Growth Performance and Rumen Microbiota of Sheep Respond to Cotton Straw Fermented with Compound Probiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Fermented Cotton Straw
2.2. Experiment Animals and Design
2.3. Diets
2.4. Feed Proximate Analyses and Measurement of Hygienic Indices
2.5. Feed Trial
2.6. Rumen Microbiota Diversity
2.7. Statistical Analyses
3. Results
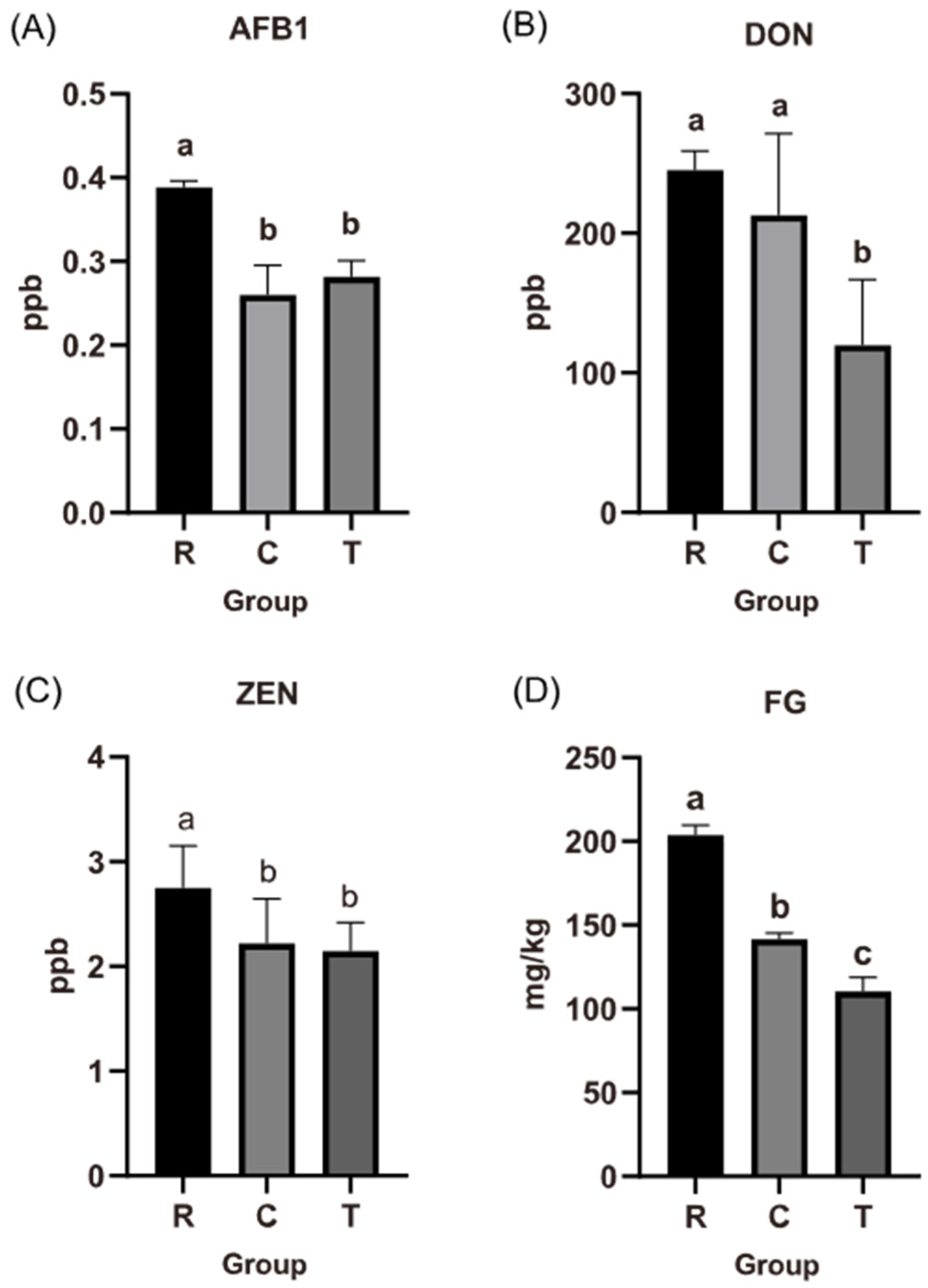
3.1. Effect of Fermented Cotton Straw with Compound Probiotics
3.2. Effects of Different Diets on Growth Performance of Sheep
3.3. Effects of Different Diets on Serum Biochemical Parameters of Sheep
3.4. Effects of Different Diets on Rumen Fluid pH of Sheep
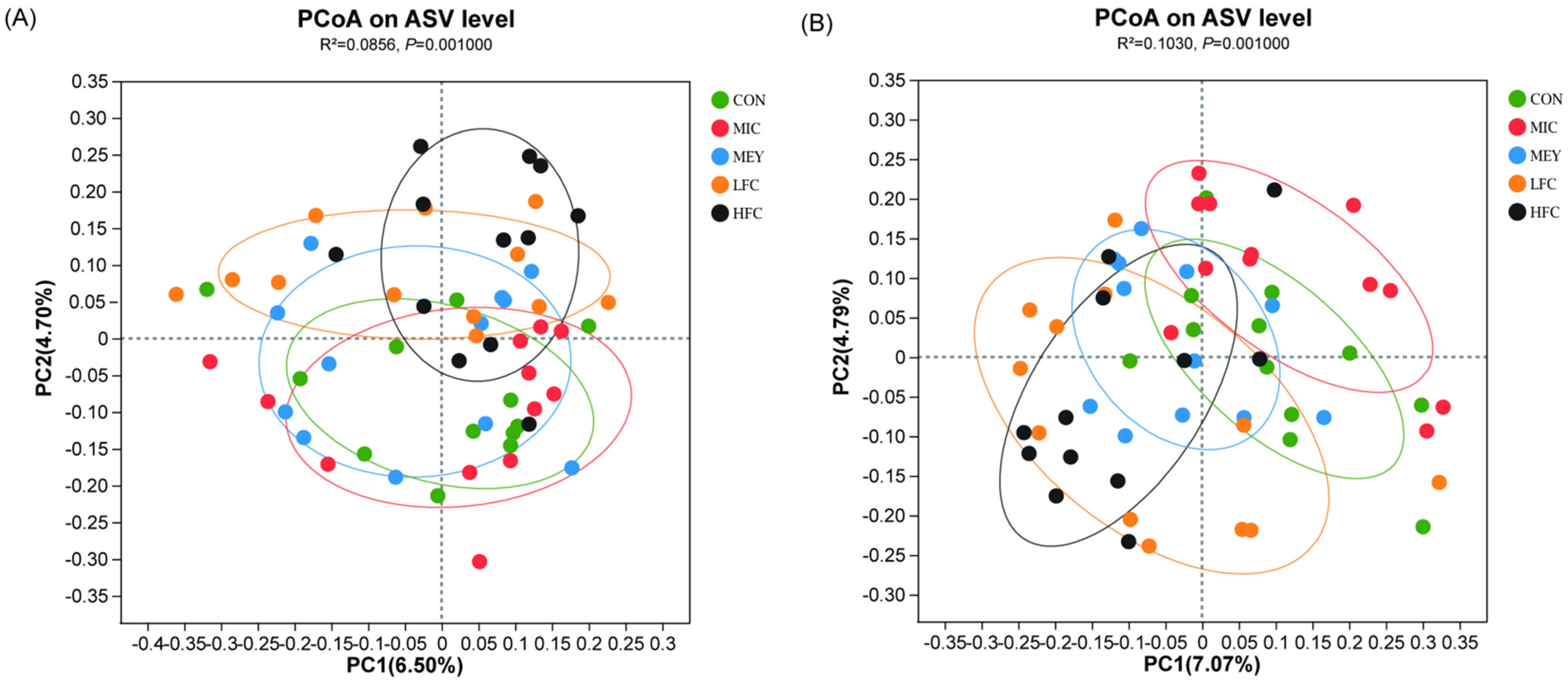
3.5. Effects of Different Diets on Rumen Microbiota Diversity of Sheep
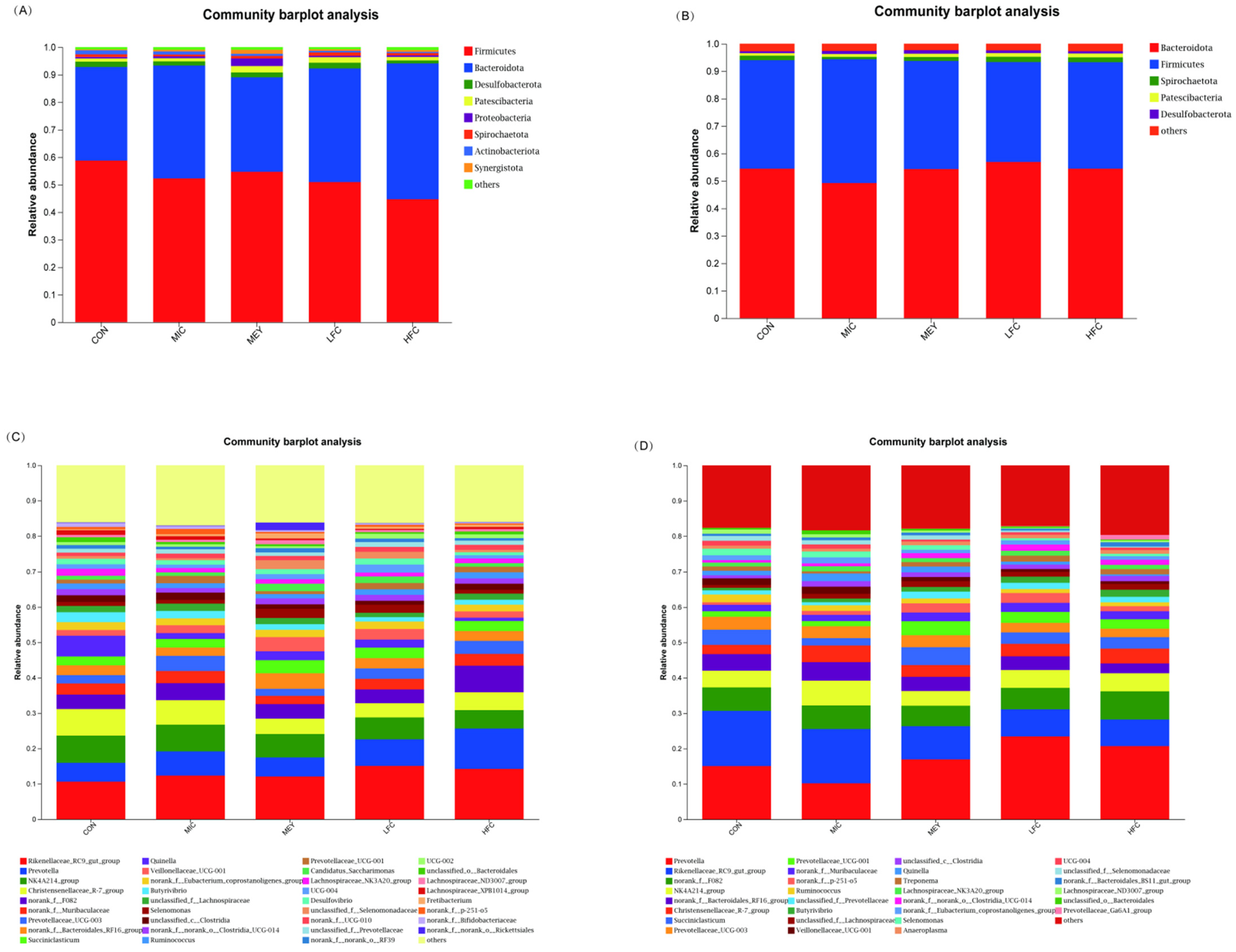
3.6. Effects of Fermented Cotton Straw on the Composition of Rumen Microbiota
4. Discussion
4.1. Effects of Fermented Cotton Straw with Compound Probiotics
4.2. Effects of Fermented Cotton Straw on Growth Performance of Sheep
4.3. Effects of Fermented Cotton Straw on Serum Biochemical Parameters of Sheep
4.4. Effects of Fermented Cotton Straw on Rumen pH and Microbiota Diversity of Sheep
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baydar, G.; Ciliz, N.; Mammadov, A. Life Cycle Assessment of Cotton Textile Products in Turkey. Resour. Conserv. Recycl. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- Qanmber, G.; Liu, Z.; Li, F.; Yang, Z. Brassinosteroids in Cotton: Orchestrating Fiber Development. New Phytol. 2024, 244, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Y.; Zhu, P.; Zhao, G.; Liu, C.; Zhao, H. Functional Characterization of Laccase Isozyme (PoLcc1) from the Edible Mushroom Pleurotus Ostreatus Involved in Lignin Degradation in Cotton Straw. Int. J. Mol. Sci. 2022, 23, 13545. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yu, P.; Xu, X. Straw Utilization in China—Status and Recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Zhang, Y. Research Progress and the Development Trend of the Utilization of Crop Straw Biomass Resources in China. Front. Chem. 2022, 10, 904660. [Google Scholar] [CrossRef]
- Datsomor, O.; Yan, Q.; Wang, K.; Mohamed, S.; Opoku-Mensah, L.; Zhao, G.; Miao, L. Effect of Ammoniated and/or Basidiomycete White-Rot Fungi Treatment on Rice Straw Proximate Composition, Cell Wall Component, and In Vitro Rumen Fermentation Characteristics. Fermentation 2022, 8, 228. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, T.; Cai, G.; Huang, X.; Zhong, S.; Li, X.; Zhang, E. Effect of Aspergillus Niger Fermentation on the Metabolites in Corn Stalks. Fermentation 2023, 9, 50. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Pfeffer, F.M.; Williams, R.; Agrawal, R. A comparative assessment of biomass pretreatment methods for the sustainable industrial upscaling of rice straw into cellulose. Cellulose 2023, 30, 4247–4261. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ke, W.C.; Bai, J.; Li, F.H.; Xu, D.M.; Ding, Z.T.; Guo, X.S. The Effect of Pediococcus Acidilactici J17 with High-Antioxidant Activity on Antioxidant, α-tocopherol, β-carotene, Fatty Acids, and Fermentation Profiles of Alfalfa Silage Ensiled at Two Different Dry Matter Contents. Anim. Feed. Sci. Technol. 2020, 268, 114614. [Google Scholar] [CrossRef]
- Yang, G.; Yang, D.; Wang, X.; Cao, W. A Novel Thermostable Cellulase-Producing Bacillus Licheniformis A5 Acts Synergistically with Bacillus Subtilis B2 to Improve Degradation of Chinese Distillers’ Grains. Bioresour. Technol. 2021, 325, 124729. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, T.; Zhou, Y.; Li, F. Effects of Enzyme + Bacteria Treatment on Growth Performance, Rumen Bacterial Diversity, KEGG Pathways, and the CAZy Spectrum of Tan Sheep. Bioengineered 2020, 11, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, X.J.; Xu, L.N.; Shao, L.W.; Zhu, B.C.; Liu, H.; Wang, Y.J.; Gao, K.Y. Effect of Whole-Plant Corn Silage Treated with Lignocellulose-Degrading Bacteria on Growth Performance, Rumen Fermentation, and Rumen Microflora in Sheep. Animal 2022, 16, 100576. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, W.; Tian, F.; Li, J.; Wang, Y.; Qi, J.; Xue, S. Corn Straw Total Mix Dietary Supplementation of Bacillus Subtilis-Enhanced Growth Performance of Lambs by Favorably Modulating Rumen Bacterial Microbiome. Fermentation 2022, 9, 32. [Google Scholar] [CrossRef]
- Vataščinová, T.; Pipová, M.; Fraqueza, M.J.R.; Maľa, P.; Dudriková, E.; Drážovská, M.; Lauková, A. Short Communication: Antimicrobial Potential of Lactobacillus Plantarum Strains Isolated from Slovak Raw Sheep Milk Cheeses. J. Dairy Sci. 2020, 103, 6900–6903. [Google Scholar] [CrossRef]
- Abdel-Kareem, M.M.; Rasmey, A.M.; Zohri, A.A. The Action Mechanism and Biocontrol Potentiality of Novel Isolates of Saccharomyces Cerevisiae against the Aflatoxigenic Aspergillus Flavus. Lett. Appl. Microbiol. 2019, 68, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Branà, M.T.; Sergio, L.; Haidukowski, M.; Logrieco, A.F.; Altomare, C. Degradation of Aflatoxin B1 by a Sustainable Enzymatic Extract from Spent Mushroom Substrate of Pleurotus Eryngii. Toxins 2020, 12, 49. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Huang, R.; Nie, C.; Niu, J.; Chen, C.; Zhang, W. Biodegradation of Free Gossypol by Helicoverpa Armigera Carboxylesterase Expressed in Pichia Pastoris. Toxins 2022, 14, 816. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2010. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Hao, Z.; Guo, X.; Zhu, B. Anaerobic Solid-State Fermentation with Bacillus Subtilis for Digesting Free Gossypol and Improving Nutritional Quality in Cottonseed Meal. Front. Nutr. 2022, 9, 1017637. [Google Scholar] [CrossRef]
- Khonkhaeng, B.; Cherdthong, A. Improving Nutritive Value of Purple Field Corn Residue and Rice Straw by Culturing with White-Rot Fungi. J. Fungi 2020, 6, 69. [Google Scholar] [CrossRef]
- Qi, X.; Li, Z.; Akami, M.; Mansour, A.; Niu, C. Fermented Crop Straws by Trichoderma Viride and Saccharomyces Cerevisiae Enhanced the Bioconversion Rate of Musca Domestica (Diptera: Muscidae). Environ. Sci. Pollut. Res. Int. 2019, 26, 29388–29396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, F.; Fang, Y.; Zhou, D.; Wang, S.; Wu, D.; Wang, L.; Zhong, R. High-Potency White-Rot Fungal Strains and Duration of Fermentation to Optimize Corn Straw as Ruminant Feed. Bioresour. Technol. 2020, 312, 123512. [Google Scholar] [CrossRef] [PubMed]
- Elolimy, A.; Vailati-Riboni, M.; Liang, Y.; Loor, J.J. Cellular Mechanisms and Epigenetic Changes: Role of Nutrition in Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Oskoueian, E.; Jahromi, M.F.; Jafari, S.; Shakeri, M.; Le, H.H.; Ebrahimi, M. Manipulation of Rice Straw Silage Fermentation with Different Types of Lactic Acid Bacteria Inoculant Affects Rumen Microbial Fermentation Characteristics and Methane Production. Vet. Sci. 2021, 8, 100. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M.; Uriyapongson, S. Fermented Sugarcane Bagasse with Lactobacillus Combined with Cellulase and Molasses Promotes in Vitro Gas Kinetics, Degradability, and Ruminal Fermentation Patterns Compared to Rice Straw. Anim. Biotechnol. 2022, 33, 116–127. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Effects of Lactic Acid Bacteria and Molasses on Fermentation Dynamics, Structural and Nonstructural Carbohydrate Composition and in Vitro Ruminal Fermentation of Rice Straw Silage. Asian-Australas. J. Anim. Sci. 2019, 32, 783–791. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Growth Performances, Nutrient Digestibility, Ruminal Fermentation and Energy Partition of Thai Native Steers Fed Exclusive Rice Straw and Fermented Sugarcane Bagasse with Lactobacillus, Cellulase and Molasses. J. Anim. Physiol. Anim. Nutr. 2022, 106, 45–54. [Google Scholar] [CrossRef]
- Cherdthong, A.; Suntara, C.; Khota, W.; Wanapat, M. Feed Utilization and Rumen Fermentation Characteristics of Thai-Indigenous Beef Cattle Fed Ensiled Rice Straw with Lactobacillus Casei TH14, Molasses, and Cellulase Enzymes. Livest. Sci. 2021, 245, 104405. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, H.; Wang, S.; Liu, Y.; Chen, M.; Liu, H. Lactobacillus-Driven Feed Fermentation Regulates Microbiota Metabolism and Reduces Odor Emission from the Feces of Pigs. mSystems 2023, 8, e00988-23. [Google Scholar] [CrossRef]
- Khattab, I.M.; Abdel-Wahed, A.M.; Khattab, A.S.; Anele, U.Y.; El-Keredy, A.; Zaher, M. Effect of Dietary Probiotics Supplementation on Intake and Production Performance of Ewes Fed Atriplex Hay-Based Diet. Livest. Sci. 2020, 237, 104065. [Google Scholar] [CrossRef]
- Schmidely, P.H.; Bahloul, L. Milk Performance and Oxidative Status Responses to Rumen Protected Methionine Supplementation in Genotyped α-S1 Casein Lactating Dairy Goats Fed Two Levels of Metabolizable Protein Diets. Small Ruminant Res. 2022, 209, 106638. [Google Scholar] [CrossRef]
- Li, M.M.; Titgemeyer, E.C.; Hanigan, M.D. A Revised Representation of Urea and Ammonia Nitrogen Recycling and Use in the Molly Cow Model. J. Dairy Sci. 2019, 102, 5109–5129. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, M.M.; Vargas-Bello-Pérez, E.; Zhuang, Y.; Fu, Y.; Zhang, N. Impact of Dietary Supplementation of β-Hydroxybutyric Acid on Performance, Nutrient Digestibility, Organ Development and Serum Stress Indicators in Early-Weaned Goat Kids. Anim. Nutr. 2022, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive Glutamine Metabolism by IDH1 Mediates Lipogenesis under Hypoxia. Nature 2012, 481, 380–384. [Google Scholar] [CrossRef]
- Sofeo, N.; Hart, J.H.; Butler, B.; Oliver, D.J.; Yandeau-Nelson, M.D.; Nikolau, B.J. Altering the Substrate Specificity of Acetyl-CoA Synthetase by Rational Mutagenesis of the Carboxylate Binding Pocket. ACS Synth. Biol. 2019, 8, 1325–1336. [Google Scholar] [CrossRef]
- Morisset, J. The Early Story of Growth Hormone-Releasing Factor in Rats, Swine, and Cattle. Can. J. Anim. Sci. 2022, 102, 211–231. [Google Scholar] [CrossRef]
- Mameri, A.; Bournine, L.; Mouni, L.; Bensalem, S.; Iguer-Ouada, M. Oxidative Stress as an Underlying Mechanism of Anticancer Drugs Cytotoxicity on Human Red Blood Cells’ Membrane. Toxicol. In Vitro 2021, 72, 105106. [Google Scholar] [CrossRef]
- Mao, C.; Xu, Y.; Shi, L.; Guo, S.; Jin, X.; Yan, S.; Shi, B. Effects of Photoperiod Change on Melatonin Secretion, Immune Function and Antioxidant Status of Cashmere Goats. Animals 2019, 9, 766. [Google Scholar] [CrossRef]
- Giorgio, D.; Di Trana, A.; Di Gregorio, P.; Rando, A.; Avondo, M.; Bonanno, A.; Valenti, B.; Di Grigoli, A. Oxidative Status of Goats with Different CSN1S1 Genotypes Fed Ad Libitum with Fresh and Dry Forages. Antioxidants 2020, 9, 224. [Google Scholar] [CrossRef]
- Angrimani, D.S.R.; Silva, R.O.C.; Losano, J.D.A.; Dalmazzo, A.; Tsunoda, R.H.; Perez, E.G.A.; Góes, P.A.A.; Barnabe, V.H.; Nichi, M. Extender Supplementation with Antioxidants Selected after the Evaluation of Sperm Susceptibility to Oxidative Challenges in Goats. Anim. Biotechnol. 2019, 30, 21–29. [Google Scholar] [CrossRef]
- Oldman, A.H.; Martin, D.S.; Feelisch, M.; Grocott, M.P.W.; Cumpstey, A.F. Effects of Perioperative Oxygen Concentration on Oxidative Stress in Adult Surgical Patients: A Systematic Review. Br. J. Anaesth. 2021, 126, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Tovar-Ramírez, D.; Esteban, M.Á.; Angulo, C. Probiotic Effects of Marine Debaryomyces Hansenii CBS 8339 on Innate Immune and Antioxidant Parameters in Newborn Goats. Appl. Microbiol. Biotechnol. 2019, 103, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Karabinas, D.; Nenov, V.; Zervas, G.; Tsiplakou, E. Dietary Supplementation of a Live Yeast Product on Dairy Sheep Milk Performance, Oxidative and Immune Status in Peripartum Period. J. Fungi 2020, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- Padilla, L. Impact of pH and Palmitic Acid on Ruminal Fermentation and Microbial Community Composition. Master’s Thesis, Utah State University, Logan, UT, USA, 5 May 2022. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Kim, S.H.; Biswas, A.A.; Yu, Z.; Cho, K.-K.; Kim, S.-B.; Lee, K.; Lee, S.S. Rumen Fermentation and Microbial Community Composition Influenced by Live Enterococcus Faecium Supplementation. AMB Express 2019, 9, 123. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.; Wei, Y. Effects of Bacillus Amyloliquefaciens and Bacillus Pumilus on Rumen and Intestine Morphology and Microbiota in Weanling Jintang Black Goat. Animals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of Rumen Bacterial Community and Feed Utilization in Camel and Sheep Using Combined Supplementation of Live Yeast and Microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef]
- Chen, H.; Guo, B.; Yang, M.; Luo, J.; Hu, Y.; Qu, M.; Song, X. Response of Growth Performance, Blood Biochemistry Indices, and Rumen Bacterial Diversity in Lambs to Diets Containing Supplemental Probiotics and Chinese Medicine Polysaccharides. Front. Vet. Sci. 2021, 8, 681389. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.-Z.; Han, J.-L.; Salekdeh, G.H. Lignocellulose Degradation by Rumen Bacterial Communities: New Insights from Metagenome Analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Guo, Y.; Fan, Z.; Li, M.; Xie, H.; Peng, L.; Yang, C. Effects of Sodium Nitrate and Coated Methionine on Lactation Performance, Rumen Fermentation Characteristics, Amino Acid Metabolism, and Microbial Communities in Lactating Buffaloes. Microorganisms 2023, 11, 675. [Google Scholar] [CrossRef]
- Xue, M.-Y.; Sun, H.-Z.; Wu, X.-H.; Liu, J.-X.; Guan, L.L. Multi-Omics Reveals That the Rumen Microbiome and Its Metabolome Together with the Host Metabolome Contribute to Individualized Dairy Cow Performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Dao, T.-K.; Do, T.-H.; Le, N.-G.; Nguyen, H.-D.; Nguyen, T.-Q.; Le, T.-T.-H.; Truong, N.-H. Understanding the Role of Prevotella Genus in the Digestion of Lignocellulose and Other Substrates in Vietnamese Native Goats’ Rumen by Metagenomic Deep Sequencing. Animals 2021, 11, 3257. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shah, A.M.; Yuan, M.; Kang, K.; Wang, Z.; Wang, L.; Xue, B.; Zou, H.; Zhang, X.; Yu, P.; et al. Effects of Dry Yeast Supplementation on Growth Performance, Rumen Fermentation Characteristics, Slaughter Performance and Microbial Communities in Beef Cattle. Anim. Biotechnol. 2022, 33, 1150–1160. [Google Scholar] [CrossRef] [PubMed]

| Items | Groups 2 | ||
|---|---|---|---|
| CON | LFC | HFC | |
| Ingredients | |||
| Cotton leaf | 43.50 | 29.00 | 14.50 |
| Fermented cotton straw | 0 | 14.50 | 29.00 |
| Silage corn | 16.90 | 16.90 | 16.90 |
| Corn | 21.70 | 21.70 | 21.70 |
| Soybean meal | 9.35 | 9.35 | 9.35 |
| Cottonseed meal | 5.75 | 5.75 | 5.75 |
| CaHPO4 | 0.80 | 0.80 | 0.80 |
| NaHCO3 | 0.50 | 0.50 | 0.50 |
| NaCl | 0.50 | 0.50 | 0.50 |
| Premix 1 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient levels 3 | |||
| CP | 14.62 | 13.65 | 12.66 |
| CF | 19.14 | 21.87 | 23.35 |
| EE | 4.36 | 3.61 | 2.65 |
| Ca | 0.81 | 0.93 | 0.98 |
| P | 0.46 | 0.49 | 0.50 |
| Items 1 | Sampling Time | p-Value | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 28 | Day 42 | Day 56 | ||
| Control | ||||||
| CP | 6.74 ± 0.02 b | 6.87 ± 0.14 b | 6.92 ± 0.03 b | 7.20 ± 0.14 a | 7.18 ± 0.08 a | 0.001 |
| NDF | 73.28 ± 2.14 a | 67.58 ± 3.14 b | 61.79 ± 1.70 c | 64.65 ± 2.63 bc | 64.15 ± 1.57 bc | <0.001 |
| ADF | 62.41 ± 1.69 a | 56.49 ± 2.68 ab | 51.04 ± 1.29 b | 54.03 ± 1.93 b | 53.27 ± 0.84 b | <0.001 |
| Treatment | ||||||
| CP | 6.74 ± 0.02 b | 7.92 ± 0.06 a | 8.07 ± 0.05 a | 8.30 ± 0.18 a | 8.20 ± 0.01 a | <0.001 |
| NDF | 73.28 ± 2.14 a | 65.96 ± 5.32 b | 66.14 ± 2.01 b | 61.05 ± 2.37 c | 60.68 ± 1.23 c | <0.001 |
| ADF | 62.41 ± 1.69 a | 57.19 ± 1.25 b | 56.87 ± 1.11 b | 52.43 ± 1.42 c | 51.54 ± 0.30 c | <0.001 |
| Items 1 | Groups 2 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | MIC | MEY | LFC | HFC | ||
| BW (kg) | ||||||
| Initial BW | 28.77 ± 3.30 | 28.73 ± 2.94 | 29.03 ± 4.26 | 28.43 ± 4.06 | 29.15 ± 2.38 | 0.988 |
| Final BW | 38.41 ± 4.42 | 39.12 ± 3.29 | 39.55 ± 4.14 | 39.26 ± 4.02 | 38.45 ± 3.16 | 0.933 |
| DMI (g/d) | ||||||
| W1–W4 | 1226.05 ± 60.07 a | 1246.93 ± 33.49 a | 1222.94 ± 33.29 a | 1188.84 ± 41.00 a | 1118.70 ± 43.88 b | 0.007 |
| W5–W7 | 1334.50 ± 108.05 a | 1365.82 ± 75.24 a | 1315.80 ± 77.55 a | 1276.57 ± 77.47 ab | 1155.61 ± 107.32 b | 0.041 |
| W1–W7 | 1273.50 ± 77.03 a | 1298.94 ± 51.71 a | 1263.56 ± 45.67 a | 1227.22 ± 50.34 a | 1134.85 ± 71.19 b | 0.014 |
| ADG (g/d) | ||||||
| W1–W4 | 243.21 ± 72.08 | 265.59 ± 18.05 | 263.12 ± 46.57 | 239.35 ± 50.67 | 225.00 ± 28.71 | 0.720 |
| W5–W7 | 146.43 ± 36.43 | 152.98 ± 26.26 | 162.70 ± 31.07 | 208.33 ± 32.16 | 153.57 ± 17.68 | 0.059 |
| W1–W7 | 200.87 ± 34.07 | 216.32 ± 14.51 | 219.18 ± 31.32 | 225.78 ± 34.29 | 193.75 ± 22.09 | 0.494 |
| F/G | ||||||
| W1–W4 | 5.36 ± 1.45 | 4.71 ± 0.24 | 4.74 ± 0.68 | 5.16 ± 1.22 | 5.03 ± 0.61 | 0.803 |
| W5–W7 | 9.55 ± 2.39 | 9.15 ± 1.81 | 8.30 ± 1.58 | 6.21 ± 0.75 | 7.54 ± 0.22 | 0.056 |
| W1–W7 | 6.43 ± 0.74 | 6.02 ± 0.35 | 5.83 ± 0.65 | 5.53 ± 0.85 | 5.89 ± 0.42 | 0.681 |
| Items 1 | Groups 2 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | MIC | MEY | LFC | HFC | ||
| W1–W4 mmol/L | ||||||
| TC | 1.34 ± 0.33 | 1.13 ± 0.35 | 1.25 ± 0.50 | 1.00 ± 0.40 | 1.52 ± 0.66 | 0.085 |
| TG | 0.31 ± 0.14 | 0.28 ± 0.12 | 0.38 ± 0.16 | 0.28 ± 0.13 | 0.44 ± 0.35 | 0.224 |
| UREA | 5.10 ± 1.83 | 4.49 ± 1.44 | 5.07 ± 1.41 | 4.43 ± 1.26 | 6.01 ± 1.53 | 0.089 |
| AcCoA | 36.76 ± 6.99 bc | 43.76 ± 2.11 a | 33.75 ± 3.94 c | 48.50 ± 8.32 a | 40.13 ± 3.16 b | <0.001 |
| BHBA | 0.30 ± 0.18 | 0.30 ± 0.06 | 0.41 ± 0.15 | 0.29 ± 0.11 | 0.36 ± 0.17 | 0.210 |
| GH (ng/mL) | 4.91 ± 0.36 d | 5.98 ± 0.38 b | 4.46 ± 0.50 e | 6.52 ± 0.49 a | 5.51 ± 0.53 c | <0.001 |
| SOD (U/mL) | 64.50 ± 16.08 bc | 73.94 ± 4.53 a | 58.84 ± 3.18 c | 75.85 ± 2.70 a | 67.92 ± 2.10 b | <0.001 |
| MDA (nmol/mL) | 4.34 ± 0.63 b | 3.13 ± 0.72 d | 4.99 ± 0.38 a | 3.50 ± 0.93 cd | 4.05 ± 0.70 bc | <0.001 |
| T-AOC (U/mL) | 8.14 ± 1.19 bc | 9.34 ± 0.62 ab | 7.65 ± 1.10 c | 10.99 ± 1.73 a | 8.86 ± 0.42 b | <0.001 |
| W5–W7 mmol/L | ||||||
| TC | 1.47 ± 0.41 a | 0.60 ± 0.32 bc | 0.41 ± 0.23 c | 0.69 ± 0.32 bc | 0.77 ± 0.32 b | <0.001 |
| TG | 0.30 ± 0.10 a | 0.13 ± 0.09 bc | 0.09 ± 0.06 c | 0.17 ± 0.10 b | 0.16 ± 0.08 b | <0.001 |
| UREA | 5.69 ± 1.25 a | 3.57 ± 1.11 cd | 3.03 ± 0.72 d | 4.00 ± 1.04 bc | 4.51 ± 1.01 b | <0.001 |
| AcCoA | 29.98 ± 7.25 d | 56.21 ± 3.55 b | 66.00 ± 4.21 a | 50.66 ± 5.25 c | 53.25 ± 5.66 bc | <0.001 |
| BHBA | 0.33 ± 0.11 a | 0.18 ± 0.08 b | 0.14 ± 0.09 b | 0.21 ± 0.10 b | 0.17 ± 0.06 b | <0.001 |
| GH (ng/mL) | 3.49 ± 0.58 d | 8.10 ± 0.47 b | 9.32 ± 0.39 a | 7.59 ± 0.35 c | 8.48 ± 0.54 b | <0.001 |
| SOD (U/mL) | 52.30 ± 2.66 d | 84.06 ± 2.71 b | 89.16 ± 2.81 a | 78.10 ± 3.93 c | 81.64 ± 3.11 b | <0.001 |
| MDA (nmol/mL) | 5.58 ± 0.91 a | 2.11 ± 0.44 c | 1.25 ± 0.40 d | 2.85 ± 0.22 b | 2.48 ± 0.30 c | <0.001 |
| T-AOC (U/mL) | 6.64 ± 0.46 d | 13.74 ± 1.49 ab | 14.65 ± 1.03 a | 11.61 ± 1.04 c | 12.84 ± 1.56 bc | <0.001 |
| Items | Groups 1 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | MIC | MEY | LFC | HFC | ||
| W1–W4 | 6.20 ± 0.33 b | 6.60 ± 0.17 a | 6.15 ± 0.26 b | 6.18 ± 0.24 b | 6.66 ± 0.16 a | <0.001 |
| W5–W7 | 6.60 ± 0.16 | 6.71 ± 0.14 | 6.86 ± 0.28 | 6.77 ± 0.35 | 6.73 ± 0.45 | 0.111 |
| Items | Groups 1 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | MIC | MEY | LFC | HFC | ||
| W1–W4 | ||||||
| Coverage | 0.9975 ± 0.008 | 0.9974 ± 0.0010 | 0.9977 ± 0.0010 | 0.9977 ± 0.0010 | 0.9973 ± 0.0006 | 0.731 |
| Sobs | 1069.00 ± 191.39 | 1146.42 ± 198.49 | 1113.83 ± 189.02 | 1089.50 ± 184.17 | 1177.75 ± 124.18 | 0.588 |
| Ace | 1090.05 ± 195.66 | 1168.28 ± 204.11 | 1132.97 ± 196.88 | 1108.59 ± 189.31 | 1200.35 ± 125.38 | 0.594 |
| Chao1 | 1089.71 ± 195.93 | 1167.73 ± 207.02 | 1131.35 ± 197.69 | 1106.45 ± 189.51 | 1199.29 ± 124.66 | 0.596 |
| Shannon | 5.86 ± 0.49 | 6.00 ± 0.33 | 5.86 ± 0.48 | 5.87 ± 0.33 | 6.04 ± 0.36 | 0.693 |
| Shannoneven | 0.841 ± 0.048 | 0.864 ± 0.028 | 0.834 ± 0.049 | 0.841 ± 0.030 | 0.854 ± 0.042 | 0.735 |
| W5–W7 | ||||||
| Coverage | 0.9977 ± 0.0007 c | 0.9996 ± 0.0003 a | 0.9990 ± 0.0005 b | 0.9981 ± 0.0006 c | 0.9982 ± 0.0007 c | <0.001 |
| Sobs | 1033.00 ± 221.07 | 910.17 ± 145.13 | 992.00 ± 170.44 | 1121.33 ± 232.48 | 1103.92 ± 167.43 | 0.057 |
| Ace | 1052.12 ± 225.00 ab | 912.39 ± 145.05 b | 998.69 ± 172.23 ab | 1135.65 ± 235.56 a | 1117.79 ± 170.72 a | 0.041 |
| Chao1 | 1049.41 ± 225.04 ab | 911.22 ± 144.92 b | 997.21 ± 172.15 ab | 1134.51 ± 235.19 a | 1115.17 ± 169.25 a | 0.041 |
| Shannon | 5.76 ± 0.48 | 5.79 ± 0.40 | 5.95 ± 0.41 | 6.04 ± 0.43 | 6.02 ± 0.28 | 0.316 |
| Shannoneven | 0.832 ± 0.042 | 0.850 ± 0.040 | 0.864 ± 0.043 | 0.861 ± 0.035 | 0.861 ± 0.025 | 0.237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Guan, M.; Liang, X.; Yuan, K.; Chen, N.; Yang, Y.; Gong, P. Growth Performance and Rumen Microbiota of Sheep Respond to Cotton Straw Fermented with Compound Probiotics. Fermentation 2025, 11, 244. https://doi.org/10.3390/fermentation11050244
Wei P, Guan M, Liang X, Yuan K, Chen N, Yang Y, Gong P. Growth Performance and Rumen Microbiota of Sheep Respond to Cotton Straw Fermented with Compound Probiotics. Fermentation. 2025; 11(5):244. https://doi.org/10.3390/fermentation11050244
Chicago/Turabian StyleWei, Peiling, Mingxuan Guan, Xuhui Liang, Kaixin Yuan, Ning Chen, Yuxin Yang, and Ping Gong. 2025. "Growth Performance and Rumen Microbiota of Sheep Respond to Cotton Straw Fermented with Compound Probiotics" Fermentation 11, no. 5: 244. https://doi.org/10.3390/fermentation11050244
APA StyleWei, P., Guan, M., Liang, X., Yuan, K., Chen, N., Yang, Y., & Gong, P. (2025). Growth Performance and Rumen Microbiota of Sheep Respond to Cotton Straw Fermented with Compound Probiotics. Fermentation, 11(5), 244. https://doi.org/10.3390/fermentation11050244








