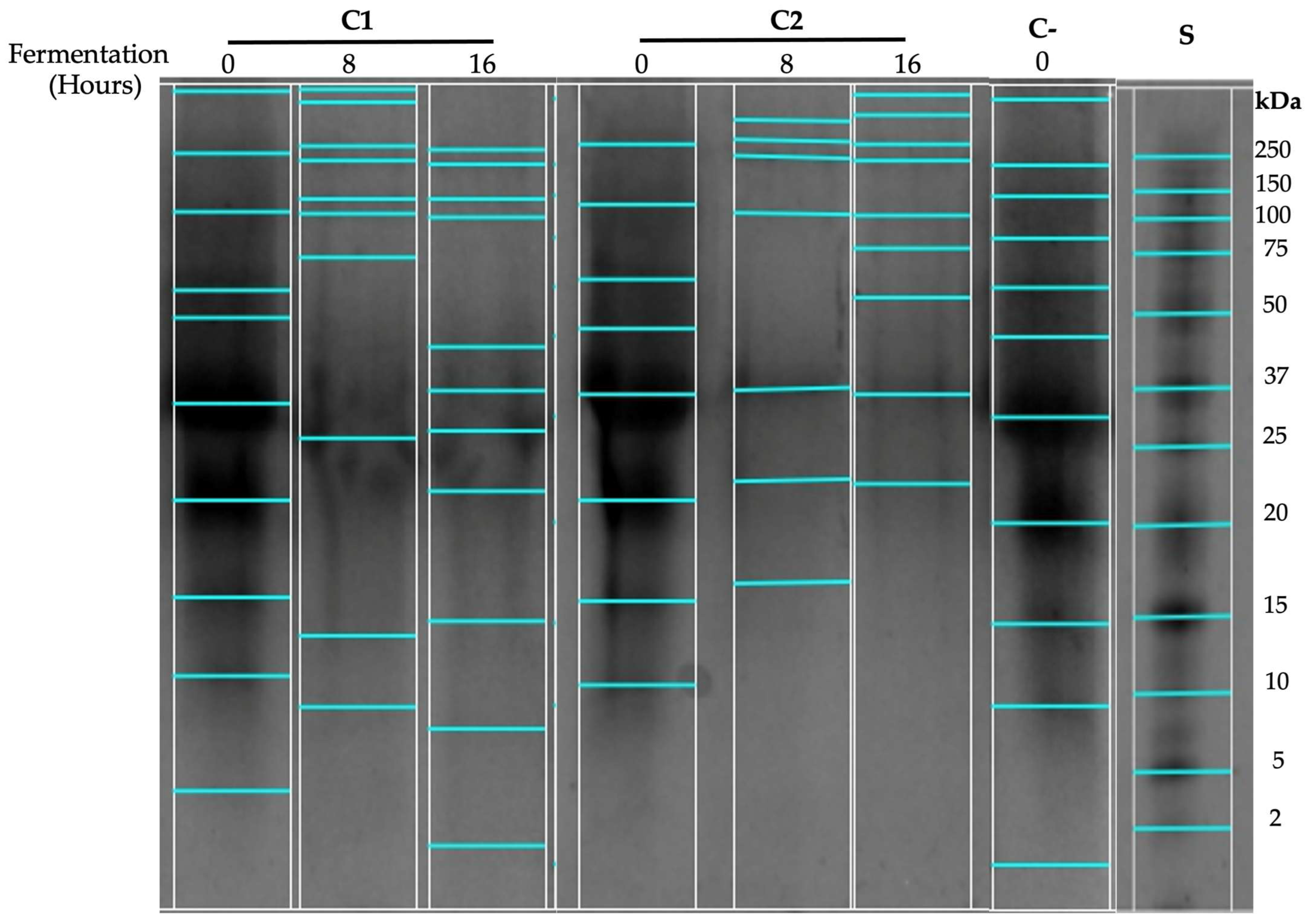
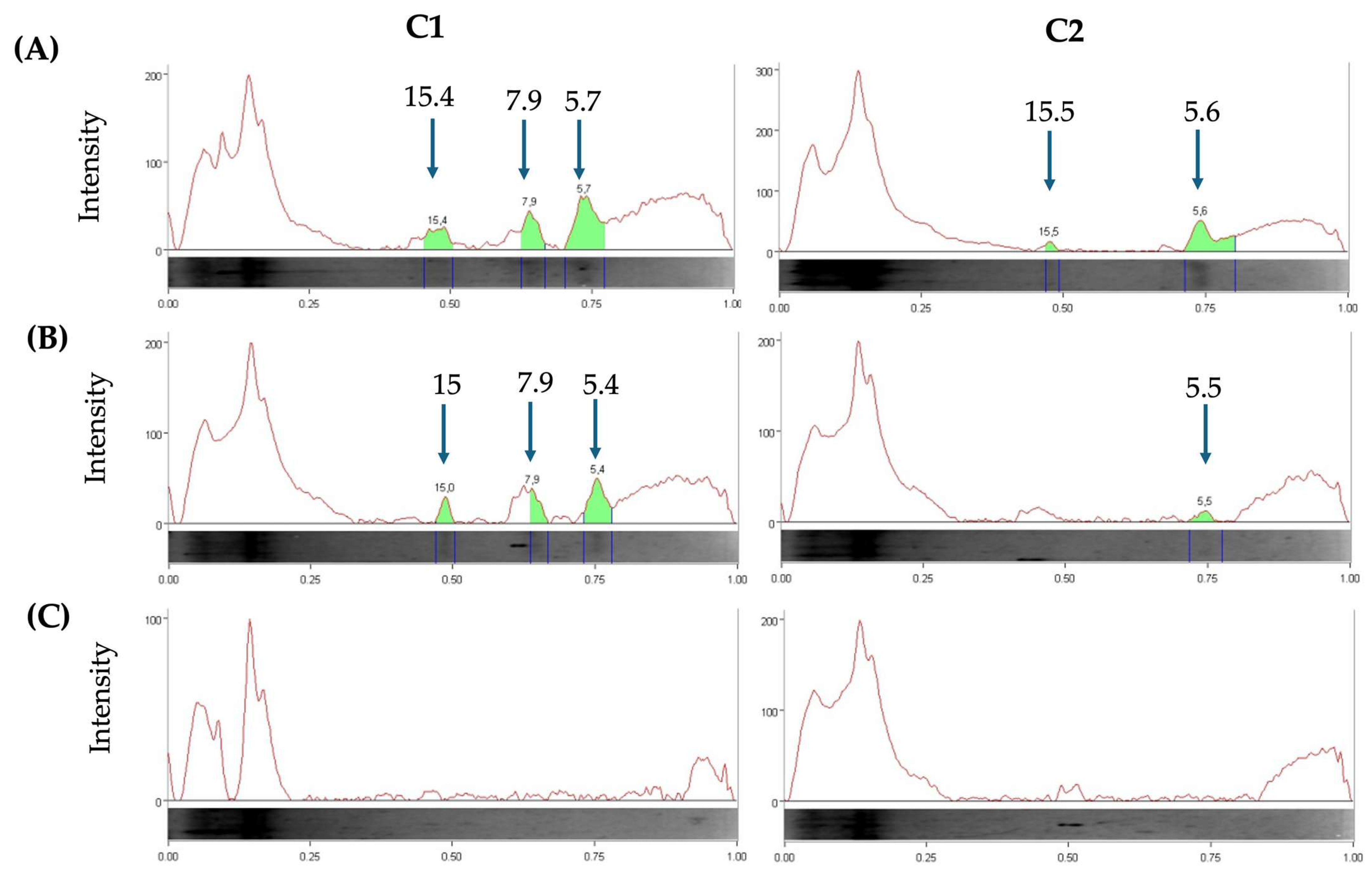
2.5.2. Low-Molecular-Weight Peptides Separation by Tris–Tricine Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (Tris–Tricine SDS-PAGE)
2.6. Organic Acids Quantification
2.7. Quantification of Phenolic Compounds
2.8. Antioxidant Capacity Assays
2.8.1. Oxygen Radical Absorbance Capacity (ORAC) Test
2.8.2. Ferric Ion-Reducing Antioxidant Power (FRAP) Assay
2.8.3. Antioxidant Activity Assay Against 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH●)
2.9. Extraction of Low-Molecular-Weight Peptides
2.10. Statistical Analysis
3. Results
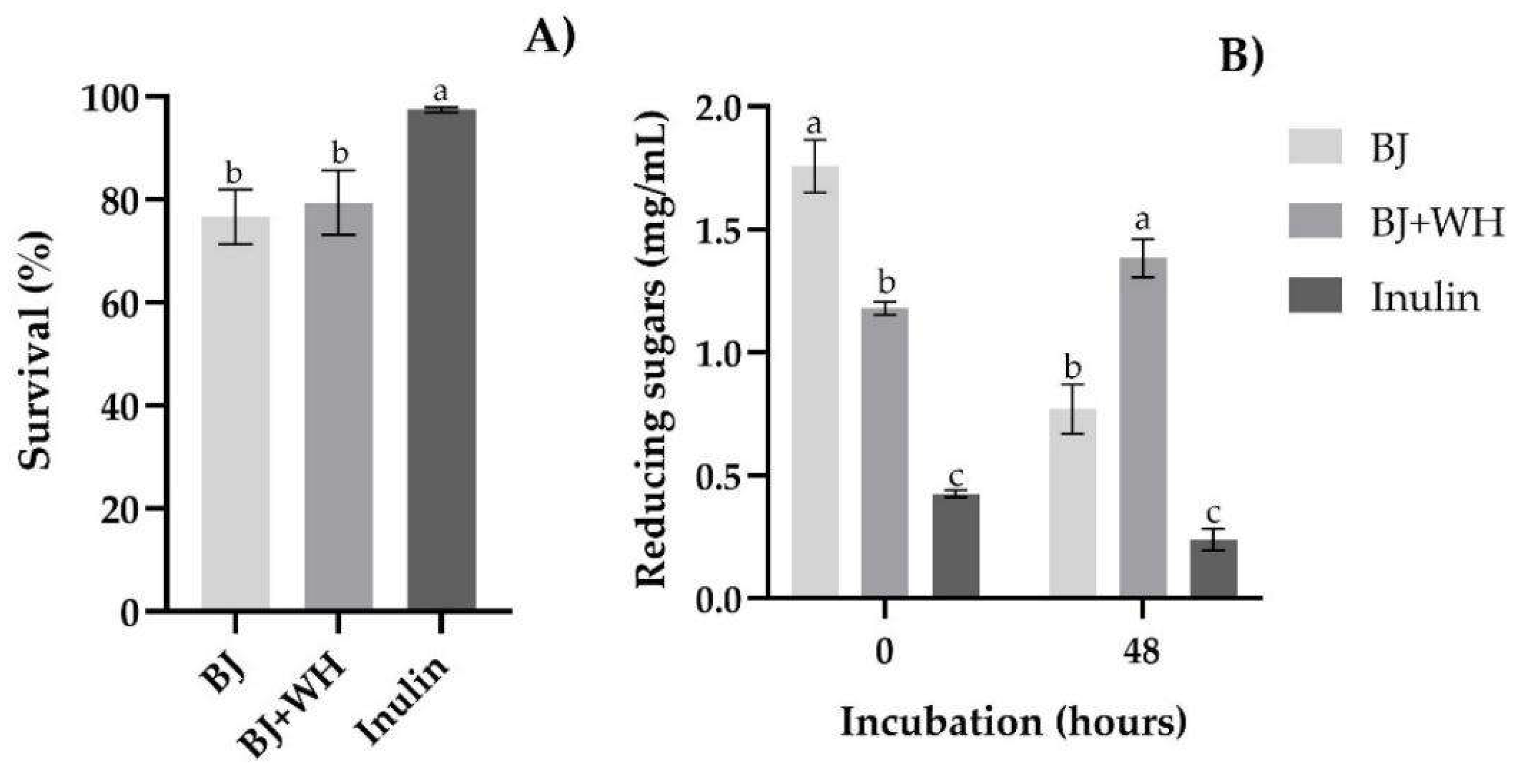
3.1. Prebiotic Activity
3.2. Proteolytic Profile
3.3. Quantification of Organic and Phenolic Compounds During Fermentation
3.4. Antioxidant Capacity
3.5. Separation of Bacteriocins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liao, H.; Sun, S.; Zhang, X.; Xie, L.; Liu, H. Research Progress on the Application of Lacticaseibacillus rhamnosus GG in Pediatric Respiratory Diseases. Front. Nutr. 2025, 12, 1553674. [Google Scholar] [CrossRef]
- Leser, T.; Baker, A. Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function. Microorganisms 2024, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2025, 17, 138–158. [Google Scholar] [CrossRef]
- Gurram, S.; Jha, D.K.; Shah, D.S.; Kshirsagar, M.M.; Amin, P.D. Insights on the Critical Parameters Affecting the Probiotic Viability During Stabilization Process and Formulation Development. AAPS PharmSciTech 2021, 22, 156. [Google Scholar] [CrossRef]
- Enck, K.; Banks, S.; Yadav, H.; Welker, M.E.; Emmanuel, C. Opara Development of a Novel Oral Delivery Vehicle for Probiotics. Curr. Pharm. Des. 2020, 26, 3134–3140. [Google Scholar] [CrossRef]
- de Oliveira, P.M.; Leite Júnior, B.R.d.C.; Martins, E.M.F.; Martins, M.L.; Vieira, É.N.R.; de Barros, F.A.R.; Cristianini, M.; de Almeida Costa, N.; Ramos, A.M. Mango and Carrot Mixed Juice: A New Matrix for the Vehicle of Probiotic Lactobacilli. J. Food Sci. Technol. 2021, 58, 98–109. [Google Scholar] [CrossRef]
- Plessas, S. Editorial: Innovations in Functional Food Production: Application of Fruit and Vegetable Juices as Vehicles for Probiotic Delivery. Front. Microbiol. 2022, 13, 854705. [Google Scholar] [CrossRef]
- Horackova, S.; Rokytova, K.; Bialasova, K.; Klojdova, I.; Slukova, M. Fruit Juices with Probiotics—New Type of Functional Foods. Czech J. Food Sci. 2018, 36, 284–288. [Google Scholar] [CrossRef]
- Buljeta, I.; Nosić, M.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules 2022, 27, 3029. [Google Scholar] [CrossRef] [PubMed]
- Barkaoui, S.; Madureira, J.; Boudhrioua, N.; Cabo Verde, S. Berries: Effects on Health, Preservation Methods, and Uses in Functional Foods: A Review. Eur. Food Res. Technol. 2023, 249, 1689–1715. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity In Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Zemel, M.B. Functional Properties of Whey, Whey Components, and Essential Amino Acids: Mechanisms Underlying Health Benefits for Active People (Review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef] [PubMed]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial Whey Products Promote Intestinal Barrier Function with Glycomacropeptide Enhanced Activity in Downregulating Bacterial Endotoxin Lipopolysaccharides (LPS)-Induced Inflammation in Vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef]
- Wang, C.; Killpatrick, A.; Humphrey, A.; Guo, M. Whey Protein Functional Properties and Applications in Food Formulation. In Whey Protein Production, Chemistry, Functionality, and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 157–204. ISBN 978-1-119-25605-2. [Google Scholar]
- Daliri, E.B.-M.; Lee, B.H.; Park, B.-J.; Kim, S.-H.; Oh, D.-H. Antihypertensive Peptides from Whey Proteins Fermented by Lactic Acid Bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef]
- Qian, Z.; Li, Y.; Hao, Z.; Zheng, Z.; Yang, H.; Li, S.; Xu, S.; Xu, Y.; Zhang, L. Enhancement of the Organic Acid Content and Antioxidant Capacity of Yellow Whey through Fermentation with Lacticaseibacillus casei YQ336. World J. Microbiol. Biotechnol. 2023, 40, 53. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Mársico, E.T.; Campelo, P.H.; Pimentel, T.C.; Cristina Silva, M.; et al. Probiotic Fermented Whey-Milk Beverages: Effect of Different Probiotic Strains on the Physicochemical Characteristics, Biological Activity, and Bioactive Peptides. Food Res. Int. Ott. Ont 2023, 164, 112396. [Google Scholar] [CrossRef]
- Ying, D.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-Demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in Whey Protein and Resistant Starch Matrices: Probiotic Survival in Fruit Juice. J. Funct. Foods 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Shao, Y.; Guo, Y.; Gu, R.; Wang, W. Cheese Whey Protein and Blueberry Juice Mixed Fermentation Enhance the Freeze-Resistance of Lactic Acid Bacteria in the Freeze-Drying Process. Foods 2024, 13, 2260. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Y.; Yang, X.; Wang, Z.; Yao, X.; Guo, Y. Enhanced Bioavailability and Anti-Hyperglycemic Activity of Young Apple Polyphenols by Complexation with Whey Protein Isolates. J. Food Sci. 2022, 87, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Zarate, L.; Delgado-Olivares, L.; Cruz-Cansino, N.D.; González-Olivares, L.G.; Castrejón-Jiménez, N.S.; Estrada-Luna, D.; Jiménez-Osorio, A.S. Blackberry Juice Fermented with Two Consortia of Lactic Acid Bacteria and Isolated Whey: Physicochemical and Antioxidant Properties during Storage. Int. J. Mol. Sci. 2024, 25, 8882. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Jaimez-Ordaz, J.; Contreras-López, E.; Ramírez-Godínez, J.; Castañeda-Ovando, A.; González-Olivares, L.G. Prebiotic Potential of Macerates Derived from Solid-State Fermentation of Barley Straw by Rhizopus Oryzae JCP024: Preliminary Evaluation. Biomass Convers. Biorefinery 2023, 13, 4797–4802. [Google Scholar] [CrossRef]
- Sebastián-Nicolas, J.L.; Contreras-López, E.; Ramírez-Godínez, J.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M.; Añorve-Morga, J.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Ayala-Niño, A.; et al. Milk Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102: Proteolytic Profile and ACE-Inhibitory Activity. Fermentation 2021, 7, 215. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanís-García, E.; Añorve-Morga, J.; Quintero-Lira, A.; Castañeda-Ovando, A.; Ramírez-Moreno, E. Organic Acids, Antioxidants, and Dietary Fiber of Mexican Blackberry (Rubus fruticosus) Residues Cv. Tupy. J. Food Qual. 2018, 2018, 5950761. [Google Scholar] [CrossRef]
- García-Falcón, M.S.; Pérez-Lamela, C.; Martínez-Carballo, E.; Simal-Gándara, J. Determination of Phenolic Compounds in Wines: Influence of Bottle Storage of Young Red Wines on Their Evolution. Food Chem. 2007, 105, 248–259. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Gómez-Sierra, T.; Medina-Campos, O.N.; Hernández-Juárez, J.; Hernández-Cruz, P.A.; Gallegos-Velasco, I.B.; Pérez-Cervera, Y.; Pedraza-Chaverri, J. Antioxidant Activity of Glucosamine and Its Effects on ROS Production, Nrf2, and O-GlcNAc Expression in HMEC-1 Cells. Curr. Res. Toxicol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Ramírez, V.; Robledo-Márquez, K.; García-Rojas, N.; Rojas-Morales, P.; Arango, N.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Pérez-Rojas, J.M.; Flores-Ramírez, R.; et al. Stenocereus Huastecorum-Fruit Juice Concentrate Protects against Cisplatin-Induced Nephrotoxicity by Nitric Oxide Pathway Activity and Antioxidant and Antiapoptotic Effects. Food Res. Int. 2022, 160, 111337. [Google Scholar] [CrossRef]
- Gaspar, C.; Donders, G.G.; Palmeira-de-Oliveira, R.; Queiroz, J.A.; Tomaz, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Bacteriocin Production of the Probiotic Lactobacillus acidophilus KS400. AMB Express 2018, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.H.; Grolle, K.; Nga, T.T.V.; Zeeman, G.; Temmink, H.; van Eekert, M. Protein Hydrolysis and Fermentation under Methanogenic and Acidifying Conditions. Biotechnol. Biofuels 2019, 12, 254. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Jaimez-Ordaz, J.; Pérez-Escalante, E.; Quintero-Lira, A.; Ramírez-Moreno, E.; Contreras-López, E.; González-Olivares, L.G. Differences in the Proteolytic System of Lactic Acid Bacteria Affect the Release of DPP-IV Inhibitory Peptides from Whey Proteins. Dairy 2023, 4, 515–526. [Google Scholar] [CrossRef]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive Peptides Derived from Whey Proteins for Health and Functional Beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative Stress Tolerance and Antioxidant Capacity of Lactic Acid Bacteria as Probiotic: A Systematic Review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Abbasiliasi, S.; Tan, J.S.; Tengku Ibrahim, T.A.; Bashokouh, F.; Ramakrishnan, N.R.; Mustafa, S.; Ariff, A.B. Fermentation Factors Influencing the Production of Bacteriocins by Lactic Acid Bacteria: A Review. RSC Adv. 2017, 7, 29395–29420. [Google Scholar] [CrossRef]
- Guerin, J.; Bacharouche, J.; Burgain, J.; Lebeer, S.; Francius, G.; Borges, F.; Scher, J.; Gaiani, C. Pili of Lactobacillus rhamnosus GG Mediate Interaction with β-Lactoglobulin. Food Hydrocoll. 2016, 58, 35–41. [Google Scholar] [CrossRef]
- Lu, W.; Fu, N.; Woo, M.W.; Chen, X.D. Exploring the Interactions between Lactobacillus rhamnosus GG and Whey Protein Isolate for Preservation of the Viability of Bacteria through Spray Drying. Food Funct. 2021, 12, 2995–3008. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Pérez-Escalante, E.; Castañeda-Ovando, A.; Contreras-López, E.; Cruz-Guerrero, A.E.; Regal-López, P.; Cardelle-Cobas, A.; González-Olivares, L.G. ACE-Inhibitory Activity of Whey Proteins Fractions Derived of Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102. Foods 2023, 12, 2416. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of Different Prebiotics on Viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus Encapsulated in Alginate Microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Santa, D.; Srbinovska, S. Whey: Source of Bioactive Peptides, Probiotics, Organic Acids, Aromatic Compounds and Enzymes. In Whey Valorization: Innovations, Technological Advancements and Sustainable Exploitation; Poonia, A., Trajkovska Petkoska, A., Eds.; Springer Nature: Singapore, 2023; pp. 239–258. ISBN 978-981-9954-59-9. [Google Scholar]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive Peptides of Whey: Obtaining, Activity, Mechanism of Action, and Further Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10351–10381. [Google Scholar] [CrossRef]
- Martín-del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Chiring Phukon, L.; Abedin, M.M.; Padhi, S.; Singh, S.P.; Rai, A.K. Bioactive Peptides in Fermented Foods and Their Application: A Critical Review. Syst. Microbiol. Biomanufacturing 2023, 3, 88–109. [Google Scholar] [CrossRef]
- Hernández-Jabalera, A.; Cortés-Giraldo, I.; Dávila-Ortíz, G.; Vioque, J.; Alaiz, M.; Girón-Calle, J.; Megías, C.; Jiménez-Martínez, C. Influence of Peptides-Phenolics Interaction on the Antioxidant Profile of Protein Hydrolysates from Brassica Napus. Food Chem. 2015, 178, 346–357. [Google Scholar] [CrossRef]
- Hu, Y.; Ling, Y.; Qin, Z.; Huang, J.; Jian, L.; Ren, D.F. Isolation, Identification, and Synergistic Mechanism of a Novel Antimicrobial Peptide and Phenolic Compound from Fermented Walnut Meal and Their Application in Rosa Roxbughii Tratt Spoilage Fungus. Food Chem. 2024, 433, 137333. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.-Y.; Wang, W.; Yan, H.; Qu, H.; Liu, Y.; Qian, Y.; Gu, R. The Effect of Different Organic Acids and Their Combination on the Cell Barrier and Biofilm of Escherichia coli. Foods 2023, 12, 3011. [Google Scholar] [CrossRef]
- Li, K.J.; Brouwer-Brolsma, E.M.; Burton-Pimentel, K.J.; Vergères, G.; Feskens, E.J.M. A Systematic Review to Identify Biomarkers of Intake for Fermented Food Products. Genes Nutr. 2021, 16, 5. [Google Scholar] [CrossRef]
- Wang, H.; Huang, T.; Liu, K.; Yu, J.; Yao, G.; Zhang, W.; Zhang, H.; Sun, T. Protective Effects of Whey Protein Hydrolysate on Bifidobacterium animalis ssp. lactis Probio-M8 during Freeze-Drying and Storage. J. Dairy Sci. 2022, 105, 7308–7321. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, X.; Li, W.; Chang, C.; Wu, J. A Protectant for Lactobacillus rhamnosus Based on Whey Protein Isolate and Isomalt: Stress Resistance and Underlying Mechanisms. Int. J. Biol. Macromol. 2024, 280, 135712. [Google Scholar] [CrossRef]
- Prosekov, A.; Lyubov, D.; Milentyeva, I.; Sykhikh, S.A.; Babich, O.; Ivanova, S.; Pavsky, V.; Shishin, M.V.; Matskova, L.V. Antioxidant and Antimicrobial Activity of Bacteriocin-Producing Strains of Lactic Acid Bacteria Isolated from the Human Gastrointestinal Tract. Prog. Nutr. 2017, 19, 67–80. [Google Scholar] [CrossRef]
- Nuryana, I.; Andriani, A.; Lisdiyanti, P.; Yopi. Analysis of Organic Acids Produced by Lactic Acid Bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012054. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile Profile of Elderberry Juice: Effect of Lactic Acid Fermentation Using L. plantarum, L. rhamnosus and L. casei Strains. Food Res. Int. Ott. Ont 2018, 105, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Zalán, Z.; Hudáček, J.; Štětina, J.; Chumchalová, J.; Halász, A. Production of Organic Acids by Lactobacillus Strains in Three Different Media. Eur. Food Res. Technol. 2010, 230, 395–404. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef]
- Fenwick, M.L. The Production of an Esterase Inhibitor from Schradan in the Fat Body of the Desert Locust. Biochem. J. 1958, 70, 373–381. [Google Scholar] [CrossRef]
- Fritsch, C.; Heinrich, V.; Vogel, R.F.; Toelstede, S. Phenolic Acid Degradation Potential and Growth Behavior of Lactic Acid Bacteria in Sunflower Substrates. Food Microbiol. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; López de Felipe, F.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food Phenolics and Lactic Acid Bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z.; Zhang, C.; Shang, C.; Gao, L.; Li, C.; Zhang, G.; Liu, L. Effect of Epigallocatechin Gallate on the Fermentative and Physicochemical Properties of Fermented Milk. J. Dairy Sci. 2022, 105, 7322–7333. [Google Scholar] [CrossRef]
- Gaur, G.; Gänzle, M.G. Conversion of (Poly)Phenolic Compounds in Food Fermentations by Lactic Acid Bacteria: Novel Insights into Metabolic Pathways and Functional Metabolites. Curr. Res. Food Sci. 2023, 6, 100448. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.D.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of Fermentation on the Antioxidant Activity in Plant-Based Foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ortiz, A.; Castaño-Tostado, E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Reynoso-Camacho, R. Anthocyanins Extraction from Hibiscus sabdariffa and Identification of Phenolic Compounds Associated with Their Stability. J. Sci. Food Agric. 2021, 101, 110–119. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods 2024, 13, 3851. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Gatri, E.; Filannino, P.; M’Hir, S.; Ayed, L. Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties. Beverages 2025, 11, 27. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Yarmand, M.S.; Ghanbarzadeh, B.; Mirzaee, H. Generation of Bioactive Peptides from Lentil Protein: Degree of Hydrolysis, Antioxidant Activity, Phenol Content, ACE-Inhibitory Activity, Molecular Weight, Sensory, and Functional Properties. J. Food Meas. Charact. 2021, 15, 5021–5035. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Bioactive Peptides and Dietary Polyphenols: Two Sides of the Same Coin. Molecules 2020, 25, 3443. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Multiple Bacteriocin Production in Lactic Acid Bacteria. J. Biosci. Bioeng. 2022, 134, 277–287. [Google Scholar] [CrossRef]
- Kowalski, R.; Gustafson, E.; Carroll, M.; Gonzalez de Mejia, E. Enhancement of Biological Properties of Blackcurrants by Lactic Acid Fermentation and Incorporation into Yogurt: A Review. Antioxidants 2020, 9, 1194. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and Sensory Characteristics of Fruit Juice and Fruit Fermented Beverages and Their Consumer Acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Li, X.; Zhang, H.; Chen, Q.; Kong, B. Application of Lactic Acid Bacteria for Improving the Quality of Reduced-Salt Dry Fermented Sausage: Texture, Color, and Flavor Profiles. LWT 2022, 154, 112723. [Google Scholar] [CrossRef]
| Fermentation | 0 h | 8 h | 16 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | C1 | C2 | C− | C1 | C2 | C− | C1 | C2 | C− |
| Phenolic acids | |||||||||
| Gallic acid | 0.356 ± 0.007 A,a | 0.394 ± 0.014 A,a | 0.353 ± 0.031 A,a | 0.144 ± 0.060 B,a | 0.171 ± 0.017 B,a | 0.349 ± 0.021 A,b | 0.147 ± 0.004 B,a | 0.163 ± 0.001 B,a | 0.347 ± 0.016 A,b |
| Hydroxybenzoic | 0.031 ± 0.001 A,a | 0.029 ± 0.002 A,a | 0.030 ± 0.001 A,a | 0.017 ± 0.002 B,a | 0.017 ± 0.004 B,a | 0.028 ± 0.002 AB,b | 0.013 ± 0.001 C,a | 0.018 ± 0.001 B,b | 0.026 ± 0.001 B,c |
| Vanillic acid | 0.072 ± 0.004 A,a | 0.046 ± 0.023 A,a | 0.063 ± 0.010 A,a | 0.042 ± 0.022 AB,ab | 0.027 ± 0.013 A,a | 0.078 ± 0.002 A,b | 0.020 ± 0.014 B,a | 0.015 ± 0.004 A,a | 0.073 ± 0.005 A,c |
| Caffeic acid | 0.069 ± 0.001 A,b | 0.060 ± 0.004 A,a | 0.064 ± 0.002 A,ab | 0.062 ± 0.016 A,a | 0.040 ± 0.009 B,a | 0.038 ± 0.004 B,a | 0.031 ± 0.006 B,a | 0.024 ± 0.002 C,a | 0.031 ± 0.012 B,a |
| Coumaric acid | 0.157 ± 0.001 A,a | 0.126 ± 0.015 A,a | 0.157 ± 0.022 A,a | 0.138 ± 0.028 A,a | 0.023 ± 0.005 B,b | 0.111 ± 0.001 B,b | 0.046 ± 0.002 B,c | 0.026 ± 0.001 B,a | 0.037 ± 0.001 C,b |
| Ferulic acid | 0.075 ± 0.005 A,b | 0.023 ± 0.006 B,a | 0.092 ± 0.004 A,c | 0.030 ± 0.003 C,a | 0.051 ± 0.008 A,b | 0.086 ± 0.008 A,c | 0.051 ± 0.002 B,a | 0.056 ± 0.004 A,a | 0.087 ± 0.004 A,b |
| Benzoic acid | 1.064 ± 0.057 A,a | 1.588 ± 0.174 A,b | 1.742 ± 0.142 A,b | 1.084 ± 0.268 A,a | 1.252 ± 0.206 AB,a | 1.407 ± 0.113 B,a | 1.324 ± 0.032 A,a | 1.126 ± 0.127 B,a | 1.211 ± 0.074 B,a |
| Ellagic acid | 0.063 ± 0.002 A,a | 0.063 ± 0.005 A,a | 0.056 ± 0.001 A,a | 0.047 ± 0.010 AB,b | 0.024 ± 0.002 B,a | 0.062 ± 0.004 A,b | 0.028 ± 0.008 B,a | 0.033 ± 0.006 B,ab | 0.059 ± 0.019 A,b |
| Flavonoids | |||||||||
| Rutin | 0.683 ± 0.029 A,a | 1.172 ± 0.143 A,b | 0.526 ± 0.031 B,a | 0.686 ± 0.081 A,b | 0.467 ± 0.052 B,a | 0.852 ± 0.021 A,c | 0.494 ± 0.019 B,a | 0.511 ± 0.067 B,a | 0.910 ± 0.079 A,b |
| Myricetin | 0.062 ± 0.016 A,a | 0.053 ± 0.004 A,a | 0.070 ± 0.005 A,a | 0.067 ± 0.005 A,b | 0.031 ± 0.002 B,a | 0.055 ± 0.016 A,b | 0.021 ± 0.002 B,b | 0.007 ± 0.001 C,a | 0.053 ± 0.009 A,c |
| Epicatechin | 0.407 ± 0.042 A,b | 0.166 ± 0.009 B,a | 0.333 ± 0.029 B,b | 0.404 ± 0.027 A,b | 0.343 ± 0.015 A,a | 0.441 ± 0.021 A,b | 0.364 ± 0.008 A,a | 0.369 ± 0.018 A,a | 0.474 ± 0.007 A,b |
| EGCG | <LOD | <LOD | <LOD | <LOD | 0.132 ± 0.005 B,a | <LOD | 0.0134 ± 0.005 b | 0.142 ± 0.005 A,a | 0.215 ± 0.008 A |
| Catechin | 3.307 ± 0.271 AB,a | 2.249 ± 0.911 A,a | 2.882 ± 0.296 B,b | 3.554 ± 0.220 A,b | 2.882 ± 0.119 A,a | 3.454 ± 0.052 A,b | 2.848 ± 0.085 B,a | 3.067 ± 0.063 A,b | 3.418 ± 0.104 A,c |
| Organic acids | |||||||||
| Acetic acid | 52.4 ± 4.26 C,b | 49.22 ± 4.77 B,b | 64.71 ± 3.81 A,a | 77.34 ± 7.65 B,a | 64.75 ± 4.78 AB,ab | 58.32 ± 6.15 A,b | 93.91 ± 3.38 A,a | 65.94 ± 6.00 A,b | 53.00 ± 2.50 B,c |
| Samples | Fermentation (Hours) | ORAC * | FRAP * | DPPH (% of Elimination) |
|---|---|---|---|---|
| C1 | 0 | 2447.8 ± 48.5 A,b | 2.7 ± 0.09 A,a | 51.71 ± 5.79 A,a |
| 8 | 2106.5 ± 274.6 A,b | 2.4 ± 0.28 AB,a | 51.27 ± 1.04 A,a | |
| 16 | 2061.5 ± 203.1 A,a | 2.04 ± 0.10 B,a | 43.36 ± 1.82 A,a | |
| C2 | 0 | 2919.8 ± 290.7 A,a | 2.75 ± 0.27 A,a | 45.31 ± 2.83 A,a |
| 8 | 2291.7 ± 148.5 B,a | 2.38 ± 0.17 AB,a | 45.27 ± 2.32 A,b | |
| 16 | 2982.6 ± 30.17 A,a | 1.99 ± 0.11 B,a | 46.27 ± 1.95 A,a | |
| C− | 0 | 2589.5 ± 90.3 A,ab | 2.51 ± 0.22 A,a | 44.39 ± 1.42 A,a |
| 8 | 2652.6 ± 92.9 A,a | 2.61 ± 0.45 A,a | 39.7 ± 2.51 A,c | |
| 16 | 2164.1 ± 165.2 B,a | 2.19 ± 0.2 A,a | 44.78 ± 2.04 A,a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugo-Zarate, L.; Jiménez-Osorio, A.S.; González-Olivares, L.G.; Pérez-Escalante, E.; Castañeda-Ovando, A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Herrera-Hernández, M.G.; Delgado-Olivares, L. Potential of Whey Protein-Fortified Blackberry Juice in Transporting and Protecting Lactic Acid Bacteria: A Proteolytic Profile Analysis and Antioxidant Activity. Fermentation 2025, 11, 252. https://doi.org/10.3390/fermentation11050252
Lugo-Zarate L, Jiménez-Osorio AS, González-Olivares LG, Pérez-Escalante E, Castañeda-Ovando A, Pedraza-Chaverri J, Medina-Campos ON, Herrera-Hernández MG, Delgado-Olivares L. Potential of Whey Protein-Fortified Blackberry Juice in Transporting and Protecting Lactic Acid Bacteria: A Proteolytic Profile Analysis and Antioxidant Activity. Fermentation. 2025; 11(5):252. https://doi.org/10.3390/fermentation11050252
Chicago/Turabian StyleLugo-Zarate, Liliana, Angélica Saraí Jiménez-Osorio, Luis Guillermo González-Olivares, Emmanuel Pérez-Escalante, Araceli Castañeda-Ovando, José Pedraza-Chaverri, Omar Noel Medina-Campos, María Guadalupe Herrera-Hernández, and Luis Delgado-Olivares. 2025. "Potential of Whey Protein-Fortified Blackberry Juice in Transporting and Protecting Lactic Acid Bacteria: A Proteolytic Profile Analysis and Antioxidant Activity" Fermentation 11, no. 5: 252. https://doi.org/10.3390/fermentation11050252
APA StyleLugo-Zarate, L., Jiménez-Osorio, A. S., González-Olivares, L. G., Pérez-Escalante, E., Castañeda-Ovando, A., Pedraza-Chaverri, J., Medina-Campos, O. N., Herrera-Hernández, M. G., & Delgado-Olivares, L. (2025). Potential of Whey Protein-Fortified Blackberry Juice in Transporting and Protecting Lactic Acid Bacteria: A Proteolytic Profile Analysis and Antioxidant Activity. Fermentation, 11(5), 252. https://doi.org/10.3390/fermentation11050252








