Abstract
This study aims to optimize the bioconversion of palm kernel cake (PKC) by Pleurotus ostreatus to improve fungal biomass production, lignocellulolytic enzyme expression, and the nutritional value of the substrate as ruminant feed. Three inorganic nitrogen sources (ammonium sulfate, ammonium nitrate, and urea) were evaluated for fungal biomass production using a central composite design (CCD) in liquid fermentations. The formulated culture medium (18.72 g/L glucose and 0.39 g/L urea) effectively yielded better fungal biomass production (8 g/L). Based on these results, an extreme vertex design, mixtures with oil palm by-products (PK, hull, and fiber) supplemented with urea, were formulated, finding that PKC stimulated the highest biomass production and laccase enzyme activity in P. ostreatus. The transcriptome of P. ostreatus was obtained, and the chemical composition of the fermented PKC was determined. Transcriptomic analysis revealed the frequency of five key domains with carbohydrate-activated enzyme (CAZy) function: GH3, GH18, CBM1, AA1, and AA5, with activities on lignocellulose. In the fermented PKC, lignin was reduced by 46.9%, and protein was increased by 69.8%. In conclusion, these results show that urea is efficient in the bioconversion of PKC with P. ostreatus as a supplement for ruminants.
1. Introduction
Bioconversion of oil palm lignocellulose biomass by Pleurotus ostreatus for ruminant feeding purposes involves optimizing fermentation conditions, particularly the carbon and nitrogen availability in the substrate [1]. These conditions directly influence the production and activity of enzymes responsible for degrading anti-nutritional compounds and releasing valuable components for animal nutrition [2]. A study suggested that the regulation of enzyme synthesis in white-rot fungi (WRF) depends on the supply of nitrogen sources in the culture medium, particularly in fermentative processes of lignocellulolytic substrates [3]. Palm kernel cake (PKC) is a high-fiber, medium-grade protein feed with a lignin content between 14–20%, holocellulose of 60%, and crude protein ≈20%, representing a C:N ratio of 20:1 approximately [4,5,6,7]. These concentrations suggest that fungal growth could be favored by the amount of protein contained in the PKC rather than in other residues; thus, this by-product seems sufficient as the only C and N source for its bioconversion into fungal biomass. However, experience shows that not supplying an external nitrogen source to the fermentation process with P. ostreatus would mean considerable decreases in crude protein in the by-products. This is possibly because P. ostreatus synthesizes proteases [8], which can hydrolyze the protein nitrogen reserves in the residues, and this is undesirable if they are to be used as animal feed. Therefore, the expression of genetic profiles in P. ostreatus is a function of the chemical composition of the growth medium, particularly the C:N ratio, which is a key factor that affects the quality of the final product used in animal nutrition programs [9,10]. This highlights the importance of supplementing the medium with nitrogenous nutrients for optimal fungal growth and colonization. Nitrogen metabolite repression (NMR) in filamentous fungi—a regulatory system that controls the expression of enzymes needed to utilize various secondary nitrogen sources—is specifically activated under nitrogen-sufficient conditions, especially when ammonium (NH₄⁺) or L-glutamine are present as the nitrogen source [11,12]. Supplementation with nitrogen-rich sources has been shown to enhance the production of lignolytic enzymes and fungal biomass in P. ostreatus [13].
The CAZy database (www.cazy.org), supported by results from omics studies, provides information on two categories of WRF-expressed proteins involved in lignocellulose degradation—first, associated modules, and second, catalytic modules. The first category corresponds to carbohydrate-binding modules (CBMs); the second is composed of five enzyme subgroups that have been reported: glycoside hydrolases (GH), glycosyltransferases (GT), polysaccharide lyases (PL), carbohydrate esterases (CE), and auxiliary activities (AA) [14]. CBM, GH, GT, PL, and CE are involved in the hydrolysis of carbohydrates such as cellulose and hemicellulose. The AA group includes lignin-modifying enzymes (LME) and lytic polysaccharide monooxygenases (LPMO) that depolymerize lignin. This last group is the most frequent CAZy found in P. ostreatus [15], mainly because this fungus selectively degrades lignin, with a minor effect on cellulose and hemicellulose under nitrogen-sufficient conditions [16,17]. Under these considerations, it is important to identify favorable carbon and nitrogen concentrations in the medium that favor biomass production and lignolytic enzymatic synthesis in P. ostreatus, improving the fermentation of lignocellulosic materials.
Statistical designs of experiments, in particular the central composite and the extreme vertex mixture design, have been consolidated as effective approaches to improve the interpretation of how interactions between nutritional factors regulate growth and enzyme production in P. ostreatus [13], as well as to reduce process times and costs [18]. In this study, an central composite design (CCD) and extreme vertex were used to optimize the culture medium C:N ratio with different sources of inorganic nitrogen that enhances mycelial development, and the expression of enzyme genes related to carbohydrate metabolism (lignocellulose) by P. ostreatus, improving the fermentation process and generating added value to oil palm by-products.
2. Materials and Methods
2.1. Optimization of Biomass Production Using a Central Composite Design (CCD)
A commercial strain of P. ostreatus (genetically compatible with P. ostreatus PC15_PC15) maintained on malt extract agar at 4 °C was used. The inoculum consisted of 4-mm diameter agar discs from the peripheral growth area of the fungus incubated at 25 °C for eight days on malt extract agar; one agar disc with mycelium was used to inoculate 20 mL of liquid culture medium. The concentrations of glucose and inorganic nitrogen sources (ammonium sulfate, ammonium nitrate, and urea) were defined according to a two-factor two-level composite central design (CCD). Here, the factors to be evaluated corresponded to the glucose concentration and the concentration of the inorganic nitrogen source at the high and low levels (Table 1). Thirteen culture media formulations were obtained (Table 2) after defining the high and low levels of the factors in the CCD. The CCD allows estimation of the curvature of a response surface for a specific variable and calculation of the terms of a first or second-order model.

Table 1.
Factors and levels of the central composite design 2k.

Table 2.
Calculated concentrations of glucose and inorganic nitrogen for a CCD 2k.
Submerged culture fermentations (SmF) of 100 mL of culture medium were carried out in 250 mL flasks at 25 °C ± 2 °C and 150 rpm for 13 days [19]. Ammonium sulfate ((NH4)2 SO4), ammonium nitrate (NH4NO3), and urea (CH4N2O) were used as sources of inorganic nitrogen. Glucose and 0.4 g/L−1 of yeast extract were used as the basal ingredients of the medium to achieve different carbon-to-nitrogen (C:N) ratios under the experimental conditions (Table 2). All SmF cultures were inoculated with five 4-mm diameter discs taken from the growth zone of eight-day-old cultures of P. ostreatus.
The elemental composition analysis of the yeast extract used in this study showed an estimated C:N ratio of 4:1; while for urea, the C:N ratio was 0.43:1.
The biomass was measured gravimetrically. For this purpose, the known culture volume was filtered through filter paper (dried and weighed previously). The filter with the retained material was dried at 70 °C for 24 h [20].
2.2. Extreme Vertex Design for Biomass Production in Solid Fermentation Using Oil Palm By-Product Blends
The solid-state fermentation (SSF) systems design was carried out using an experimental extreme vertex mixing design. The C:N ratio of each formulation was adjusted based on the composition that yielded the highest fungal biomass production under SmF, using urea as the selected inorganic nitrogen source. The mixtures were adjusted to 60% moisture content and an initial pH of 5.5, followed by sterilization at 121 °C and 120 kPa for 15 min. After sterilization, the substrate was aseptically inoculated at a rate of 4% (dry matter basis) using 4-mm diameter pellets of Pleurotus ostreatus and incubated at 30 °C for 13 days under dark conditions [21]. To facilitate aerobic metabolism during the incubation, the bags were sealed with a semi-permeable adhesive membrane that allowed gas exchange. Upon completion of the incubation period, the fermented material was dried at 50 °C for 48 h. The components of the mixtures were palm kernel, fiber and hulls, and urea. The amounts established for the blends are shown in Table 3.

Table 3.
By-product amounts in the extreme vertex design.
The elemental composition in terms of the C:N ratio of palm kernel cake, fiber, and palm kernel shells was 18:1, 52:1, and 86:1, respectively.
Since, in solid-state fermentation, the fungal biomass of the fungus grows attached to the lignocellulose biomass, the fungal biomass was measured indirectly by quantifying the amount of N-acetyl-D-glucosamine (NAGA), which is a precursor of chitin, a major component of the fungal cell wall [22].
Determination of Laccase Enzyme Activity
The evaluation of laccase activity was carried out following the methodology of Zhou et al. [23]. For this, 1 g of the fermented residue was suspended in 9 mL of distilled water and incubated at 200 rpm for 10 min. Subsequently, the suspension was centrifuged at 4000 rpm for 15 min. Next, 5 µL of the supernatant was taken and mixed with 1 mL of a 1:100 solution of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) in sodium citrate buffer (pH 4). Finally, absorbance was measured at 436 nm using a spectrophotometer, as reported by Durán-Sequeda et al. [19].
2.3. Transcriptome Analysis
For transcriptomic analysis, the fungus was grown on the optimized medium, enriched with palm kernel cake at 20 g/L. The media were incubated for 8 days at 28 °C and 200 rpm. At the end of the incubation time, the fungal biomass was separated from the culture medium by filtration on sterile 0.45 µm cellulose paper. 100 ng of biomass was sprayed with liquid nitrogen and immediately suspended in Trizol TM Reagent for RNA extraction. Total RNA extraction was performed following the standard RNA extraction protocol described by the manufacturer (Trizol TM Reagent, Invitrogen, Waltham, MA USA; Total RNA extraction protocol). RNA integrity was measured by calculating the RIN value by capillary electrophoresis. Once the RNA quality was verified, RNA-seq sequencing was carried out. A TruSeq stranded mRNA library was prepared, and 150-base paired reads were read on the Illumina Novaseq 6000 platform (San Diego, CA, USA). CUTADAPT v3.5 was used to clean the reads with a Q30 quality threshold, and reads with ambiguous bases were excluded (Ns). Trinity v2.13.2 with default values was used for de novo transcriptome assembly. The overall statistics of the transcriptome were calculated with a proprietary script written in Python (https://www.python.org/). Functional annotation of the assembled sequences was processed with EggNOG-mapper [24], using stringent parameters for filtering and coverage (E-value ≤ 0. 001, minimum score of 60, minimum identity of 40%, and minimum coverage of 20%) which generated GO (Gene Ontology), KEGG (Kyoto Encyclopedia of Genes and Genomes) CAZy (Carbohydrate-Active enZYmes), PFAMs (Protein Families) annotations, the functional description, short name and Enzyme Commission Number (EC) available for each sequence. Additionally, KEGG Mapper Reconstruction of GhostKOALA was used to determine the frequencies of KEGG Categories identified in the transcriptome.
2.4. Determination of the Chemical Composition of PKC Fermented in Urea-Enriched Solid Medium
The PKC, together with the fungal biomass, was dried at 60 °C until a constant weight was reached at the end of the solid-state fermentation. Subsequently, the chemical composition of the fermented substrate in terms of crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), and lignin (LIG) was determined by NIRS (near infrared spectroscopy) [25].
2.5. Statistical Analysis
Minitab® 18 was used to construct the statistical design, assess statistical significance, obtain the regression models, and find the simultaneous local optimum of one or more response variables [26].
3. Results and Discussion
3.1. Biomass Production Optimization
Figure 1 shows the results of biomass production of P. ostreatus (gL−1) growing in different concentrations of glucose and inorganic nitrogen sources in a liquid medium. CCD results indicated that biomass production was affected by both glucose concentrations and different sources and ratios of inorganic nitrogen in the medium (Figure 1). Maximum biomass production using ammonium sulfate (8 g/L), ammonium nitrate (10.5 g/L), and urea (8 g/L) was obtained at C:N ratios of 10:1, 11:1 and 34:1, respectively (Figure 2), with no significant statistical differences (p < 0.05). However, it is suggested that the nitrogen available in urea is more assimilable by the fungus for biomass production, given the higher C:N ratio needed to produce a similar amount of biomass to that of the other nitrogen sources (Figure 2).
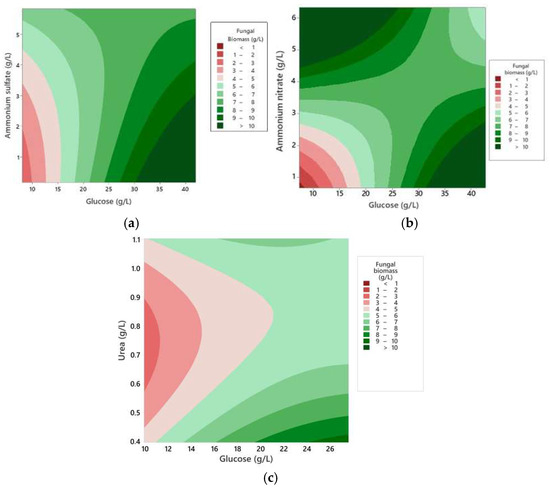
Figure 1.
Boundary plots of biomass production of P. ostreatus. (a) Culture at different concentrations of ammonium sulfate. (b) Culture at different concentrations of ammonium nitrate. (c) Culture at different concentrations of urea.
Figure 2.
Maximum biomass production of P. ostreatus. (a) Synthetic with ammonium sulfate (glucose 41.97 g/L and ammonium sulfate 3 g/L) results in 8 g/L of biomass, and a C:N ratio of 10.3:1. (b) Synthetic with ammonium nitrate (glucose 37.5 g/L and ammonium nitrate 1.5 g/L) results in 10.5 g/L of biomass and a C:N: ratio of 11:1. (c) Synthetic with urea (glucose 18.72 g/L and urea 0.39 g/L) results in 8 g/L of biomass and a C:N: ratio of 34:1.
These findings indicate that urea provides a higher amount of assimilable nitrogen in the form of ammonia, facilitating a faster and more efficient uptake of nitrogen, supporting increased cell growth, and, consequently, greater biomass accumulation [27]. Furthermore, the high ratio of carbon to nitrogen in the case of urea may allow for an optimal balance between energy availability and nitrogen supply, which is crucial for the synthesis of essential biomolecules such as proteins and nucleic acids. This balance could explain why urea, although requiring a higher C ratio, is more effective in promoting the biomass production of P. ostreatus.
Some authors have reported that supplementing the substrate with urea not only increases the productivity of P. ostreatus but also enhances its nutritional value, particularly in terms of protein and β-glucans [28]. A recent study found that growing P. ostreatus on a spent oyster substrate mixed with wheat straw (1:1, w/w mixture) enriched with urea in doses between 3g and 5g per kilogram of substrate increased the protein and essential amino acid contents of the fungus, reaching up to 0.8% and 0.31%, respectively [29]. This suggests a significant improvement in the nutritional value of P. ostreatus.
Using response surface methodology, another study identified that the optimal urea concentrations for higher laccase enzyme production in P. ostreatus were between 0.8% and 1.2% (w/v), similar to the findings observed in this research regarding biomass production [30]. When evaluating the effects of four lignocellulolytic substrates and urea dosage on the growth and yield of P. ostreatus, Déo, and Faustin [31] found that the highest yield and mushroom diameter were observed with a corn cob substrate supplemented with 100 g of urea per kg of substrate. This dose is significantly higher than that reported in this study, possibly due to differences in experimental conditions, such as substrate variety, mushroom species, or nutrient availability, all of which may influence the response to urea fertilization.
In this regard, the use of urea as a source of inorganic nitrogen for P. ostreatus growth maximizes fungal biomass production. It presents an economical and easy-to-use alternative compared to other inorganic nitrogen sources evaluated in this study, making it a preferred option for large-scale production of mycelial protein from P. ostreatus.
3.2. Biomass Production and Laccase Enzyme Activity in Solid-State Fermentation
In Figure 3, each vertex of the triangle represents one of the three components of the mixture. The figure shows 10 points in the design space, corresponding to the mixtures defined in Table 3. The data indicate that both fungal biomass (2.4 g/L) and laccase enzyme activity (1200–1400 U/L) reached the highest values in the mixtures with the highest proportion of palm kernel cake. This suggests that fungal growth may benefit from the higher amount of protein present in the palm kernel cake compared to fiber and kernel hulls. Therefore, this by-product seems to be a suitable option for bioconversion into fungal biomass.
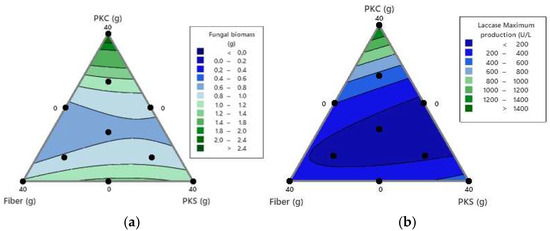
Figure 3.
(a) Mixing boundary plot of maximum biomass production and (b) maximum laccase enzyme production of P. ostreatus growing on urea-enriched oil palm by-product mixtures. PKC: palm kernel cake; PKS: palm kernel shells.
Recent studies have also shown that urea is an efficient source of inorganic nitrogen, stimulating growth and ligninolytic enzyme synthesis in P. ostreatus [32,33]. Additionally, urea supplementation not only stimulates the growth of WRF but also enhances lignin degradation by increasing the activity of ligninolytic enzymes, thus promoting the bioconversion of lignocellulosic residues [34].
3.3. Transcriptomic Analysis
Identification of metabolic pathways activated in P. ostreatus under urea- and palm kernel cake-enriched conditions, related to the fungus’s ability to bioconvert lignocellulosic and metabolic components, was carried out. 7029 KEGG_ko terms were filtered from the genome with 13,635 coding fragments, and the frequency of each unique term (3167) was established. Table 4 presents the top 20 most frequent terms, totaling 389 annotations, and Figure 4 the most frequent domains. The results show a clear activation of carbohydrate metabolism, highlighting proteins associated with complex polysaccharide hydrolytic pathways such as chitinase (K01183) and B-glucosidase (K05349) with the highest frequencies, of 26 and 24, respectively; as well as cellulose 1,4-B-cellobiosidase (K01225) and pectate lyase (K01728). Unclassified metabolic pathways also support this activity, as oxidative proteins like glyoxal oxidase (K20929) and lytic cellulose monooxygenase (K19356) are frequently found and are key for lignin degradation [35,36]. Similarly, the activation of xenobiotics metabolism is highlighted, with enzymes like salicylate hydroxylase (K00480) and microsomal epoxide hydrolase (K01253) showing capabilities in degrading aromatic or toxic compounds derived from the processing of lignin’s aromatic monomers [37,38]. These results show that P. ostreatus is highly efficient in the molecular activation of enzymes involved in the bioconversion of agro-industrial wastes such as palm kernel cake.

Table 4.
Top 20 most frequent KEGG terms in the transcriptome of P. ostreatus grown in GY medium enriched with urea and palm kernel cake.
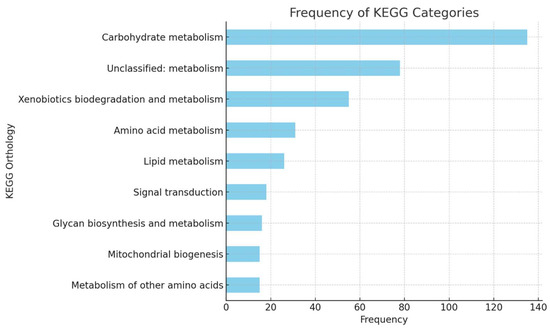
Figure 4.
Frequency of KEGG categories identified in the transcriptome of P. ostreatus grown on GY medium enriched with urea and palm kernel cake.
Table 5 presents a comparison between the CAZymes identified in P. ostreatus transcriptome and frequencies reported in the literature, aiming to explore similarities and recurrent patterns across different studies and experimental contexts. While the frequencies in both cases are not directly comparable due to differences in methodologies and experimental conditions, the results highlight consistent trends that reinforce the relevance of certain CAZymes in the degradation of lignocellulosic substrates. On one hand, the frequencies reported in the transcriptome (405 genes identified) specifically reflect gene expression under the current experimental conditions (urea-enriched medium and palm kernel cake). On the other hand, the frequencies in the literature represent an aggregate of several studies (n = 9) *, covering different experimental conditions, methodologies (transcriptome, secretome, proteome), and lignocellulosic substrates. In both datasets (Table 5), the most frequently reported domains are related to holocellulose: GH3, GH7, CBM1, and AA9 are prominent in both lists and suggest their key role in cellulose and hemicellulose hydrolysis. For lignin degradation, AA1, AA2, AA3, and AA5 stand out, reinforcing P. ostreatus’s ability to depolymerize lignin through oxidative pathways. These findings indicate that the most relevant enzymes in lignocellulosic processing are consistently expressed under various experimental conditions.

Table 5.
Frequencies of CAZymes identified in the transcriptome of P. ostreatus and comparison with published literature on lignocellulosic substrates.
Figure 5 illustrates the relationship between the CAZyme domains identified in the P. ostreatus transcriptome and those reported in the literature. It shows 23 common domains, including key families such as GH3, GH7, AA9, AA1, and AA5, essential for holocellulose and lignin degradation. The 36 unique domains in the transcriptome suggest specific adaptations to the experimental conditions with palm kernel cake, while the 29 unique domains in the literature reflect methodological and substrate differences used in other studies. This overlap validates the relevance of the identified CAZymes and highlights new opportunities to explore specific enzymes with biotechnological potential in the bioconversion of lignocellulosic residues, positioning P. ostreatus as a robust model in this field.
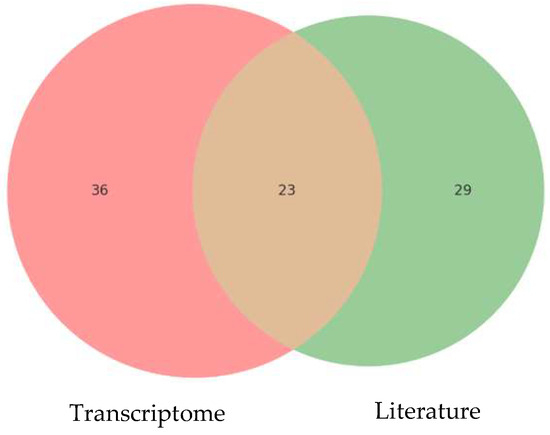
Figure 5.
Comparison of CAZyme domains identified in the transcriptome of P. ostreatus and reported in the literature.
Figure 6 presents an integrated analysis of the CAZyme domains identified in the P. ostreatus transcriptome compared to the frequencies reported in the literature. A summary of the most frequently mentioned domains in previous studies is provided, emphasizing their relevance in lignin, cellulose, and hemicellulose processing. The figure also highlights the most expressed domains in the transcriptome under specific experimental conditions, such as the palm kernel cake enriched medium. Additionally, Figure 6 visualizes key findings, including the high frequency of GH3, AA1, AA5, and CBM1, which validate the experimental results in the context of existing knowledge. Furthermore, unique domains of the transcriptome, such as GH18, were identified, which might reflect specific adaptations to the experimental environment and are primarily involved in the degradation of fungal cell wall components, such as chitin [15,46]. This integration of results underlines both the consistency and novel contributions of the present study, reinforcing the importance of P. ostreatus as a model for the bioconversion of lignocellulosic residues.
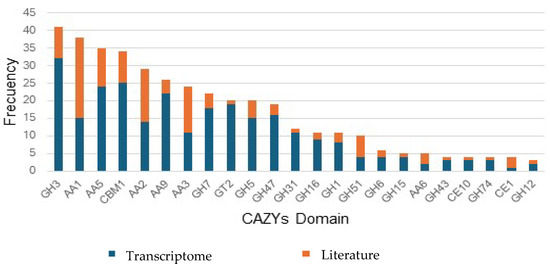
Figure 6.
Comparison of CAZyme domains in the transcriptome and the literature.
Based on omics data from the literature and the findings of this study, we propose a general mechanism to elucidate the enzymatic degradation of lignocellulose by Pleurotus sp. This mechanism is supported by the structural composition of the lignocellulosic complex, represented by the carbohydrate cellulose and hemicellulose, and the lignin fraction. CBM1 interacts with the cellulose or hemicellulose fraction without exhibiting catalytic activity, thereby enhancing the function of GH7 (which includes EC 3.2.1.4 and cellulose endo-β-1,4-glucosidase). Additional GHs, such as GH3 (including EC 3.2.1.58 and glucan exo-β-1,3-glucosidase) and GH51 (including EC 3.2.1.8, xylan endo-β-1,4-xylosidase or EC 3.2.1.37, and xylan exo-β-1,4-xylosidase, among others), may contribute to hemicellulose fraction modification. AA9 (EC 1.14.99.54; lytic cellulose monooxygenase) plays a role in cellulose degradation. AA1_1 (laccases) and AA2 (including manganese peroxidase EC 1.11.1.13 and versatile peroxidase EC 1.11.1.16) are classified as LMEs. Hydrogen peroxide (H₂O₂), generated by AA3_2 (which includes aryl alcohol oxidase and glucose 1-oxidase) and AA5_1 (including EC 1.2.3.15, glyoxal oxidase serves as a co-substrate for AA2 and AA9.
Table 6 presents a set of KEGG orthology (KO) genes identified in this study that are potentially involved in the regulation of nitrogen metabolism, particularly in the utilization of urea as a nitrogen source during the growth of P. ostreatus.

Table 6.
Proposed metabolic pathway for nitrogen assimilation from urea in Pleurotus ostreatus, including key enzymes and corresponding KEGG orthologs (KOs).
Based on the findings, the proposed pathway for urea assimilation in P. ostreatus involves the use of urea as an accessible nitrogen source, which is initially hydrolyzed into ammonia and carbon dioxide by the enzyme urease. The resulting ammonia is then efficiently incorporated into organic molecules through the glutamine synthetase–glutamate synthase (GS-GOGAT) cycle, a high-affinity system essential under low-ammonia conditions. Subsequently, the glutamine produced is converted into glutamate, which acts as a key nitrogen donor in transamination reactions catalyzed by aminotransferases. These reactions enable the incorporation of nitrogen into amino acids and other nitrogen-containing compounds, thereby supporting fungal growth and biomass production. The identification of corresponding KEGG orthologs provides additional evidence supporting the functionality of these enzymatic steps in P. ostreatus.
3.4. Changes in the Chemical Composition of PKC Fermented
Table 7 presents the chemical composition of palm kernel cake (PKC), both unfermented (NFPKC) and fermented with P. ostreatus (PoFPKC). Liquid fermentation of palm kernel cake with P. ostreatus resulted in significant improvements to its nutritional profile, including a 26.3% reduction in neutral detergent fiber (NDF), a 20.4% reduction in acid detergent fiber (ADF), and a 46.9% reduction in lignin (LIG). This indicates that pretreatment of palm kernel cake with P. ostreatus facilitates the breakdown of bonds between cellulose, hemicellulose, and lignin, as a result of the lignocellulosic enzymes secreted by the fungus [47], as supported by the transcriptome findings reported in this study.

Table 7.
Chemical composition of PKC after fermentation with P. ostreatus.
In a previous study, it was observed that the production of ligninolytic enzymes by P. ostreatus significantly impacted the lignocellulosic structure of maize stover, leading to an 18.3% reduction in NDF and a 6.8% reduction in ADF content, along with a 118% increase in non-fiber carbohydrates [48]. Similarly, treatment of oil palm empty fruit bunches (RFV) with WRF, including Pleurotus sp., resulted in up to a 20% decrease in lignin content, alongside increases of between 7.49 and 16.67% in cellulose levels [49].
Simultaneously, a 69.8% increase in CP content was recorded, which could be attributed to the production of mycelial biomass by the fungus, which is particularly rich in protein [50]. In this regard, protein enrichment of the lignocellulosic substrate occurs because approximately 70% of the nitrogen present in P. ostreatus is in the form of protein [51]. Similarly, increases of up to 71% in crude protein content were found in corn cob waste pretreated with P. ostreatus [52], while fermentation of maize straw with Pleurotus spp. led to an increase in the protein content of up to 38% [17].
In parallel, it has been demonstrated that, beyond improving the nutritional composition of the substrate, the fermentation of lignocellulosic material with P. ostreatus enhances the final product with bioactive secondary metabolites. Among these is lovastatin, an inhibitor of the enzyme (3S)-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which plays a key role in the mevalonate pathway for the synthesis of isoprenoid ether lipid precursors involved in the formation of cell membranes in methanogenic archaea [53]. Lovastatin also interferes with the synthesis of coenzyme F420, which participates in electron transport during methanogenesis, thereby contributing to a reduction in methane production [54]. The presence of this metabolite in palm kernel cake (PKC) may be associated with the identification of KEGG orthologs K05607, K02267, and K00507 in the transcriptomic data from this study, corresponding to the enzymatic activities of enoyl-CoA hydra-tase/isomerase, cytochrome c oxidase, and cytochrome b5-like heme/steroid-binding domain proteins, respectively.
Therefore, the enrichment of PKC with anti-methanogenic metabolites may represent a sustainable approach to mitigating emissions of enteric methane—a potent greenhouse gas generated during rumen fermentation—without compromising animal nutrition [55,56]. These results support the potential of PoFPKC as a functional feed supplement for ruminants.
4. Conclusions
Optimization of fermentation conditions using statistical methods determined that the use of urea as a source of inorganic nitrogen and palm kernel cake as a by-product improved biomass production and the expression of genes involved in lignocellulose hydrolysis in Pleurotus ostreatus. This approach contributes to understanding the biochemical processes that regulate the bioconversion of palm kernel cake into a functional feed enriched with microbial protein from P. ostreatus for use in ruminant feed.
Author Contributions
A.I.-R.: conceptualization, resources, methodology, data analysis, writing—original draft, writing—review and editing. D.E.D.-S.: methodology, validation, formal analysis, writing—original draft, data curation, writing—review and editing. A.C.C.-P.: methodology, validation, formal analysis. P.F.-C.: formal analysis, writing—original draft, resources. R.B.-R.: writing—review and editing, resources, formal analysis. J.E.M.-R.: writing—original draft, writing—review and editing, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Popular del Cesar, grant number 054-2019, and the APC was funded by Universidad Popular del Cesar.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author José Edwin Mojica-Rodríguez was employed by the company Corporación Colombiana de Investigación Agropecuaria (AGROSAVIA). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kumar, V.; Goala, M.; Kumar, P.; Singh, J.; Kumar, P. Integration of treated agro-based wastewaters (TAWs) management with mushroom cultivation. In Environmental Degradation: Causes and Remediation Strategies; Agro Environ Media, Agriculture and Environmental Science Academy: Haridwar, India, 2020; pp. 63–75. [Google Scholar] [CrossRef]
- Conceição, A.A.; Mendes, T.D.; Mendonça, S.; Quirino, B.F.; de Almeida, E.G.; de Siquiera, F.G. Nutraceutical Enrichment of Animal Feed by Filamentous Fungi Fermentation. Fermentation 2022, 8, 402. [Google Scholar] [CrossRef]
- Thamvithayakorn, P.; Phosri, C.; Pisutpaisal, N.; Krajangsang, S.; Whalley, A.J.S.; Suwannasai, N. Utilization of oil palm decanter cake for valuable laccase and manganese peroxidase enzyme production from a novel white-rot fungus, Pseudolagarobasidium sp. PP17-33. 3 Biotech 2019, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Azzahra, Y.R.; Toharmat, T.; Prihantoro, I. Bio-processing Plantation by-products with White Oyster Mushroom (Pleurotus ostreatus) to Improve Fermentability and Digestibility Based on Substrate Type and Fermentation Time. Bul. Peternak. 2022, 46, 228. [Google Scholar] [CrossRef]
- Santos, L.V.; Silva, R.R.; Silva, F.F.; Silva, J.W.D.; Barroso, D.S.; Silva, A.P.; Souza, S.O.; Santos, M.C. Increasing levels of palm kernel cake (Elaeis guineensis jacq.) in diets for feedlot cull cows. Chil. J. Agric. Res. 2019, 79, 628–635. [Google Scholar] [CrossRef]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Amorim, G.M.; Azevêdo, J.A.G.; Godoy, M.G.; Freire, D.M.G. Solid-state fermentation of co-products from palm oil processing: Production of lipase and xylanase and effects on chemical composition. Biocatal Biotransform. 2018, 36, 381–388. [Google Scholar] [CrossRef]
- Cruz-Vázquez, A.; Tomasini, A.; Armas-Tizapantzi, A.; Marcial-Quino, J.; Montiel-González, A.M. Extracellular proteases and laccases produced by Pleurotus ostreatus PoB: The effects of proteases on laccase activity. Int. Microbiol. 2022, 25, 495–502. [Google Scholar] [CrossRef]
- Nur-Nazratul, F.M.Y.; Rakib, M.R.M.; Zailan, M.Z.; Yaakub, H. Enhancing in vitro ruminal digestibility of oil palm empty fruit bunch by biological pre-treatment with Ganoderma lucidum fungal culture. PLoS ONE 2021, 16, e0258065. [Google Scholar] [CrossRef]
- Boadu, K.B.; Nsiah-Asante, R.; Antwi, R.T.; Obirikorang, K.A.; Anokye, R.; Ansong, M. Influence of the chemical content of sawdust on the levels of important macronutrients and ash composition in Pearl oyster mushroom (Pleurotus ostreatus). PLoS ONE 2023, 18, e0287532. [Google Scholar] [CrossRef]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef]
- Davis, M.A.; Wong, K.H. Nitrogen Metabolism in Filamentous Fungi. In Cellular and Molecular Biology of Filamentous Fungi; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 325–338. [Google Scholar] [CrossRef]
- Velásquez-Quintero, C.; Merino-Restrepo, A.; Hormaza-Anaguano, A. Production, extraction, and quantification of laccase obtained from an optimized solid-state fermentation of corncob with white-rot fungi. J. Clean. Prod. 2022, 370, 133598. [Google Scholar] [CrossRef]
- Xu, F.; Chen, P.; Li, H.; Qiao, S.; Wang, J.; Wang, Y.; Wang, X.; Wu, B.; Liu, H.; Wang, C.; et al. Comparative transcriptome analysis reveals the differential response to cadmium stress of two Pleurotus fungi: Pleurotus cornucopiae and Pleurotus ostreatus. J. Hazard. Mater. 2021, 416, 125814. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; López-Lucendo, M.F.; Pérez-Boada, M.; Rencoret, J.; Gutiérrez, A.; Pisabarro, A.G.; Ramírez, L.; Martínez, A.T. A secretomic view of woody and nonwoody lignocellulose degradation by Pleurotus ostreatus. Biotechnol Biofuels 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Datsomor, O.; Gou-qi, Z.; Miao, L. Effect of ligninolytic axenic and coculture white-rot fungi on rice straw chemical composition and in vitro fermentation characteristics. Sci. Rep. 2022, 12, 1129. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Luo, L.; Zhang, H.; Liao, Y.; Gou, C. Enhancement of the nutritional value of fermented corn stover as ruminant feed using the fungi Pleurotus spp. Sci. Rep. 2021, 11, 11961. [Google Scholar] [CrossRef]
- Venkateswarulu, T.C.; Prabhakar, K.V.; Kumar, R.B.; Krupanidhi, S. Modeling and optimization of fermentation variables for enhanced production of lactase by isolated Bacillus subtilis strain VUVD001 using artificial neural networking and response surface methodology. 3 Biotech 2017, 7, 186. [Google Scholar] [CrossRef]
- Durán-Sequeda, D.; Suspes, D.; Maestre, E.; Alfaro, M.; Pérez, G.; Ramírez, L.; Pisabarro, A.G.; Sierra, R. Effect of Nutritional Factors and Copper on the Regulation of Laccase Enzyme Production in Pleurotus ostreatus. J. Fungi 2022, 8, 7. [Google Scholar] [CrossRef]
- Tinoco-Valencia, R.; Gómez-Cruz, C.; Galindo, E.; Serrano-Carreón, L. Toward an understanding of the effects of agitation and aeration on growth and laccases production by Pleurotus ostreatus. J. Biotechnol. 2014, 177, 67–73. [Google Scholar] [CrossRef]
- Rubiano-Orozco, L.A.; Castro-Pacheco, A.C.; Jiménez-Rojas, F.; Fragoso-Castilla, P.J.; Ibarra-Rondón, A.J.; Rodríguez-Jiménez, D.M. Evaluación de la actividad lignocelulolítica de hongos cultivados en subproductos de la palma de aceite (Elaeis guineensis). Biotecnol. Sect. Agropecu. Agroind. 2024, 22, 70–86. [Google Scholar]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization pase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Zhou, S.; Raouche, S.; Grisel, S.; Navarro, D.; Sigoillot, J.C.; Herpoël-Gimbert, I. Solid-state fermentation in multi-well plates to assess pretreatment efficiency of rot fungi on lignocellulose biomass. Microb. Biotechnol. 2015, 8, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Ariza Nieto, C.; Mayorga Mogollón, O.L.; Parra Forero, D.M.; Camargo Hernández, D.B.; Buitrago Albarado, C.P.; Moreno Rodríguez, J.M. Tecnología NIRS para el Análisis Rápido y Confiable de la Composición Química de Forrajes Tropicales; Corporación Colombiana de Investigación Agropecuaria (Agrosavia): Agustín Codazzi, Colombia, 2020. [Google Scholar] [CrossRef]
- Minitab Inc, “Minitab® 18” [Software de Análisis Estadístico]. 2017. Available online: https://www.minitab.com/es-mx/products/minitab/ (accessed on 30 October 2024).
- Naim, L.; Alsanad, M.A.; El Sebaaly, Z.; Shaban, N.; Abou Fayssal, S.; Sassine, Y.N. Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to different doses and timings of nano-urea. Saudi J. Biol. Sci. 2020, 27, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.D.; Rodríguez da Luz, J.M.; Albino Paes, S.; Oliveira Ribeiro, J.J.; Soares da Silva, M.C.; Megumi Kasuya, M.C. Nitrogen Supplementation on the Productivity and the Chemical Composition of Oyster Mushroom. J. Food Res. 2012, 1, 113. [Google Scholar] [CrossRef]
- Sassine, Y.N.; Naim, L.; El Sebaaly, Z.; Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H. Nano urea effects on Pleurotus ostreatus nutritional value depending on the dose and timing of application. Sci. Rep. 2021, 11, 5588. [Google Scholar] [CrossRef]
- Chiranjeevi, P.V.; Rajasekara, M.; Sathish, T. Enhancement of Laccase Production from Pleurotus ostreatus PVCRSP-7 by altering the Nutritional Conditions using Response Surface Methodology. BioResources 2014, 9, 4212–4225. [Google Scholar] [CrossRef]
- Déo, N.; Faustin, K. Effect of substrates and doses of urea on growth and yield of an oyster mushroom (Pleurotus ostreatus) in greenhouse. Int. J. Agric. Policy Res. 2015, 3, 314–322. [Google Scholar] [CrossRef]
- Sośnicka, A.; Kózka, B.; Makarova, K.; Giebułtowicz, J.; Klimaszewska, M.; Turło, J. Optimization of White-Rot Fungi Mycelial Culture Components for Bioremediation of Pharmaceutical-Derived Pollutants. Water 2022, 14, 1374. [Google Scholar] [CrossRef]
- Haider, A.; Alam, M.M.; Khan, A.A.; Zulfiqar, M.A. Optimization of Cultural Conditions for the Treatment of Pulp and Paper Industrial Effluent by Pleurotus ostreatus (L.). Pak. J. Agric. Res. 2019, 32, 507–513. [Google Scholar] [CrossRef]
- Agustin, F.; Elihasridas; Juliyarsi, I. The effect of urea supplementation and incubation time in fermentation process of bagasse by using Ganoderma lucidum on the growth of G. lucidum and the nutritive value of bagasse. IOP Conf. Ser. Earth Environ. Sci. 2019, 287, 012016. [Google Scholar] [CrossRef]
- Daou, M.; Faulds, C.B. Glyoxal oxidases: Their nature and properties. World J. Microbiol. Biotechnol. 2017, 33, 87. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.J.; Wei, F.; Wan, Z.; Dong, Y.; Lu, Y.; Yang, P.; Jin, Y.; Saddler, J. Elucidating the synergistic action between sulfonated lignin and lytic polysaccharide monooxygenases (LPMOs) in enhancing cellulose hydrolysis. Int. J. Biol. Macromol. 2025, 296, 139674. [Google Scholar] [CrossRef]
- Erickson, E.; Bleem, A.; Kuatsjah, E.; Werner, A.Z.; DuBois, J.L.; McGeehan, J.E.; Eltis, L.D.; Beckham, G.T. Critical enzyme reactions in aromatic catabolism for microbial lignin conversión. Nat. Catal. 2022, 5, 86–98. [Google Scholar] [CrossRef]
- Ning, D.; Wang, H.; Ding, C.; Lu, H. Novel evidence of cytochrome P450-catalyzed oxidation of phenanthrene in Phanerochaete chrysosporium under ligninolytic conditions. Biodegradation 2010, 21, 889–901. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Zhou, Y.; Zheng, S.; Hu, Q.; Zou, Y. Label-free comparative proteomic analysis of Pleurotus eryngii grown on sawdust, bagasse, and peanut shell substrates. J. Proteom. 2024, 294, 105074. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, G.; Yang, M.; Yang, X.; Li, T.; Chen, G. De novo transcriptome analysis of Pleurotus djamor to identify genes encoding CAZymes related to the decomposition of corn stalk lignocellulose. J. Biosci. Bioeng. 2019, 128, 529–536. [Google Scholar] [CrossRef]
- Xie, C.; Gong, W.; Zhu, Z.; Zhou, Y.; Xu, C.; Yan, L.; Hu, Z.; Ai, L.; Peng, Y. Comparative secretome of white-rot fungi reveals co-regulated carbohydrate-active enzymes associated with selective ligninolysis of ramie stalks. Microb. Biotechnol. 2021, 14, 911–922. [Google Scholar] [CrossRef]
- Valadares, F.; Gonçalves, T.A.; Damasio, A.; Milagres, A.M.F.; Squina, F.M.; Segato, F.; Ferraz, A. The secretome of two representative lignocellulose-decay basidiomycetes growing on sugarcane bagasse solid-state cultures. Enzyme Microb. Technol. 2019, 130, 109370. [Google Scholar] [CrossRef]
- Wu, H.; Nakazawa, T.; Xu, H.; Yang, R.; Bao, D.; Kawauchi, M.; Sakamoto, M.; Honda, Y. Comparative transcriptional analyses of Pleurotus ostreatus mutants on beech wood and rice straw shed light on substrate-biased gene regulation. Appl. Microbiol. Biotechnol. 2021, 105, 1175–1190. [Google Scholar] [CrossRef]
- Peña, A.; Peña, A.; Babiker, R.; Chaduli, D.; Lipzen, A.; Wang, M.; Chovatia, M.; Rencoret, J.; Marques, G.; Sánchez-Ruiz, M.I.; et al. A multiomic approach to understand how Pleurotus eryngii transforms non-woody lignocellulosic material. J. Fungi 2021, 7, 426. [Google Scholar] [CrossRef]
- Alfaro, M.; Oguiza, J.A.; Ramírez, L.; Pisabarro, A.G. Comparative analysis of secretomes in basidiomycete fungi. J. Proteom. 2014, 6, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sossah, F.L.; Li, Z.; Hyde, K.D.; Li, D.; Xiao, S.; Fu, Y.; Yuan, X.; Li, Y. Genome-Wide Identification and Analysis of Chitinase GH18 Gene Family in Mycogone perniciosa. Front. Microbiol. 2021, 11, 596719. [Google Scholar] [CrossRef]
- Yarden, O.; Zhang, J.; Marcus, D.; Changwal, C.; Mabjeesh, S.J.; Lipzen, A.; Zhang, Y.; Savage, E.; Ng, V.; Grigoriev, I.V.; et al. Altered Expression of Two Small Secreted Proteins (ssp4 and ssp6) Affects the Degradation of a Natural Lignocellulosic Substrate by Pleurotus ostreatus. Int. J. Mol. Sci. 2023, 24, 16828. [Google Scholar] [CrossRef]
- Olagunju, L.K.; Isikhuemhen, O.S.; Dele, P.A.; Anike, F.N.; Essick, B.G.; Holt, N.; Udombang, N.S.; Ike, K.A.; Shaw, Y.; Brice, R.M.; et al. Pleurotus ostreatus Can Significantly Improve the Nutritive Value of Lignocellulosic Crop Residues. Agriculture 2023, 13, 1161. [Google Scholar] [CrossRef]
- Sabariyah, S.; Rusdi; Damry; Hasanuddin, A. The nutritional value enhancement of oil palm empty fruit bunches as animal feed using the fungus Coprinus comatus, with different numbers of inoculums and incubation times. Int. J. Des. Nat. Ecodyn. 2021, 16, 269–274. [Google Scholar] [CrossRef]
- van Dam, L.; Cruz-Morales, P.; Valerón, N.R.; de Carvalho, A.C.; Vásquez, D.P.; Lübke, M.; Pedersen, L.K.; Munk, R.; Sommer, M.O.A.; Jahn, L.J. GastronOmics: Edibility and safety of mycelium of the oyster mushroom Pleurotus ostreatus. Curr. Res. Food Sci. 2024, 9, 100866. [Google Scholar] [CrossRef]
- Jaworska, G.; Berna, E. Comparison of amino acid content in canned Pleurotus ostreatus and Agaricus bisporus mushrooms. Veg. Crops Res. Bull. 2011, 74, 107–115. [Google Scholar] [CrossRef]
- Castorina, G.; Cappa, C.; Negrini, N.; Criscuoli, F.; Casiraghi, M.C.; Marti, A.; Rollini, M.; Consonni, G.; Erba, D. Characterization and nutritional valorization of agricultural waste corncobs from Italian maize landraces through the growth of medicinal mushrooms. Sci. Rep. 2023, 13, 21148. [Google Scholar] [CrossRef]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochem. Rev. 2022, 21, 339–383. [Google Scholar] [CrossRef]
- Ábrego-Gacía, A.; Poggi-Varaldo, H.M.; Robles-González, V.; Ponce-Noyola, T.; Calva-Calva, G.; Ríos-Leal, E.; Estrada-Bárcenas, D.; Mendoza-Vargas, A. Lovastatin as a supplement to mitigate rumen methanogenesis: An overview. J. Anim. Sci. Biotechnol. 2021, 12, 123. [Google Scholar] [CrossRef]
- Astudillo-Neira, R.; Suescun-Ospina, S.; Vera-Aguilera, N.; Alarcon-Enos, J.; Ávila-Stagno, J. Biodegraded hay with graded addition of Pleurotus ostreatus improves dry matter disappearance and reduces methane production of diets incubated in vitro. Ital. J. Anim. Sci. 2023, 22, 347–358. [Google Scholar] [CrossRef]
- Khonkhaeng, B.; Cherdthong, A. Improving nutritive value of purple field corn residue and rice straw by culturing with white-rot fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).