Utilizing Agrobyproducts: Potential Alternative Substrates for Cultivation of Lentinula edodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Treatments
2.2. Spawning and Incubation
2.3. Fruiting and Harvest
2.4. Analytical Tests on Mushrooms
2.5. Statistical Analysis
3. Results
3.1. Mycelial Growth and Development of L. edodes Under Different Treatments
3.2. Effect of Treatment on the Yield of L. edodes
3.3. Effect of Treatment on the Nutritional Value of L. edodes
3.4. Correlation Between Various Paraments of L. edodes Grown on Different Formulas
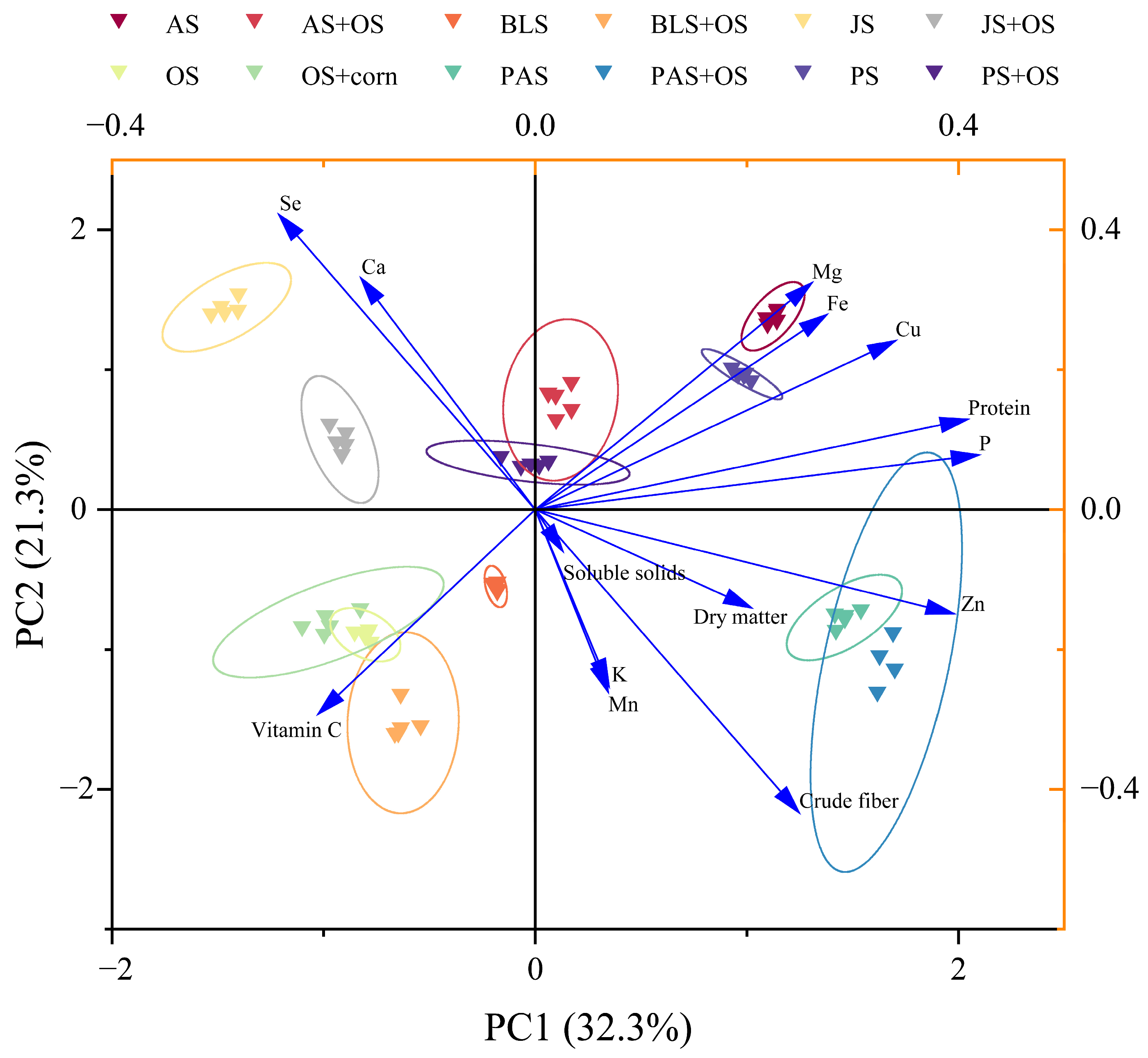
3.5. Principal Component Analysis Among Nutrient Composition of L. edodes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III-world production of different mushroom species in 21st Century. Mushroom Res. 2021, 29, 75–111. [Google Scholar] [CrossRef]
- Yu, H.; Li, Q.; Shen, X.; Zhang, L.; Liu, J.; Tan, Q.; Li, Y.; Lv, B.; Shang, X. Transcriptomic analysis of two Lentinula edodes genotypes with different cadmium accumulation ability. Front. Microbiol. 2020, 11, 558104. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Ely, M.R. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 05, 1095–1105. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Chang, S.; Philip, M.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2004. [Google Scholar]
- Wang, Z.; Tao, K.; Yuan, J.; Liu, X. Design and experiment on mechanized batch harvesting of shiitake mushrooms. Comput. Electron. Agric. 2024, 217, 108593. [Google Scholar] [CrossRef]
- Sud, D.; Sharma, S.; Dhiman, R. Significance and cultivation techniques of shiitake mushroom (Lentinula edodes (Berk.) Pegler). Indian Phytopathol. 2024, 77, 925–945. [Google Scholar] [CrossRef]
- Frey, G.E.; Durmus, T.; Sills, E.O.; Isik, F.; Comer, M.M. Potential alternative tree species as substrates for forest farming of log-grown shiitake mushrooms in the Southeastern United States. HortTechnology 2020, 30, 741–744. [Google Scholar] [CrossRef]
- Zhao, S.; Zheng, Y.; Huang, Y. Log cultivation of Lentinula edodes. Edible Med. Mushrooms 2024, 32, 162–171. [Google Scholar]
- Huang, Y.; Zheng, Y.; Zhang, C. Assessing mushroom-forest conflicts as bottlenecks in the development of shiitake and Auricularia heimuer industries. Edible Med. Mushrooms 2018, 26, 333–337. [Google Scholar]
- Saldanha, A.; Gomes, L.; Pinela, J.; Coimbra, M.; Barros, L.; Inês, M.; Pereira, C. Sustainable cultivation of edible mushrooms: Preserving biodiversity and ensuring product quality. Biol. Life Sci. Forum. 2023, 27, 6. [Google Scholar] [CrossRef]
- Desisa, B.; Muleta, D.; Dejene, T.; Jida, M.; Goshu, A.; Negi, T.; Martin-Pinto, P. Utilization of local agro-industrial by-products based substrates to enhance production and dietary value of mushroom (P. ostreatus) in Ethiopia. World J. Microbiol. Biotechnol. 2024, 40, 277. [Google Scholar] [CrossRef]
- Barua, B.; Nigaki, A.; Kataoka, R. A new recycling method through mushroom cultivation using food waste: Optimization of mushroom bed medium using food waste and agricultural use of spent mushroom substrates. Recycling 2024, 9, 58. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Abiakshara, V.; Perumal, C. Utilization of lignocellulosic substrates on oyster mushroom (Pleurotus citrinopileatus) cultivation. Agric. Sci. Dig. Res. J. 2023, D-5777, 1–7. [Google Scholar] [CrossRef]
- Baktemur, G.; Kara, E.; Yarar, M.; Yilmaz, N.; AĞÇAm, E.; Akyildiz, A.; TaŞKin, H. Yield, quality and enzyme activity of shiitake mushroom (Lentinula edodes) grown on different agricultural wastes. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12553. [Google Scholar] [CrossRef]
- Zhou, T.; Jian, H.; Wan, E.; Gao, Y.; Pei, L.; Ding, J.; Lei, W.; Li, J. Experimental study on using fruit tree branches instead of oak sawdust for shiitake mushroom cultivation. Edible Mushroom 2025, 47, 45–48. [Google Scholar]
- Li, W.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Yu, H.; Zhou, S.; Li, X.; Liu, Y. Effects of culture substrates on taste component content and taste quality of Lentinula edodes. Int. J. Food Sci. Technol. 2017, 52, 981–991. [Google Scholar] [CrossRef]
- Sinha, N. Effect of different substrates on the growth and yield of oyster mushrooms. Int. J. Pure App. Biosci. 2018, 6, 6. [Google Scholar]
- Baktemur, G.; Çelik, Z.D.; Kara, E.; Taşkın, H. The effect of different agricultural wastes on aroma composition of shiitake (Lentinula edodes (Berk.) Pegler) mushroom. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1540–1547. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Zhang, L.; Li, Q.; Song, C.; Shang, X.; Bao, D.; Tan, Q.; Chen, H.; Lv, B. Corncob as a substrate for the cultivation of Lentinula edodes. Waste Biomass Valorization 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Zhong, G.; Wang, Y.; Xiao, Y.; Zhang, C. Study on formula experiment of Lentinual edodes cultivation with waste tangerine branches. Acta Agric. Jiangxi 2024, 36, 55–62. [Google Scholar] [CrossRef]
- Feng, F.; Zhu, H.; Wang, J.; Li, J.; Meng, F.; Wang, S.; Liang, H.; Liu, F.; Wang, Y.; Liu, H. Effect of armeniaca sawdust substitute cultivation on yield and quality of Lentinula edodes. Edible Fungi China 2024, 43, 43–47. [Google Scholar] [CrossRef]
- Luo, J.; Shen, X.; Liu, L.; Feng, J.; Zhang, M.; Song, C.; Zhang, J.; Liu, Y. Effects of strain and cultivation site on polysaccharide content and molecular weight distribution characteristics of Lentinula edodes fruiting bodies. J. Edible Fungi 2024, 31, 77–86. [Google Scholar] [CrossRef]
- Hebei Provincial Bureau of Statistics. Hebei Statistical Yearbook 2022; China Statistics Press: Beijing, China, 2022. [Google Scholar]
- Zhang, L.; Wang, Y. Hebei Provincial Forestry Bureau; China Forestry Publishing House: Beijing, China, 2015. [Google Scholar]
- National Forestry and Grassland Administration of China. China Forestry and Grassland Statistical Yearbook 2022; China Forestry Publishing House: Beijing, China, 2023. [Google Scholar]
- Chen, A.W. Shiitake Cultivation. In Mushroom Growers’ Handbook 2, 2nd ed.; Mush World: Seoul, Republic of Korea, 2005. [Google Scholar]
- Sebaaly, Z.E.; Nabhan, S.; Outayek, J.; Nedelin, T.; Sassine, Y.N. Mixing oak and eucalyptus sawdusts improves shiitake (Lentinula edodes) yield and nutritional value. PLoS ONE 2024, 19, e0309787. [Google Scholar] [CrossRef] [PubMed]
- Gregory, F.E. The Basics of Hardwood-Log Shiitake Mushroom Production and Marketing; Virginia State University: Petersburg, VA, USA, 2020. [Google Scholar]
- Chai, M. The Influence of Different Cultivation Medium and Patterns to the Mycelium Growth and the Nutrition Content and the Yield of the Shitake Mushroom. Master’s Thesis, Shanxi Agricultural University, Taigu, China, 2016. [Google Scholar]
- Aafi, E.; Reza, M.; Mirabzadeh, M. Jujube (Ziziphus jujuba Mill. (Rhamnaceae)): A review on its pharmacological properties and phytochemistry. Tradit. Med. Res. 2022, 7, 38. [Google Scholar] [CrossRef]
- Rajaei, A.; Salarbashi, D.; Asrari, N.; Fazly Bazzaz, B.S.; Aboutorabzade, S.M.; Shaddel, R. Antioxidant, antimicrobial, and cytotoxic activities of extracts from the seed and pulp of Jujube (Ziziphus jujuba) grown in Iran. Food Sci. Nutr. 2020, 9, 682–691. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Zhang, H. Peach gum polysaccharides-based edible coatings extend shelf life of cherry tomatoes. 3 Biotech 2017, 7, 168. [Google Scholar] [CrossRef]
- Tian, L. Study on the Effects of Adding Peach Sawdust on the Growth of Lentinula edodes. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar]
- Endara, A.G.; Moncayo, M.F.G.; Verdugo, D.N.; Sulca, R.L.; Cobo, J.C.; Viejó, F.G.; Cobos, J.D.V. Chemical and productivity characterization of parental and hybrid strains of Lentinula edodes cultivated in different agricultural residues. Emir. J. Food Agric. 2021, 33, 260–265. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Yang, Q.; Li, X.; Xiang, Q.; Zhao, K.; Ma, M.; Yu, X.; Chen, Q.; Zeng, X.; et al. Cultivating Lentinula edodes on substrate containing composted sawdust affects the expression of carbohydrate and aromatic amino acid metabolism-related genes. mSystems 2022, 7, e0082721. [Google Scholar] [CrossRef]
- Thompson, D.N.; Houghton, T.P.; Lacey, J.A.; Shaw, P.G.; Hess, J.R. Preliminary investigation of fungal bioprocessing of wheat straw for production of straw-thermoplastic composites. Appl. Biochem. Biotechnol. 2003, 106, 423–436. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Han, P.; Chai, M.; Zhao, Y.; Wang, W.; Li, Y. Influence of different fruit cultivation medium on the yield and the main nutrient transformation of Lentinula edodes. Edible Fungi China 2018, 37, 25–28+33. [Google Scholar] [CrossRef]
- Yu, G.; Li, X.; Zhao, S.; Sun, S.; Yu, Y.; Chen, J.; Cheng, X.; Li, W. Waste apple wood: A safe and economical alternative substrate for the cultivation of Pleurotus ostreatus and Lentinula edodes. Folia Hortic. 2022, 34, 173–185. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; He, P.; Feng, H.; Xie, J.; Yu, L. Yield and nutritional quality of Lentinula edodes cultivated by wood of nine species of Fagaceae. Mycosystema 2023, 42, 1404–1412. [Google Scholar] [CrossRef]
- Muñoz-González, Y.; Arriagada-Acuña, R.; Soto-Garrido, G.; García-Lovera, R. Activated carbons from peach stones and pine sawdust by phosphoric acid activation used in clarification and decolorization processes. J. Chem. Technol. Biotechnol. 2010, 84, 39–47. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Yang, Y.; Zhou, F.; Liu, Y.; Zhou, S.; Yu, H. Effects of different carbon sources and C/N values on nonvolatile taste components of Pleurotus eryngii. Int. J. Food Sci. Technol. 2015, 50, 2360–2366. [Google Scholar] [CrossRef]
- Han, J.; Sun, R.; Huang, C.; Xie, H.; Gao, X.; Yao, Q.; Yang, P.; Li, J.; Gong, Z. Effects of different carbon and nitrogen ratios on yield, nutritional value, and amino acid contents of Flammulina velutipes. Life 2024, 14, 598. [Google Scholar] [CrossRef]
- Siwulski, M.; Budka, A.; Budzyńska, S.; Gsecka, M.; Kala, P.; Niedzielski, P.; Mleczek, M. Mineral composition of traditional and organic-cultivated mushroom Lentinula edodes in Europe and Asia–similar or different? LWT 2021, 147, 111570. [Google Scholar] [CrossRef]
- Cai, J.; Chang, Y.; Xiao, Y.; Li, J.; Li, W.; Zhang, J.; Li, S.; Liu, J.; Tian, J.; Wang, L.; et al. Comparative experiment on the formulation of cultivating shiitake mushrooms with Robinia pseudoacacia sawdust. Edible Mushrooms 2020, 42, 45–46. [Google Scholar]
- Wang, Y.; Lin, Q.; Xu, H.; Zha, Y.; Yang, F.; Chen, J. Preliminary study on Lentinus edodes cultured with Robinia pseudoacacia. Hubei Agric. Sci. 2016, 55, 5114–5116. [Google Scholar] [CrossRef]
- Carvalho, M.A.; Costa, L.M.A.S.; Santos, D.M.E.; Dias, D.; Zied, D.; Souza Dias, E. Ligninase and cellulase activity of Lentinula edodes (Berk.) pegler strains in different culture media. J. Pure Appl. Microbiol. 2016, 10, 1683–1691. [Google Scholar]
- Landínez-Torres, A.Y.; Pérez Fagua, C.; Sanabria López, A.C.; Deaquiz Oyola, Y.A.; Girometta, C.E. Pruning wastes from fruit trees as a substrate for Pleurotus ostreatus. Acta Mycol. 2021, 56, 568. [Google Scholar] [CrossRef]
- Simas-Tosin, F.; Barraza, R.; Petkowicz, C.; Silveira, J.; Sassaki, G.; Santos, E.; Gorin, P.; Iacomini, M. Rheological and structural characteristics of peach tree gum exudate. Food Hydrocoll. 2010, 24, 486–493. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Masaphy, S.; Levanon, D. The effect of lignocellulose on lignocellulolytic activity of Pleurotus pulmonarius in submerged culture. Appl. Microbiol. Biotechnol. 1992, 36, 828–832. [Google Scholar] [CrossRef]
- Matjuškova, N.; Okmane, L.; Zaļā, D.; Rozenfelde, L.; Puķe, M.; Krūma, I.; Vederņikovs, N.; Rapoport, A. Effect of lignin-containing media on growth of medicinal mushroom Lentinula edodes. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2017, 71, 38–42. [Google Scholar] [CrossRef]
- Royse, D.J.; Sanchez, J.E. Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresour. Technol. 2007, 98, 2137–2141. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; del Río, J.C.; Rencoret, J.; Gutiérrez, A.; de Ruijter, N.C.A.; Cone, J.W. Chemical changes and increased degradability of wheat straw and oak wood chips treated with the white rot fungi Ceriporiopsis subvermispora and Lentinula edodes. Biomass Bioenergy 2017, 105, 381–391. [Google Scholar] [CrossRef]
- Wu, D.; Luo, X.; Liang, M.; He, L.; Huang, X.; Liu, J.; Shen, Y. Study on the formulation of cultivating shiitake mushrooms using mango branch. Edible Mushrooms 2019, 41, 34–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Duan, L.; Ding, J.; Li, Y.; Wang, Y.; Xu, H.; Qin, B. Effects of different orchard tree pruning residues on the yield and nutrient composition of Lentinus edodes. Front. Nutr. 2024, 11, 1477586. [Google Scholar] [CrossRef]
- Pineda-Insuasti, J.A.; Ramos-Sánchez, L.B.; Soto-Arroyave, C.P.; Patrícia, C.; Freitas-Fragata, A.; Pereira-Cruz, L. Growth of Pleurotus ostreatus on non-supplemented agro-industrial wastes. Rev. Técnica Fac. Ing. Univ. Zulia 2015, 38, 41–49. [Google Scholar] [CrossRef]
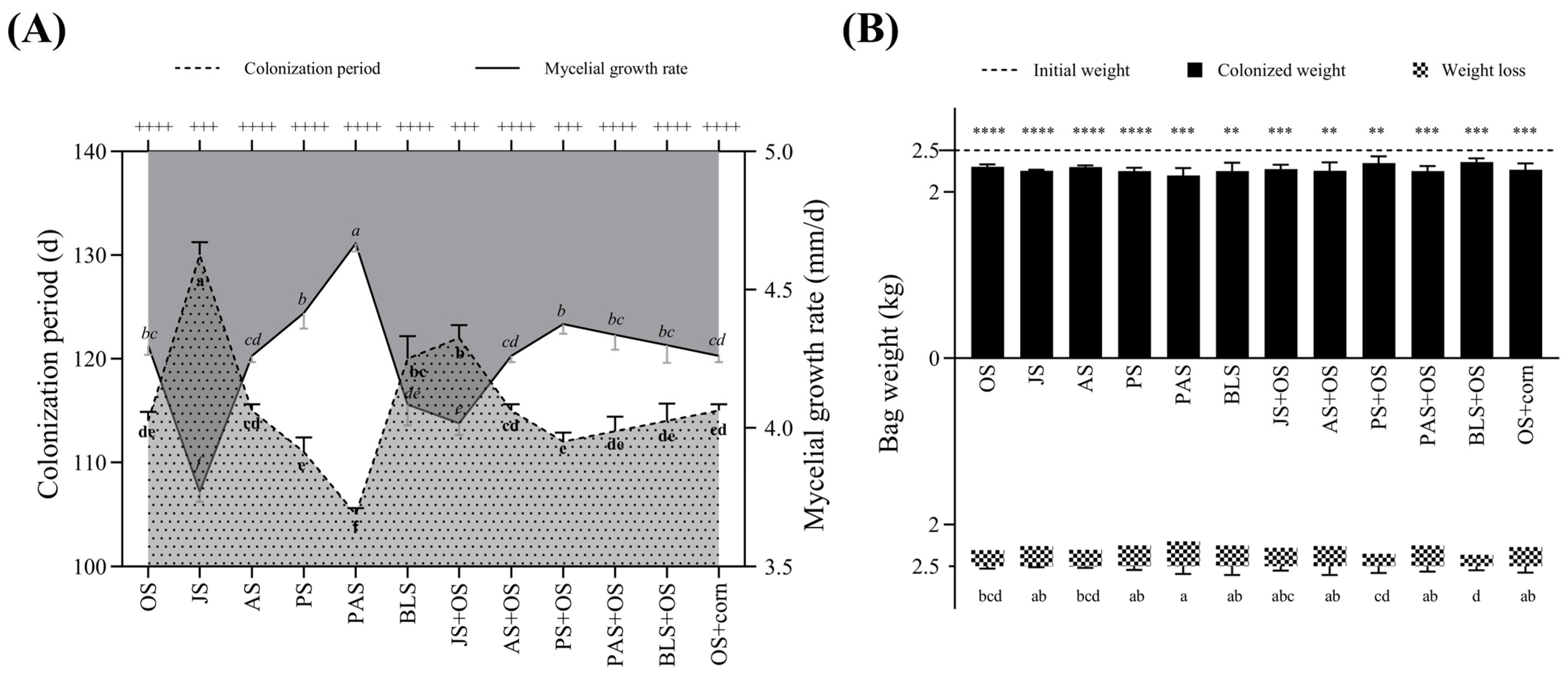
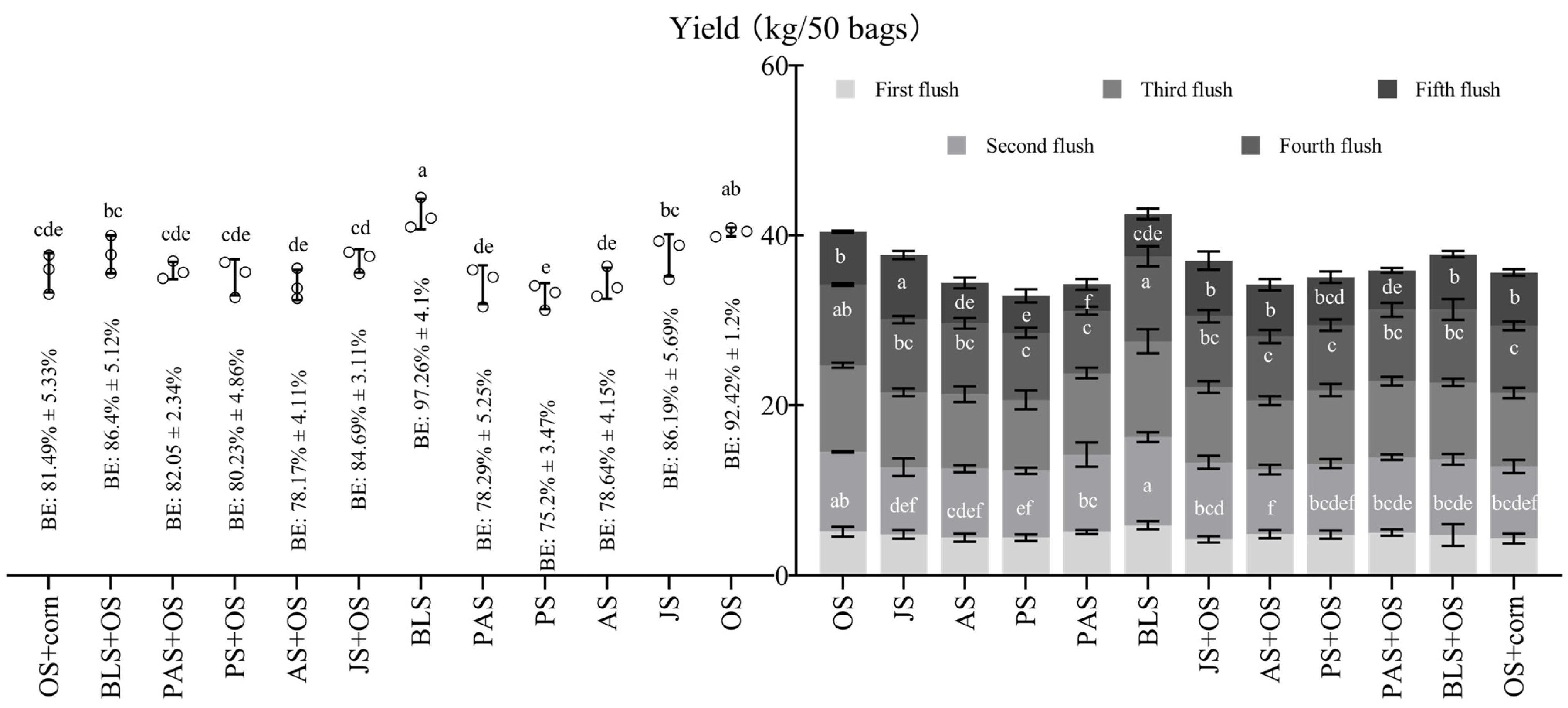
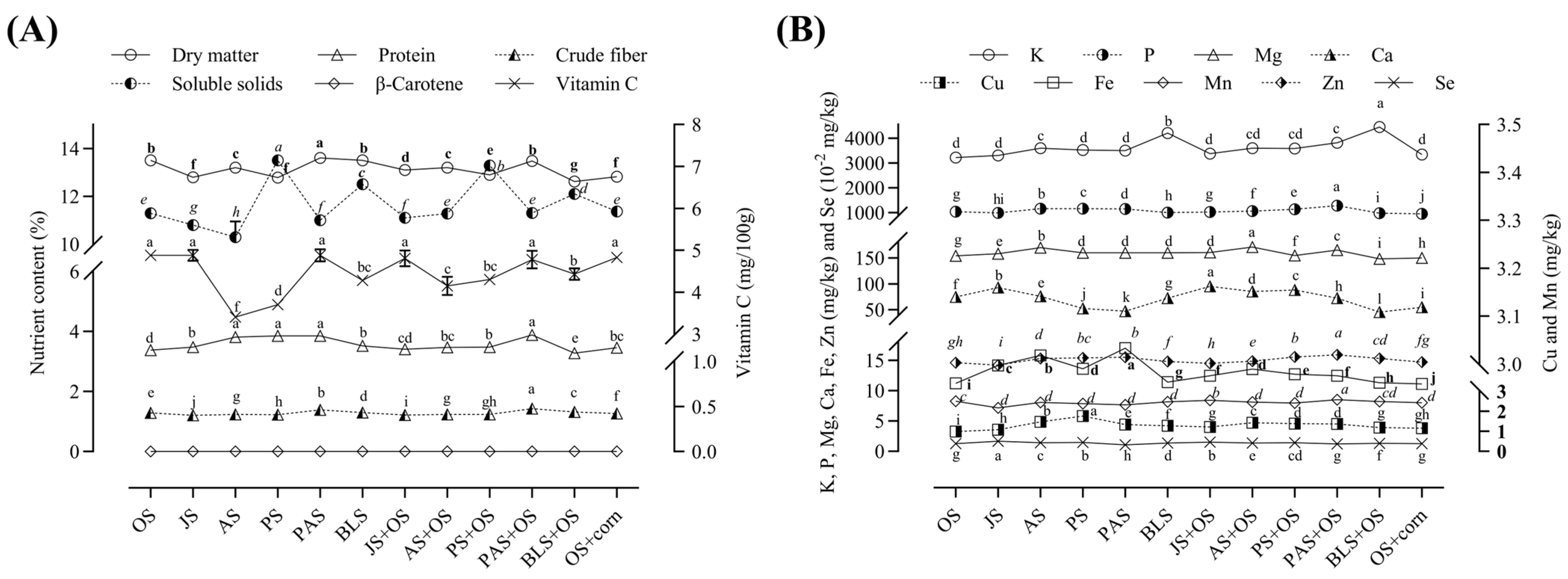
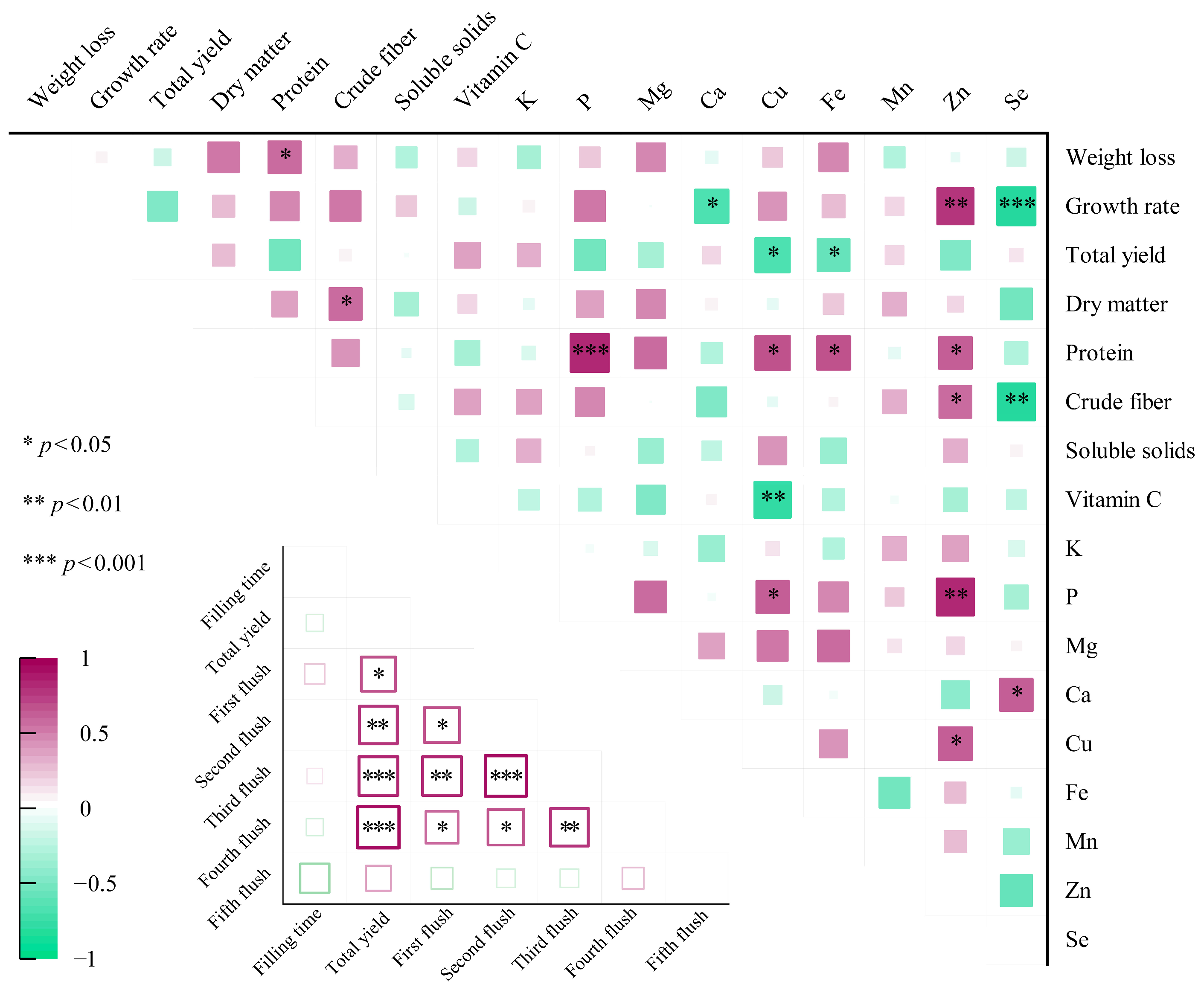
| Treatments | Formulas |
|---|---|
| OS | os (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| JS | js (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| AS | as (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| PS | ps (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| PAS | pas (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| BLS | bls (78%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| JS + OS | js (39%) + os (39%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| AS + OS | as (39%) + os (39%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| PS + OS | ps (39%) + os (39%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| PAS + OS | pas (39%) + os (39%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| BLS + OS | bls (39%) + os (39%) + gypsum (1.5%) + lime (0.5%) + wb (20%) |
| OS + corn | os (78%) + gypsum (1.5%) + lime (0.5%) + wb (17%) + corn meal (3%) |
| Principal Component | Eigenvalue | Percentage of Variance (%) | Cumulative (%) |
|---|---|---|---|
| PC1 | 4.52261 | 32.30436 | 32.30436 |
| PC2 | 2.98708 | 21.33626 | 53.64062 |
| PC3 | 2.48301 | 17.7358 | 71.37641 |
| PC4 | 1.50716 | 10.76543 | 82.14184 |
| PC5 | 0.8459 | 6.04213 | 88.18397 |
| PC6 | 0.57493 | 4.10663 | 92.29061 |
| PC7 | 0.46435 | 3.3168 | 95.6074 |
| PC8 | 0.26674 | 1.90527 | 97.51268 |
| PC9 | 0.17499 | 1.24996 | 98.76264 |
| PC10 | 0.09894 | 0.70673 | 99.46937 |
| PC11 | 0.04701 | 0.33578 | 99.80515 |
| PC12 | 0.01295 | 0.09248 | 99.89763 |
| PC13 | 0.01121 | 0.08007 | 99.9777 |
| PC14 | 0.00312 | 0.0223 | 100 |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| Dry matter | 0.20389 | −0.14018 | 0.41876 | 0.25361 | 0.02566 | 0.06756 |
| Protein | 0.40849 | 0.12898 | 0.08017 | −0.17052 | 0.10091 | −0.01931 |
| Crude fiber | 0.24918 | −0.43284 | 0.17533 | −0.0644 | −0.05254 | 0.28786 |
| Soluble solids | 0.02587 | −0.05909 | −0.51703 | 0.00437 | 0.46153 | 0.19259 |
| Vitamin C | −0.2049 | −0.29201 | 0.33642 | −0.18067 | 0.33049 | 0.31522 |
| K | 0.06958 | −0.25247 | −0.33558 | 0.1914 | −0.58987 | 0.51645 |
| P | 0.41891 | 0.0781 | 0.04291 | 0.08401 | 0.33596 | 0.18874 |
| Mg | 0.26128 | 0.324 | 0.20667 | 0.32944 | −0.23438 | 0.01762 |
| Ca | −0.16454 | 0.33075 | 0.20921 | 0.41282 | 0.25879 | 0.41309 |
| Cu | 0.34037 | 0.24149 | −0.30573 | 0.01881 | 0.06887 | −0.13456 |
| Fe | 0.27514 | 0.27835 | 0.21911 | −0.342 | −0.22628 | 0.09427 |
| Mn | 0.0687 | −0.26009 | −0.03785 | 0.65075 | 0.07019 | −0.34826 |
| Zn | 0.3953 | −0.14864 | −0.20695 | −0.02062 | 0.14237 | 0.15541 |
| Se | −0.24209 | 0.42173 | −0.14242 | 0.07706 | −0.01761 | 0.36059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Cheng, G.; Chen, W.; Xie, C.; Hou, G.; An, X. Utilizing Agrobyproducts: Potential Alternative Substrates for Cultivation of Lentinula edodes. Fermentation 2025, 11, 245. https://doi.org/10.3390/fermentation11050245
Zhou Z, Cheng G, Chen W, Xie C, Hou G, An X. Utilizing Agrobyproducts: Potential Alternative Substrates for Cultivation of Lentinula edodes. Fermentation. 2025; 11(5):245. https://doi.org/10.3390/fermentation11050245
Chicago/Turabian StyleZhou, Zhiguo, Guohui Cheng, Wenjie Chen, Chunyan Xie, Guisen Hou, and Xiaoya An. 2025. "Utilizing Agrobyproducts: Potential Alternative Substrates for Cultivation of Lentinula edodes" Fermentation 11, no. 5: 245. https://doi.org/10.3390/fermentation11050245
APA StyleZhou, Z., Cheng, G., Chen, W., Xie, C., Hou, G., & An, X. (2025). Utilizing Agrobyproducts: Potential Alternative Substrates for Cultivation of Lentinula edodes. Fermentation, 11(5), 245. https://doi.org/10.3390/fermentation11050245






