Applications of Yeasts in Heavy Metal Remediation
Abstract
1. Introduction
2. Mechanisms of Environmental Remediation by Yeasts
2.1. Cell Wall
2.2. Biosorption by Cell Wall
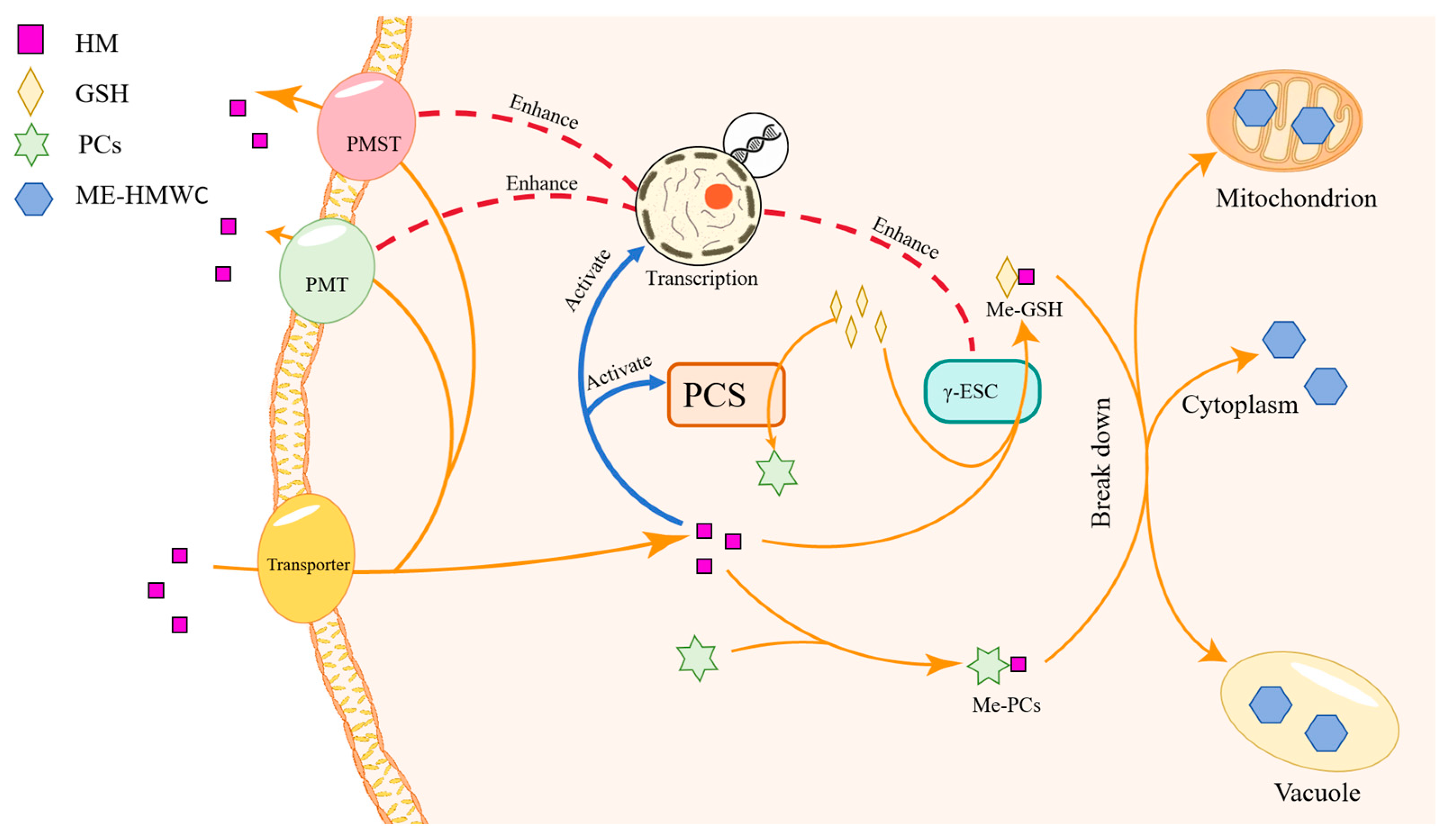
2.3. Intracellular Bioaccumulation
2.4. Biosorption by EPSs
3. Remediation of Heavy Metals by Yeasts
3.1. Heavy Metals
3.2. Metalloid
3.3. Factors Influencing Heavy Metal Remediation by Yeasts
4. Industrial Applications
4.1. Surface and Genetic Modification
4.2. Composite Materials
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartkowiak, B. Influence of heavy metals on quality of raw materials, animal products, and human and animal health status. In Environmental Impact and Remediation of Heavy Metals; Hosam, M.S., Amal, I.H., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Sarker, A.; Kim, J.-E.; Islam, A.R.M.T.; Bilal, M.; Rakib, R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy metals contamination and associated health risks in food webs—A review focuses on food safety and environmental sustainability in Bangladesh. Environ. Sci. Pollut. Res. 2021, 29, 3230–3245. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Bhavya, G.; De Britto, S.; Satapute, P.; Geetha, N.; Jogaiah, S. Biofabricated yeast: Super-soldier for detoxification of heavy metals. World J. Microbiol. Biotechnol. 2023, 39, 148. [Google Scholar] [CrossRef] [PubMed]
- Campaña-Pérez, J.F.; Barahona, P.P.; Martín-Ramos, P.; Barriga, E.J.C. Ecuadorian yeast species as microbial particles for Cr(VI) biosorption. Environ. Sci. Pollut. Res. 2019, 26, 28162–28172. [Google Scholar] [CrossRef]
- Siddiquee, S.; Rovina, K.; Azad, S.A.; Naher, L.; Suryani, S.; Chaikaew, P. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem. Technol. 2015, 7, 384–393. [Google Scholar] [CrossRef]
- Li, Z.; Meng, L.; Chen, H.; Jin, F. Heavy metals in agricultural soils: From bioavailability to productivity. In Soil Constraints and Productivity; CRC: Boca Raton, FL, USA, 2023. [Google Scholar]
- Ma, X. Heavy metals remediation through lactic acid bacteria: Current status and future prospects. Sci. Total Environ. 2024, 946, 174455. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Quirin, M.E.C.; Mukherjee, A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol. Eng. 2017, 103, 106–117. [Google Scholar] [CrossRef]
- Said, O.B.; da Silva, M.M.; Hannier, F.; Beyrem, H.; Chícharo, L. Using Sarcocornia fruticosa and Saccharomyces cerevisiae to remediate metal contaminated sediments of the Ria Formosa lagoon (SE Portugal). Ecohydrol. Hydrobiol. 2019, 19, 588–597. [Google Scholar] [CrossRef]
- Soares, E.V.; Soares, H.M.V.M. Cleanup of industrial effluents containing heavy metals: A new opportunity of valorising the biomass produced by brewing industry. Appl. Microbiol. Biotechnol. 2013, 97, 6667–6675. [Google Scholar] [CrossRef]
- van der Klei, I.J.; Veenhuis, M. Yeast and filamentous fungi as model organisms in microbody research. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 1364–1373. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Chang, S.; Li, M.; Sun, J. Evaluation of yeast inoculum seeding on the remediation of water and sediment in an urban river. Can. J. Chem. Eng. 2017, 95, 2038–2047. [Google Scholar] [CrossRef]
- Elahian, F.; Heidari, R.; Charghan, V.R.; Asadbeik, E.; Mirzaei, S.A. Genetically modified Pichia pastoris, a powerful resistant factory for gold and palladium bioleaching and nanostructure heavy metal biosynthesis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 259–265. [Google Scholar] [CrossRef]
- Massoud, R.; Hadiani, M.R.; Hamzehlou, P.; Khosravi-Darani, K. Bioremediation of heavy metals in food industry: Application of Saccharomyces cerevisiae. Electron. J. Biotechnol. 2019, 37, 56–60. [Google Scholar] [CrossRef]
- Jiang, B.H.; He, H.; Zhao, Y.; Li, L.; Hu, X.M. Absorption behaviour of Pb(II) by Rhodotorula mucilaginosa. Mater. Res. Innov. 2015, 19, 1026–1030. [Google Scholar] [CrossRef]
- El-Bestawy, E.A.; El-Batouti, M.M.; Zabermawi, N.M.; Zaghlol, H.M. Removal of heavy metals, turbidity and coliform from contaminated raw drinking water using Saccharomyces cerevisiae, the baker’s yeast. Sustain. Chem. Pharm. 2023, 33, 101131. [Google Scholar] [CrossRef]
- Fadel, M.; Hassanein, N.M.; Elshafei, M.M.; Mostafa, A.H.; Ahmed, M.A.; Khater, H.M. Biosorption of manganese from groundwater by biomass of Saccharomyces cerevisiae. HBRC J. 2017, 13, 106–113. [Google Scholar] [CrossRef]
- Han, R.; Li, H.; Li, Y.; Zhang, J.; Xiao, H.; Shi, J. Biosorption of copper and lead ions by waste beer yeast. J. Hazard. Mater. 2006, 137, 1569–1576. [Google Scholar] [CrossRef]
- Machado, M.D.; Janssens, S.; Soares, H.; Soares, E. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: Advantages of using dead biomass. J. Appl. Microbiol. 2009, 106, 1792–1804. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Shi, J.; Rosen, B.P. Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 2000, 275, 21149–21157. [Google Scholar] [CrossRef]
- Wei, Q.; Yan, J.; Chen, Y.; Zhang, L.; Wu, X.; Shang, S.; Ma, S.; Xia, T.; Xue, S.; Zhang, H. Cell Surface Display of MerR on Saccharomyces cerevisiae for Biosorption of Mercury. Mol. Biotechnol. 2018, 60, 12–20. [Google Scholar] [CrossRef]
- Ruas, F.A.D.; Amorim, S.S.; Leão, V.A.; Guerra-Sá, R. Rhodotorula mucilaginosa Isolated from the Manganese Mine Water in Minas Gerais, Brazil: Potential Employment for Bioremediation of Contaminated Water. Water Air Soil Pollut. 2020, 231, 527. [Google Scholar] [CrossRef]
- Ertuğrul, S.; San, N.O.; Dönmez, G. Treatment of dye (Remazol Blue) and heavy metals using yeast cells with the purpose of managing polluted textile wastewaters. Ecol. Eng. 2009, 35, 128–134. [Google Scholar] [CrossRef]
- Grujić, S.; Vasić, S.; Radojević, I.; Čomić, L.; Ostojić, A. Comparison of the Rhodotorula mucilaginosa Biofilm and Planktonic Culture on Heavy Metal Susceptibility and Removal Potential. Water Air Soil Pollut. 2017, 228, 73. [Google Scholar] [CrossRef]
- Tsezos, M. The role of chitin in uranium adsorption by R. arrhizus. Biotechnol. Bioeng. 1983, 25, 2025–2040. [Google Scholar] [CrossRef]
- Chen, X.; Tian, Z.; Cheng, H.; Xu, G.; Zhou, H. Adsorption process and mechanism of heavy metal ions by different components of cells, using yeast (Pichia pastoris) and Cu2+ as biosorption models. RSC Adv. 2021, 11, 17080–17091. [Google Scholar] [CrossRef]
- Khan, Z.; Rehman, A.; Hussain, S.Z. Resistance and uptake of cadmium by yeast, Pichia hampshirensis 4Aer, isolated from industrial effluent and its potential use in decontamination of wastewater. Chemosphere 2016, 159, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.S.; Ruas, F.A.D.; Barboza, N.R.; Neves, V.G.d.O.; Leão, V.A.; Guerra-Sá, R. Manganese (Mn2+) tolerance and biosorption by Meyerozyma guilliermondii and Meyerozyma caribbica strains. J. Environ. Chem. Eng. 2018, 6, 4538–4545. [Google Scholar] [CrossRef]
- He, M.; Xu, Y.; Qiao, Y.; Zhang, Z.; Liang, J.; Peng, Y.; Liao, J.; Shang, C.; Guo, Z.; Chen, S. A novel yeast strain Geotrichum sp. CS-67 capable of accumulating heavy metal ions. Ecotoxicol. Environ. Saf. 2022, 236, 113497. [Google Scholar] [CrossRef]
- Amin, A.; Latif, Z. Detoxification of mercury pollutant by immobilized yeast strain Candida xylopsoci. Pak. J. Bot. 2013, 45, 1437–1443. [Google Scholar] [CrossRef]
- Muter, O.; Lubinya, I.; Millers, D.; Grigorjeva, L.; Ventinya, E. Rapoport A Cr(VI) sorption by intact and dehydrated Candida utilis cells in the presence of other metals. Process Biochem. 2002, 38, 123–131. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Chintalpudi, V.K.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. In Biosorption; Intech: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Bemiller, J.N. Polysaccharides: Occurrence, Significance, and Properties. In Glycoscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1413–1435. ISBN 978-3-540-36154-1. [Google Scholar] [CrossRef]
- Ernst, J.F.; Pla, J. Signaling the glycoshield: Maintenance of the Candida albicans cell wall. Int. J. Med Microbiol. 2011, 301, 378–383. [Google Scholar] [CrossRef]
- Hazen, K.C.; Hazen, B.W.; Allietta, M.M. Cell-wall anchoring to cytoplasmic membrane of candida-albicans. FEMS Microbiol. Lett. 1995, 125, 143–147. [Google Scholar] [CrossRef]
- Teparic, R.; Lozancic, M.; Mrsa, V. Evolutionary overview of molecular interactions and enzymatic activities in the yeast cell walls. Int. J. Mol. Sci. 2020, 21, 8996. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Sentandreu, R. Fungal cell wall synthesis and assembly. Curr. Top. Med. Mycol. 1989, 3, 168–217. [Google Scholar]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Guerin, H.; Kulakauskas, S.; Chapot-Chartier, M.-P. Structural variations and roles of rhamnose-rich cell wall polysaccharides in gram-positive bacteria. J. Biol. Chem. 2022, 298, 102488. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Impey, R.E.; Hawkins, D.A.; Sutton, J.M.; Soares Da Costa, T.P. Overcoming Intrinsic and Acquired Resistance Mechanisms Associated with the Cell Wall of Gram-Negative Bacteria. AIMS Microbiol. 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; Francois, J. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- de Bièvre, C. 60 figures + 28 tableaux Yeasts in natural and artificial habitats, J.F.T. Spencer, O.M. Spencer, DM 198, Springer, New Mexico (1997), 381 + ix pages. Bull. L’institut Pasteur 1998, 96, 65–66. [Google Scholar] [CrossRef]
- Schiavone, M.; Déjean, S.; Sieczkowski, N.; Castex, M.; Dague, E.; François, J.M. Integration of Biochemical, Biophysical and Transcriptomics Data for Investigating the Structural and Nanomechanical Properties of the Yeast Cell Wall. Front. Microbiol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mounes, H.A.; Shady, S.H.; Ahmed, K.M. Effects of yucca, yucca schidigera, extract and/or yeast, Saccharomyces cerevisiae, as water additives on growth, biochemical, and antioxidants/oxidant biomarkers of Nile tilapia, oreochromis niloticus. Aquaculture 2021, 533, 736122. [Google Scholar] [CrossRef]
- Tuon, F.F.; Costa, S.F. Rhodotorula infection. A systematic review of 128 cases from literature. Rev. Iberoam. Micol. 2008, 25, 135–140. [Google Scholar] [CrossRef]
- Salinas, E.; de Orellano, M.E.; Rezza, I.; Martinez, L.; Marchesvky, E.; de Tosetti, M.S. Removal of cadmium and lead from dilute aqueous solutions by Rhodotorula rubra. Bioresour. Technol. 2000, 72, 107–112. [Google Scholar] [CrossRef]
- Lee, T.H.; Arai, M.; Murao, S. Structure of fucogalactomannan of red yeast cell wall. Agric. Biol. Chem. 2014, 45, 1301–1309. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, H. Characterization of cadmium removal by Rhodotorula sp. Y11. Appl. Microbiol. Biotechnol. 2006, 73, 458–463. [Google Scholar] [CrossRef]
- Cho, D.H.; Chae, H.J.; Kim, E.Y. Synthesis and characterization of a novel extracellular polysaccharide by Rhodotorula glutinis. Appl. Biochem. Biotechnol. 2001, 95, 183–193. [Google Scholar] [CrossRef]
- Monika, P.; Surajit, D. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2020, 9, 104686. [Google Scholar] [CrossRef]
- Mustapha MU, Halimoon N Microorganisms and biosorption of heavy metals in the environment: A review paper. J. Microb. Biochem. Technol. 2015, 7, 253–256. [CrossRef]
- Tobin, J.M.; White, C.; Gadd, G.M. Metal accumulation by fungi—Applications in environmental biotechnology. J. Ind. Microbiol. 1994, 13, 126–130. [Google Scholar] [CrossRef]
- Davidova, E.G.; Kasparova, S.G. Adsorption of metals by yeast-cell walls. Microbiology 1992, 61, 716–719. [Google Scholar]
- Ding, Y.; Jing, D.; Gong, H.; Zhou, L.; Yang, X. Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour. Technol. 2012, 114, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Shibasaki, S.; Ueda, M.; Tanaka, A. Cell surface-engineered yeast displaying a histidine oligopeptide (hexa-His) has enhanced adsorption of and tolerance to heavy metal ions. Appl. Microbiol. Biotechnol. 2001, 57, 697–701. [Google Scholar] [CrossRef]
- Amirnia, S.; Margaritis, A.; Ray, M.B. Adsorption of Mixtures of Toxic Metal Ions Using Non-Viable Cells of Saccharomyces cerevisiae. Adsorpt. Sci. Technol. 2012, 30, 43–63. [Google Scholar] [CrossRef]
- Velasquez, L.; Dussan, J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef]
- Dey, P.; Malik, A.; Singh, D.K.; Haange, S.-B.; von Bergen, M.; Jehmlich, N. Unveiling fungal strategies: Mycoremediation in multi-metal pesticide environment using proteomics. Sci. Rep. 2024, 14, 23171. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Sorce, C.; Andreucci, A.; Vitelli, V.; Saba, A.; Li, M.; Varotto, C.; di Toppi, L.S. Intracellular and extracellular thiol-peptides modulate the response of Marchantia polymorpha to physiological needs, excess, and starvation of zinc, copper, and iron. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2024, 158, 754–762. [Google Scholar] [CrossRef]
- Dey, P.; Malik, A.; Singh, D.K.; Haange, S.-B.; von Bergen, M.; Jehmlich, N. Insight Into the Molecular Mechanisms Underpinning the Mycoremediation of Multiple Metals by Proteomic Technique. Front. Microbiol. 2022, 13, 872576. [Google Scholar] [CrossRef]
- García-García, J.D.; Sánchez-Thomas, R.; Moreno-Sánchez, R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 2016, 34, 859–873. [Google Scholar] [CrossRef]
- de Paz, G.A.; Martinez-Gutierrez, H.; Ramirez-Granillo, A.; Lopez-Villegas, E.O.; Medina-Canales, M.G.; Rodriguez-Tovar, A.V. Rhodotorula mucilaginosa YR29 is able to accumulate Pb2+ in vacuoles: A yeast with bioremediation potential. World J. Microbiol. Biotechnol. 2023, 39, 238. [Google Scholar] [CrossRef]
- Shim, D.; Kim, S.; Choi, Y.-I.; Song, W.-Y.; Park, J.; Youk, E.S.; Jeong, S.-C.; Martinoia, E.; Noh, E.-W.; Lee, Y. Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere 2013, 90, 1478–1486. [Google Scholar] [CrossRef]
- Mrvcic, J.; Stanzer, D.; Solic, E.; Stehlik-Tomas, V. Interaction of lactic acid bacteria with metal ions: Opportunities for improving food safety and quality. World J. Microbiol. Biotechnol. 2012, 28, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption and bioaccumulation—The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef]
- Malgorzata, G.; Stanislaw, B.; Joanna, R.; Wanda, D.-R. A study on Saccharomyces cerevisiae and Candida utilis cell wall capacity for binding magnesium. Eur. Food Res. Technol. 2006, 224, 49–54. [Google Scholar] [CrossRef]
- Mesquita, V.A.; Machado, M.D.; Silva, C.F.; Soares, E.V. Influence of the metabolic state on the tolerance of Pichia kudriavzevii to heavy metals. J. Basic Microbiol. 2016, 56, 1244–1251. [Google Scholar] [CrossRef]
- Bannon, D.I.; Portnoy, M.E.; Olivi, L.; Lees, P.S.; Culotta, V.C.; Bressler, J.P. Uptake of lead and iron by divalent metal transporter 1 in yeast and mammalian cells. Biochem. Biophys. Res. Commun. 2002, 295, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Dwivedi, S.K. Fungi mediated detoxification of heavy metals: Insights on mechanisms, influencing factors and recent developments. J. Water Process Eng. 2023, 53, 103800. [Google Scholar] [CrossRef]
- Kirecci, O.A.; Ozsahin, A.D.; Yilmaz, O.; Erdem, F.; Sarigul, H. Heavy metals (Chromium, Copper, Lead and Aluminum) on some biochemical parameters in Saccharomyces cerevisiae culture medium. Fresenius Environ. Bull. 2017, 26, 2179–2189. [Google Scholar]
- Teng, Y.; Yang, Y.; Wang, Z.; Guan, W.; Liu, Y.; Yu, H.; Zou, L. The cadmium tolerance enhancement through regulating glutathione conferred by vacuolar compartmentalization in Aspergillus sydowii. Chemosphere 2024, 352, 141500. [Google Scholar] [CrossRef]
- Steiger, M.G.; Patzschke, A.; Holz, C.; Lang, C.; Causon, T.; Hann, S.; Mattanovich, D.; Sauer, M. Impact of glutathione metabolism on zinc homeostasis in Saccharomyces cerevisiae. FEMS Yeast Res. 2017, 17, 4. [Google Scholar] [CrossRef]
- Cobbett, C.S. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zhao, X.; Sun, Z.; Li, G.; Hussain, S.; Li, X.; Zhang, L.; Wang, Z.; Gong, H.; et al. Effects of SpGSH1 and SpPCS1 overexpression or co-overexpression on cadmium accumulation in yeast and Spirodela polyrhiza. Plant Physiol. Biochem. 2024, 216, 109097. [Google Scholar] [CrossRef]
- Khan, I.U.; Aqsa, A.; Jamil, Y.; Khan, N.; Iqbal, A.; Ali, S.; Hamayun, M.; Alrefaei, A.F.; Faraj, T.K.; Lee, B.; et al. Anti-Oxidative and Anti-Apoptotic Oligosaccharides from Pichia pastoris-Fermented Cress Polysaccharides Ameliorate Chromium-Induced Liver Toxicity. Pharmaceuticals 2024, 17, 958. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tan, Y.; Liu, H.; Jin, Z.; Zhang, Y.; He, F.; Yan, Z.; Liu, H.; Meng, G.; Liu, H. Extracellular polymeric sub-stances secreted by marine fungus Aspergillus terreus: Full characterization and detailed effects on aluminum alloy corrosion. Corros. Sci. 2022, 209, 110703. [Google Scholar] [CrossRef]
- Christensen, B.E. Physical and chemical properties of extracellular polysaccharides associated with biofilms and related systems. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 143–154. [Google Scholar]
- Lazarova, V.; Manem, J. Biofilm characterization and activity analysis in water and wastewater treatment. Water Res. 1995, 29, 2227–2245. [Google Scholar] [CrossRef]
- Tsuneda, S.; Aikawa, H.; Hayashi, H.; Yuasa, A.; Hirata, A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 2003, 223, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huo, H.; Shi, Y.; Zhang, F.; Gu, T.; Li, Z. Chapter three—Extraction and application of extracellular polymeric substances from fungi. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: New York, NY, USA, 2023; Volume 125, pp. 79–106. [Google Scholar]
- Dash, H.R.; Das, S. Interaction between mercuric chloride and extracellular polymers of biofilm-forming mercury resistant marine bacterium Bacillus thuringiensis PW-05. RSC Adv. 2016, 6, 109793–109802. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Debnath, C.; Vijayaraghavan, R.; Muthuraj, M. Trends in bioremediation of heavy metal contaminations. Environ. Eng. Res. 2023, 28, 220631. [Google Scholar] [CrossRef]
- Xu, H.; He, E.; Peijnenburg, W.J.; Song, L.; Zhao, L.; Xu, X.; Cao, X.; Qiu, H. Contribution of pristine and reduced microbial extracellular polymeric substances of different sources to Cu(II) reduction. J. Hazard. Mater. 2021, 415, 125616. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Pruthi, P.A.; Pruthi, V. Role of exopolysaccharides in biofilm formation. In Intro-Duction to Biofilm Engineering; Rathinam, N.K., Sani, R.K., Eds.; ACS Symposium Series: Washington, DC, USA, 2019; Volume 1323, pp. 17–57. [Google Scholar]
- Gientka, I.; Blazejak, S.; Stasiak-Rozanska, L.; Chlebowska-Smigiel, A. Exopolysaccharides from yeast: Insight into optimal conditions for biosynthesis, chemical composition and functional properties—Review. Acta Sci. Pol. Technol. Aliment. 2015, 14, 283–292. [Google Scholar] [CrossRef]
- Oyetibo, G.O.; Miyauchi, K.; Suzuki, H.; Ishikawa, S.; Endo, G. Extracellular mercury sequestration by exopolymeric substances produced by Yarrowia spp.: Thermodynamics, equilibria, and kinetics studies. J. Biosci. Bioeng. 2016, 122, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Maalej, H.; Hmidet, N.; Boisset, C.; Buon, L.; Heyraud, A.; Nasri, M. Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J. Appl. Microbiol. 2015, 118, 356–367. [Google Scholar] [CrossRef]
- Han, F.; Wu, S.; He, Y.; Li, X.; Xu, M.; Xian, M.; Weng, H.; Nli, Y.; Chen, J.; Li, Z. Weakened fungus-assisted remediation of Cd(II) after addition of fibrous palygorskite. J. Appl. Microbiol. 2025, 136, 4. [Google Scholar] [CrossRef]
- Ke, X.; Xu, J.; Wang, X.; Zhu, B.; Han, F.; Tang, L.; Jiang, Z.; Gu, T.; Li, Z. Extracting extracellular polymeric substances from fungi in contrasts: From quantity to quality. Appl. Microbiol. Biotechnol. 2023, 107, 943–954. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Wang, X.; Wang, Z.; Tang, L.; Chen, H.; Li, Z. Detoxification of Cu(II) by the red yeast Rhodotorula mucilaginosa: From extracellular to intracellular. Appl. Microbiol. Biotechnol. 2020, 104, 10181–10190. [Google Scholar] [CrossRef]
- Florence, L.; Nicole, J.-R. Cell-based electrochemical biosensors for water quality assessment. Anal. Bioanal. Chem. 2011, 400, 947–964. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, L.; Mu, G.; Zhao, M.; Zou, P.; Zhang, Y. A facile strategy for fabrication of nano-ZnO/yeast composites and their adsorption mechanism towards lead(II) ions. Appl. Surf. Sci. 2016, 378, 196–206. [Google Scholar] [CrossRef]
- Vidal, N.F.; Dávila, J.W. Lead and cadmium removal with native yeast from coastal wetlands. Open Chem. 2022, 20, 1096–1109. [Google Scholar] [CrossRef]
- Ferraz, A.I.; Teixeira, J.A. The use of flocculating brewer’s yeast for Cr(III) and Pb(II) removal from residual wastewaters. Bioprocess Eng. 1999, 21, 431–437. [Google Scholar] [CrossRef]
- Yekta, G.; Sibel, U.; Ulgar, G. Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour. Technol. 2005, 96, 103–109. [Google Scholar] [CrossRef]
- Skountzou, P.; Soupioni, M.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.; Marchant, R.; Banat, I. Lead(II) uptake during baker’s yeast production by aerobic fermentation of molasses. Process Biochem. 2003, 38, 1479–1482. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A new insight into lead(II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2019, 21, 471–479. [Google Scholar] [CrossRef]
- Tian, D.; Cheng, X.; Wang, L.; Hu, J.; Zhou, N.; Xia, J.; Xu, M.; Zhang, L.; Gao, H.; Ye, X.; et al. Remediation of Lead-Contaminated Water by Red Yeast and Different Types of Phosphate. Front. Bioeng. Biotechnol. 2022, 10, 775058. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, X.; He, Y.; Yan, Y.; Zhang, L.; Wang, Z.; Zhang, L.; Tian, D. Lead remediation by geological fluorapatite combined with Penicillium Oxalicum and red yeast. Microb. Cell Factories 2024, 23, 64. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, Q.; Ren, J.; Zheng, Z.; Xiao, Y. Structure characteristics, solution properties and morphology of oxidized yeast β-glucans derived from controlled TEMPO-mediated oxidation. Carbohydr. Polym. 2020, 250, 116924. [Google Scholar] [CrossRef]
- Jensen, L.T.; Culotta, V.C. Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J. Mol. Biol. 2002, 318, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Vido, K.; Spector, D.; Lagniel, G.; Lopez, S.; Toledano, M.B.; Labarre, J. A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 2001, 276, 8469–8474. [Google Scholar] [CrossRef]
- Perrin, D.D.; Watt, A.E. Complex formation of zinc and cadmium with glutathione. Biochim. Biophys. Acta 1971, 230, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.S.; Fragoso, L.C.; Riger, C.J.; Panek, A.D.; Eleutherio, E.C.A. Regulation of cadmium uptake by Saccharomyces cerevisiae. Biochim. Biophys. Acta-Gen. Subj. 2002, 1573, 21–25. [Google Scholar] [CrossRef]
- Alam, M.Z.; Ahmad, S. Toxic chromate reduction by resistant and sensitive bacteria isolated from tannery effluent contaminated soil. Ann. Microbiol. 2012, 62, 113–121. [Google Scholar] [CrossRef]
- Poljsak, B.; Pocsi, I.; Raspor, P.; Pesti, M. Interference of chromium with biological systems in yeasts and fungi: A review. J. Basic Microbiol. 2010, 50, 21–36. [Google Scholar] [CrossRef]
- Muneer, B.; Rehman, A.; Shakoori, F.R.; Shakoori, A.R. Evaluation of consortia of microorganisms for efficient removal of hexavalent chromium from industrial wastewater. Bull. Environ. Contam. Toxicol. 2009, 82, 597–600. [Google Scholar] [CrossRef]
- Sathvika, T.; Manasi Rajesh, V.; Rajesh, N. Microwave assisted immobilization of yeast in cellulose biopolymer as a green adsorbent for the sequestration of chromium. Chem. Eng. J. 2015, 279, 38–46. [Google Scholar] [CrossRef]
- Asri, M.; Ouafi, R.; Bahafid, W.; Elabed, S.; Koraichi, S.I.; Costa, F.; Tavares, T.; El Ghachtouli, N. Chromium removal by newly developed microbial consortia supported on wood husk. Desalination Water Treat. 2023, 289, 80–91. [Google Scholar] [CrossRef]
- Tumolo, M.; Volpe, A.; Leone, N.; Cotugno, P.; De Paola, D.; Losacco, D.; Locaputo, V.; de Pinto, M.C.; Uricchio, V.F.; Ancona, V. Enhanced natural attenuation of groundwater Cr(VI) pollution using electron donors: Yeast extract vs. polyhydroxybutyrate. Int. J. Environ. Res. Public Health 2022, 19, 9622. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Wang, Y.; Wang, X. Using the inherent elements in yeast biomass to produce Ni2+ P/N-doped biocarbon composites for efficient hexavalent chromium reduction. Environ. Sci. Pollut. Res. 2023, 30, 119343–119355. [Google Scholar] [CrossRef] [PubMed]
- Oyetibo, G.O.; Miyauchi, K.; Suzuki, H.; Endo, G. Mercury removal during growth of mercury tolerant and self-aggregating Yarrowia spp. AMB Express 2016, 6, 99. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Ahmed, S.B.; Osman, M.M.; Abdel-Fattah, T.M. A novel composite of nanomagnetite-immobilized-baker’s yeast on the surface of activated carbon for magnetic solid phase extraction of Hg(II). Fuel 2015, 139, 614–621. [Google Scholar] [CrossRef]
- Lozovaia, O.G.; Kasatkina, T.P.; Podgorskii, V.S. Search of heavy metals biosorbents among yeasts of different taxonomic groups. Mikrobiolohichnyi Zhurnal (Kiev Ukr. 1993) 2004, 66, 92–101. [Google Scholar]
- Sun, X.; Liu, L.; Zhao, Y.; Ma, T.; Zhao, F.; Huang, W.; Zhan, J. Effect of copper stress on growth characteristics and fermentation properties of Saccharomyces cerevisiae and the pathway of copper adsorption during wine fermentation. Food Chem. 2016, 192, 43–52. [Google Scholar] [CrossRef]
- Cojocaru, C.; Diaconu, M.; Cretescu, I.; Savic, J.; Vasic, V. Biosorption of copper(II) ions from aqua solutions using dried yeast biomass. Colloids Surf. A Physicochem. Eng. Asp. 2009, 335, 181–188. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Fan, Y.; Wang, Y.; Ma, Y.; Gu, D.; Lu, Y.; Zhang, S.; Chen, X.; Zhang, W. Pear metal transport protein PbMTP8.1 confers manganese tolerance when expressed in yeast and Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2021, 208, 111687. [Google Scholar] [CrossRef] [PubMed]
- Duerr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump PMR1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef]
- Mandal, D.; Woolf, T.B.; Rao, R. Manganese selectivity of PMR1, the yeast secretory pathway ion pump, is defined by residue Q783 in transmembrane segment 6. Residue D778 is essential for cation transport. J. Biol. Chem. 2000, 275, 23933–23938. [Google Scholar] [CrossRef]
- Rathod, J.; Tu, H.-P.; Chang, Y.-I.; Chu, Y.-H.; Tseng, Y.-Y.; Jean, J.-S.; Wu, W.-S. YARG: A repository for arsenic-related genes in yeast. PLoS ONE 2018, 13, e0201204. [Google Scholar] [CrossRef]
- Bertoldi, D.; Román, T.; Guzzon, R.; Santato, A.; Malacarne, M.; Nicolini, G.; Larcher, R. Vitality and detoxification ability of yeasts in naturally As-rich musts. Eur. Food Res. Technol. 2016, 242, 1655–1662. [Google Scholar] [CrossRef]
- Talemi, S.R.; Jacobson, T.; Garla, V.; Navarrete, C.; Wagner, A.; Tamas, M.J.; Schaber, J. Mathematical modelling of arsenic transport, distribution and detoxification processes in yeast. Mol. Microbiol. 2014, 92, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Lagniel, G.; Kristiansson, E.; Junot, C.; Nerman, O.; Labarre, J.; Tamás, M.J. Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol Genom. 2007, 30, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef]
- Kumar, N.V.; Yang, J.; Pillai, J.K.; Rawat, S.; Solano, C.; Kumar, A.; Grøtli, M.; Stemmler, T.L.; Rosen, B.P.; Tamás, M.J. Arsenic Directly Binds to and Activates the Yeast AP-1-Like Transcription Factor Yap8. Mol. Cell. Biol. 2015, 36, 913–922. [Google Scholar] [CrossRef]
- Menezes, R.A.; Amaral, C.; Batista-Nascimento, L.; Santos, C.; Ferreira, R.B.; Devaux, F.; Eleutherio, E.C.A.; Rodrigues-Pousada, C. Contribution of Yap1 towards Saccharomyces cerevisiae adaptation to arsenic-mediated oxidative stress. Biochem. J. 2008, 414, 301–311. [Google Scholar] [CrossRef]
- West, K.L.; Byrum, S.D.; Mackintosh, S.G.; Edmondson, R.D.; Taverna, S.D.; Tackett, A.J. Proteomic characterization of the arsenic response locus in S. cerevisiae. Epigenetics 2019, 14, 130–145. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, V.; Srivastava, S.; Bist, V.; Tripathi, P.; Naseem, M.; Nand, S.; Anshu; Khare, P.; Srivastava, P.K.; et al. Yeast strain Debaryomyces hansenii for amelioration of arsenic stress in rice. Ecotoxicol. Environ. Saf. 2020, 195, 110480. [Google Scholar] [CrossRef]
- Verma, S.; Verma, P.K.; Meher, A.K.; Dwivedi, S.; Bansiwal, A.K.; Pande, V.; Srivastava, P.K.; Verma, P.C.; Tripathi, R.D.; Chakrabarty, D. A novel arsenic methyltransferase gene of Westerdykella aurantiaca isolated from arsenic contaminated soil: Phylogenetic, physiological, and biochemical studies and its role in arsenic bioremediation. Met. Integr. Biometal Sci. 2016, 8, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Verma, P.K.; Chakrabarty, D. Arsenic bio-volatilization by engineered yeast promotes rice growth and reduces arsenic accumulation in grains. Int. J. Environ. Res. 2019, 13, 475–485. [Google Scholar] [CrossRef]
- Kaur, J.; Anand, V.; Srivastava, S.; Bist, V.; Naseem, M.; Singh, P.; Gupta, V.; Singh, P.C.; Saxena, S.; Bisht, S.; et al. Mitigation of arsenic toxicity in rice by the co-inoculation of arsenate reducer yeast with multifunctional arsenite oxidizing bacteria. Environ. Pollut. 2023, 320, 120975. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Isner, J.; Isayenkov, S.V.; Liu, W.; Zhao, F.; Maathuis, F.J.M. Heterologous expression of the yeast arsenite efflux system ACR3 improves Arabidopsis thaliana tolerance to arsenic stress. New Phytol. 2012, 194, 716–723. [Google Scholar] [CrossRef]
- Hosseini, F.; Hadian, M.; Lashani, E.; Moghimi, H. Simultaneous bioreduction of tellurite and selenite by Yarrowia lipolytica, Trichosporon cutaneum and their co-culture along with characterization of biosynthesized Te–Se nanoparticles. Microb. Cell Factories 2023, 22, 193. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Zhou, J.; Luo, X. Uranium biosorption mechanism model of protonated Saccharomyces cerevisiae. J. Hazard. Mater. 2020, 385, 121588. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Kana, M.T.H.A.; Hendy, A.A. Synthesis and implementation of nano-chitosan and its acetophenone derivative for enhanced removal of metals. Int. J. Biol. Macromol. 2015, 81, 672–680. [Google Scholar] [CrossRef]
- Stanescu, A.M.; Stoica, L.; Constantin, C.; Lacatusu, I.; Oprea, O.; Miculescu, F. Physicochemical characterization and use of heat pretreated commercial instant dry baker’s yeast as a potential biosorbent for Cu(II) removal. Clean–Soil Air Water 2014, 42, 1632–1641. [Google Scholar] [CrossRef]
- Enrique, T. Biosorption: A review of the latest advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, H.L.; Yang, Z.L.; Xu, M. Dual-responsive copolymer hydrogel as broad-spectrum adsorbents for metal ions. Polym. Test. 2019, 77, 105887. [Google Scholar] [CrossRef]
- Yao, G.H.; Bi, W.D.; Liu, H. pH-responsive magnetic graphene oxide/poly(NVI-co-AA) hydrogel as an easily recyclable adsorbent for cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124393. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.T.; Li, S.Y.; Tian, Y.Y.; Zhu, G.S. Porous aromatic framework modified electrospun fiber membrane as a highly efficient and reusable adsorbent for pharmaceuticals and personal care products removal. Ace Appl. Mater. Interfaces 2019, 11, 16662–16673. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yang, Q.L.; Huang, L.L.; Chen, L.H.; Ni, Y.H.; Xiao, H.N. Temperature and pH responsive cellulose filament/poly (NIPAM-co-AAc) hybrids as novel adsorbent towards Pb(II) removal. Carbohydr. Polym. 2018, 195, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Khamis, F.; Hegab, H.M.; Banat, F.; Arafat, H.A.; Hasan, S.W. Comprehensive review on pH and temperature-responsive polymeric adsorbents: Mechanisms, equilibrium, kinetics, and thermodynamics of adsorption processes for heavy metals and organic dyes. Chemosphere 2024, 349, 140801. [Google Scholar] [CrossRef]
- Nicula, N.-O.; Lungulescu, E.-M.; Rimbu, G.A.; Marinescu, V.; Corbu, V.M.; Csutak, O. Bioremediation of wastewater using yeast strains: An assessment of contaminant removal efficiency. Int. J. Environ. Res. Public Health 2023, 20, 4795. [Google Scholar] [CrossRef]
- Mapolelo, M.; Torto, N. Trace enrichment of metal ions in aquatic environments by Saccharomyces cerevisiae. Talanta 2004, 64, 39–47. [Google Scholar] [CrossRef]
- Rao, M.M.; Rao, G.C.; Seshaiah, K.; Choudary, N.; Wang, M. Activated carbon from ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag. 2008, 28, 849–858. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere 2005, 59, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; de Larraya, U.P.; Liu, P.; Pfenninger, N.; Mathew, A.P.; Zimmermann, T.; Tingaut, P. Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose 2014, 21, 2831–2844. [Google Scholar] [CrossRef]
- Emilia, B.; Milan, C.; Annamaria, K.; Tomas, G. Biosorption of nickel by yeasts in an osmotically unsuitable environment. Z. Für Naturforsch. C 2008, 63, 873–878. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium(II) ions from aqueous solution by a new low-cost adsorbent-bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- Gaikani, H.K.; Stolar, M.; Kriti, D.; Nislow, C.; Giaever, G. From beer to breadboards: Yeast as a force for biological innovation. Genome Biol. 2024, 25, 10. [Google Scholar] [CrossRef]
- Kuroda, K.; Ueda, M. Cell surface engineering of yeast for applications in white biotechnology. Biotechnol. Lett. 2011, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.I.; Matis, K.A.; Lazaridis, N.K. Removal of metal ions from simulated wastewater by Saccharomyces yeast biomass: Combining biosorption and flotation processes. Sep. Sci. Technol. 2001, 36, 349–365. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Saravanan, A.; Vo, D.-V.N. Advances in biosorbents for removal of environmental pollutants: A review on pretreatment, removal mechanism and future outlook. J. Hazard. Mater. 2021, 420, 126596. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.V.; Soares, H.M.V.M. Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: A review. Environ. Sci. Pollut. Res. 2012, 19, 1066–1083. [Google Scholar] [CrossRef]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Ferraz, A.I.; Tavares, T.; Teixeira, J.A. Cr(III) Removal and recovery from Saccharomyces cerevisiae. Chem. Eng. J. 2004, 105, 11–20. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V.; Soares, H.M.V.M. Selective recovery of chromium, copper, nickel, and zinc from an acid solution using an environmentally friendly process. Environ. Sci. Pollut. Res. 2011, 18, 1279–1285. [Google Scholar] [CrossRef][Green Version]
- Sieber, A.; Jelic, L.R.; Kremser, K.; Guebitz, G.M. Spent brewer’s yeast as a selective biosorbent for metal recovery from polymetallic waste streams. Front. Bioeng. Biotechnol. 2024, 12, 1345112. [Google Scholar] [CrossRef]
- Machado, M.D.; Santos, M.S.; Gouveia, C.; Soares, H.M.; Soares, E.V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: The flocculation as a separation process. Bioresour. Technol. 2008, 99, 2107–2115. [Google Scholar] [CrossRef]
- Ma, X.; Cui, W.; Yang, L.; Yang, Y.; Chen, H.; Wang, K. Efficient biosorption of lead(II) and cadmium(II) ions from aqueous solutions by functionalized cell with intracellular CaCO3 mineral scaffolds. Bioresour. Technol. 2015, 185, 70–78. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.-L.; Wang, J.-H. Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends Anal. Chem. 2015, 66, 90–102. [Google Scholar] [CrossRef]
- Ileana Cornelia, F.; Lavinia Liliana, R. Metallothioneins, Saccharomyces cerevisiae, and heavy metals: A biotechnology triad? In Old Yeasts–New Questions; Candida, L., Celia, P., Eds.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef][Green Version]
- Sun, G.L.; Reynolds, E.E.; Belcher, A.M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nat. Commun. 2019, 10, 5080. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, M.; Daneshi, A.; Younesi, H. Biosorption equilibria of binary Cd(II) and Ni(II) systems onto Saccharomyces cerevisiae and Ralstonia eutropha cells: Application of response surface methodology. J. Hazard. Mater. 2009, 168, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Xu, M.; Zheng, F.; Zhao, M. Study of the mechanisms of Cu2+ biosorption by ethanol/caustic-pretreated baker’s yeast biomass. J. Hazard. Mater. 2010, 178, 1085–1093. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, Y.-G.; Peng, Q.-Q.; Hu, X.-J.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Lata, R.; Sourja, G.; Swachchha, M. Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: A review. Appl. Biochem. Biotechnol. 2016, 180, 41–78. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Sonmez, H.B.; Senkal, B.F.; Bicak, N. Poly(acrylamide) grafts on spherical bead polymers for extremely selective removal of mercuric ions from aqueous solutions. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3068–3078. [Google Scholar] [CrossRef]
- Takafuji, M.; Ide, S.; Ihara, H.; Xu, Z. Preparation of poly(1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem. Mater. 2004, 16, 1977–1983. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E. Surface properties of yeast cells during heavy metal biosorption. Open Chem. 2011, 9, 348–351. [Google Scholar] [CrossRef]
- Yu, J.; Tong, M.; Sun, X.; Li, B. Enhanced and selective adsorption of Pb2+ and Cu2+ by EDTAD-modified biomass of baker’s yeast. Bioresour. Technol. 2008, 99, 2588–2593. [Google Scholar] [CrossRef]
- Madrid, Y.; Camara, C. Biological substrates for metal preconcentration and speciation. Trac-Trends Anal. Chem. 1997, 16, 36–44. [Google Scholar] [CrossRef]
- Yang, C.E.; Chu, I.M.; Wei, Y.H.; Tsai, S.L. Surface display of synthetic phytochelatins on Saccharomyces cerevisiae for enhanced ethanol production in heavy metal-contaminated substrates. Bioresour. Technol. 2017, 245, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Kan, G.F.; Ju, Y.; Zhou, Y.; Shi, C.J.; Qiao, Y.P.; Yang, Y.; Wang, R.Q.; Wang, X.F. Cloning and functional characterization of a novel metallothionein gene in Antarctic sea-ice yeast (Rhodotorula mucilaginosa). J. Basic Microbiol. 2019, 59, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Pavel, K.; Tomas, R. Surface display of metal fixation motifs of bacterial P1-type ATPases specifically promotes biosorption of Pb2+ by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2010, 76, 2615–2622. [Google Scholar] [CrossRef]
- Wei, Q.G.; Zhang, H.H.; Guo, D.G.; Ma, S.S. Cell surface display of four types of Solanum nigrum metallothionein on Saccharomyces cerevisiae for biosorption of cadmium. J. Microbiol. Biotechnol. 2016, 26, 846–853. [Google Scholar] [CrossRef]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Tao, H.C.; Li, P.S.; Liu, Q.S.; Su, J.; Qiu, G.Y.; Li, Z.G. Surface-engineered Saccharomyces cerevisiae cells displaying redesigned CadR for enhancement of adsorption of cadmium(II). J. Chem. Technol. Biotechnol. 2016, 91, 1889–1895. [Google Scholar] [CrossRef]
- Valls, M.; Atrian, S.; de Lorenzo, V.; Fernández, L.A. Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nat. Biotechnol. 2000, 18, 661–665. [Google Scholar] [CrossRef]
- Wegrzyn, A.; Tsurtsumia, A.; Witkowski, S.; Freitas, O.; Figueiredo, S.; Cybinska, J.; Stawinski, W. Vermiculite as a potential functional additive for water treatment bioreactors inhibiting toxic action of heavy metal cations upsetting the microbial balance. J. Hazard. Mater. 2022, 433, 128812. [Google Scholar] [CrossRef]
- Xia, Y.; Meng, L.; Jiang, Y.; Zhang, Y.; Dai, X.; Zhao, M. Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for cadmium. Chem. Eng. J. 2015, 259, 927–935. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Y.; Tang, L.; Sun, X.; Huo, H.; He, Y.; Huang, Y.; Shao, Q.; Pan, S.; Li, Z. Effects of temperature-related changes on charred bone in soil: From P release to microbial community. Curr. Res. Microb. Sci. 2024, 6, 100221. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhao, L.; Du, K. Construction of hierarchically porous chitin microspheres via a novel dual-template strategy for rapid and high-capacity removal of heavy metal ions. Chem. Eng. J. 2020, 393, 124818. [Google Scholar] [CrossRef]
- Shahzadi, I.; Wu, Y.; Lin, H.; Huang, J.; Zhao, Z.; Chen, C.; Shi, X.; Deng, H. Yeast biomass ornamented macro-hierarchical chitin nanofiber aerogel for enhanced adsorption of cadmium(II) ions. J. Hazard. Mater. 2023, 453, 131312. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.R.; Ando, R.A.; Correa, B. Bio-separator and bio-synthesizer of metallic nanoparticles—A new vision in bioremediation. Mater. Lett. 2022, 306, 130878. [Google Scholar] [CrossRef]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef]
- Wen, J.L.; He, P.P.; Lei, C.; Lv, E.J.; Wu, Y.H.; Gao, J.K.; Yao, J.M. Fabrication of metal-organic framework@Yeast composite materials for efficient removal of Pb2+ in water. J. Solid State Chem. 2019, 274, 26–31. [Google Scholar] [CrossRef]
- De, M.; Azargohar, R.; Dalai, A.K.; Shewchuk, S.R. Mercury removal by bio-char based modified activated carbons. Fuel 2013, 103, 570–578. [Google Scholar] [CrossRef]
- Yao, Y.X.; Velpari, V.; Economy, J. Design of sulfur treated activated carbon fibers for gas phase elemental mercury removal. Fuel 2014, 116, 560–565. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Osman, M.M.; Ahmed, S.B.; Abdel-Fattah, T.M. Improved adsorptive removal of cadmium from water by hybrid chemically and biologically carbonaceous sorbents. Chem. Eng. J. 2011, 175, 84–94. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Z.Q.; Hou, H.; Bai, Z.K.; Yuan, Y.; Su, Z.J.; Wang, G.Y. The effect of combined ecological remediation (plant microorganism modifier) on rare earth mine wasteland. Environ. Sci. Pollut. Res. 2020, 27, 13679–13691. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Melear, N.; Harris, K.; Das, K.C. Biochar as bulking agent for poultry litter composting. Carbon Manag. 2011, 2, 227–230. [Google Scholar] [CrossRef]
- Strotmann, U.; Durand, M.J.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological toxicity tests using standardized ISO/OECD methods—Current state and outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar] [CrossRef] [PubMed]

| Strains | Sorbed Ions | Conditions | pH | References |
|---|---|---|---|---|
| Saccharomyces cerevisiae | As5+, As3+, Cd2+, Cr6+, Cu2+, Hg2+, Mn2+, Ni2+, Pb2+, Zn2+ | 0.5 g yeast dosage and incubation time 30 min (Cu2+) 30 °C, 0.1 g/L yeast biomass concentration (Mn2+) 4.5 g dry weight/L biomass concentration (Pb2+, Cr6+) 28 °C (As5+, As3+) | pH 5–7 | [17,18,19,20,21,22] |
| Rhodotorulamucilaginosa | Cd2+, Cr6+, Cu2+, Hg2+, Pb2+, Mn2+, U6+, Zn2+ | 28 °C | - | [23,24,25,26] |
| Pichia pastoris | Cu2+ | 25 °C | pH 6 | [27] |
| Pichia hampshirensis | Zn2+, Cd2+, Pb2+, Ni2+, Cr6+, As2+, Cu2+, Hg2+ | 30–37 °C (Cd2+) | pH 7 | [28] |
| Meyerozyma guilliermondii | Mn2+, Cr6+, Ag+, Cu2+ | incubation time 2 h, 28 °C | - | [29] |
| Meyerozyma caribbica | Mn2+ | incubation time 2 h, 28 °C | - | [29] |
| Geotrichum sp. | Zn2+, Ni2+, Cu2+ | initial metal concentration 80 mg/L, 28 °C, incubation time 48 h (Cu2+) 28 °C, incubation time 60 h (Zn2+, Ni2+) | - | [30] |
| Candida xylopsoci | Hg2+ | 30 °C, incubation time 36 h | - | [31] |
| Candida utilis | Cr6+, Cu2+, Zn2+, Cd2+, Pb2+, Mg2+ | 28–30 °C, accumulation time 24 h | pH 5 | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Q.; Yan, S.; Sun, X.; Chen, H.; Lu, Y.; Li, S.; Huang, Y.; Wang, S.; Zhang, M.; Li, Z. Applications of Yeasts in Heavy Metal Remediation. Fermentation 2025, 11, 236. https://doi.org/10.3390/fermentation11050236
Shao Q, Yan S, Sun X, Chen H, Lu Y, Li S, Huang Y, Wang S, Zhang M, Li Z. Applications of Yeasts in Heavy Metal Remediation. Fermentation. 2025; 11(5):236. https://doi.org/10.3390/fermentation11050236
Chicago/Turabian StyleShao, Qi, Shihui Yan, Xin Sun, Hongming Chen, Yixiao Lu, Siqi Li, Yunjie Huang, Shimei Wang, Min Zhang, and Zhen Li. 2025. "Applications of Yeasts in Heavy Metal Remediation" Fermentation 11, no. 5: 236. https://doi.org/10.3390/fermentation11050236
APA StyleShao, Q., Yan, S., Sun, X., Chen, H., Lu, Y., Li, S., Huang, Y., Wang, S., Zhang, M., & Li, Z. (2025). Applications of Yeasts in Heavy Metal Remediation. Fermentation, 11(5), 236. https://doi.org/10.3390/fermentation11050236








