Evaluation of Sensory Properties and Short-Chain Fatty Acid Production in Fermented Soymilk on Addition of Fructooligosaccharides and Raffinose Family of Oligosaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Reagents
2.2. Preparation of Synbiotic Soymilk
2.3. Measurement of pH and Titratable Acidity (TA)
2.4. Estimation of Glucose, Sucrose, and RFOs
2.5. Estimation of Fructose
2.6. Measurement of Cell Viability
2.7. Kinetics of Cell Growth with Different Carbon Sources
2.8. Fatty Acyl Methyl Ester (FAME) Preparation and GC Analysis
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results
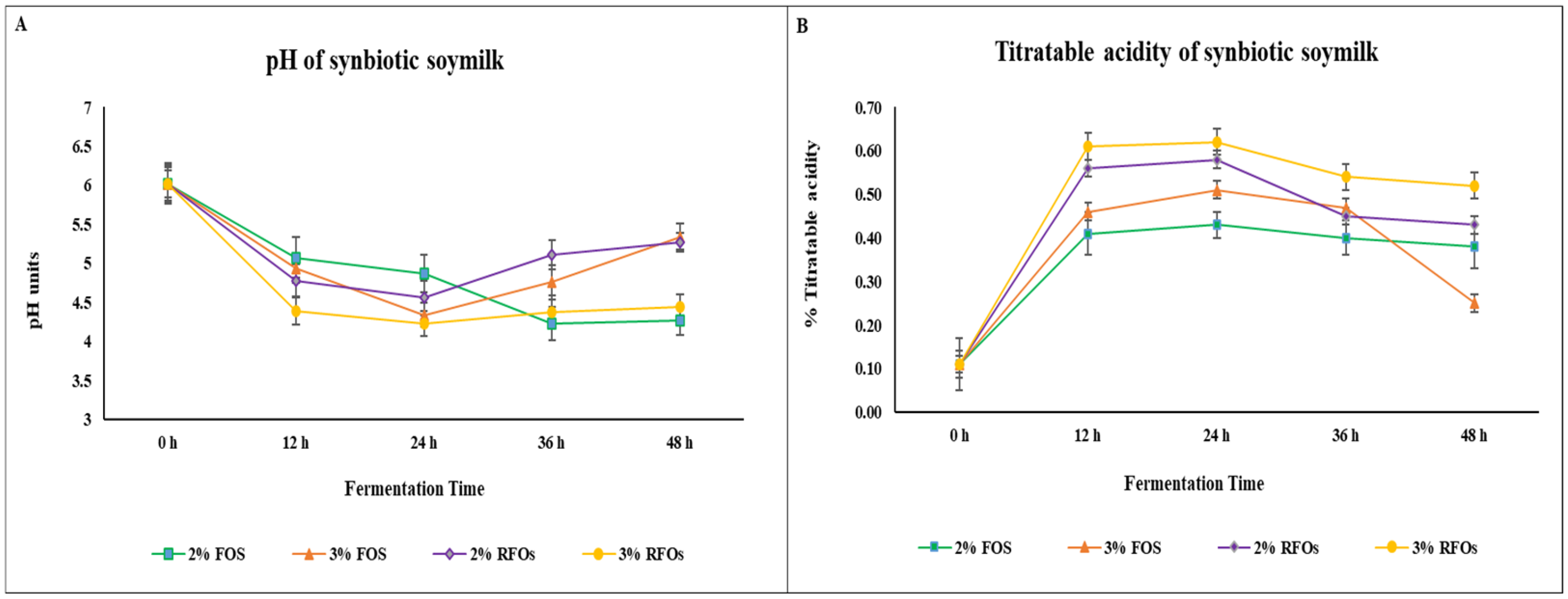
3.1. pH and TA Change During Fermentation and Storage
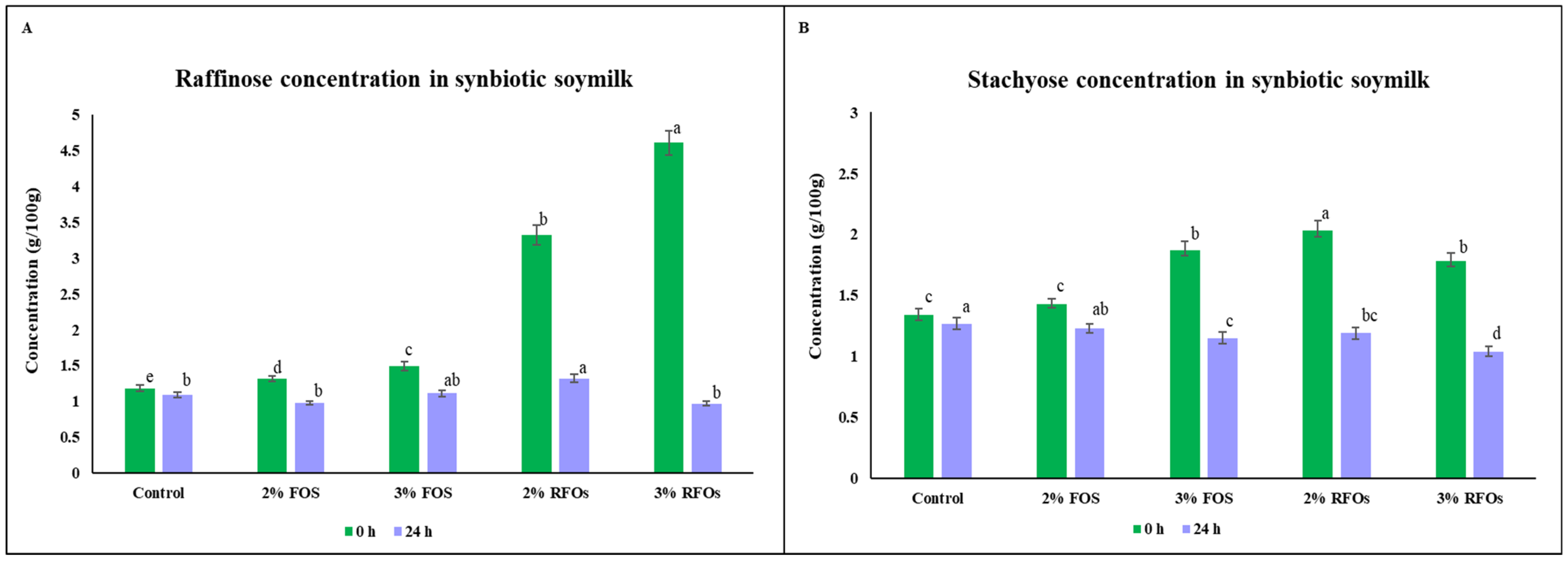
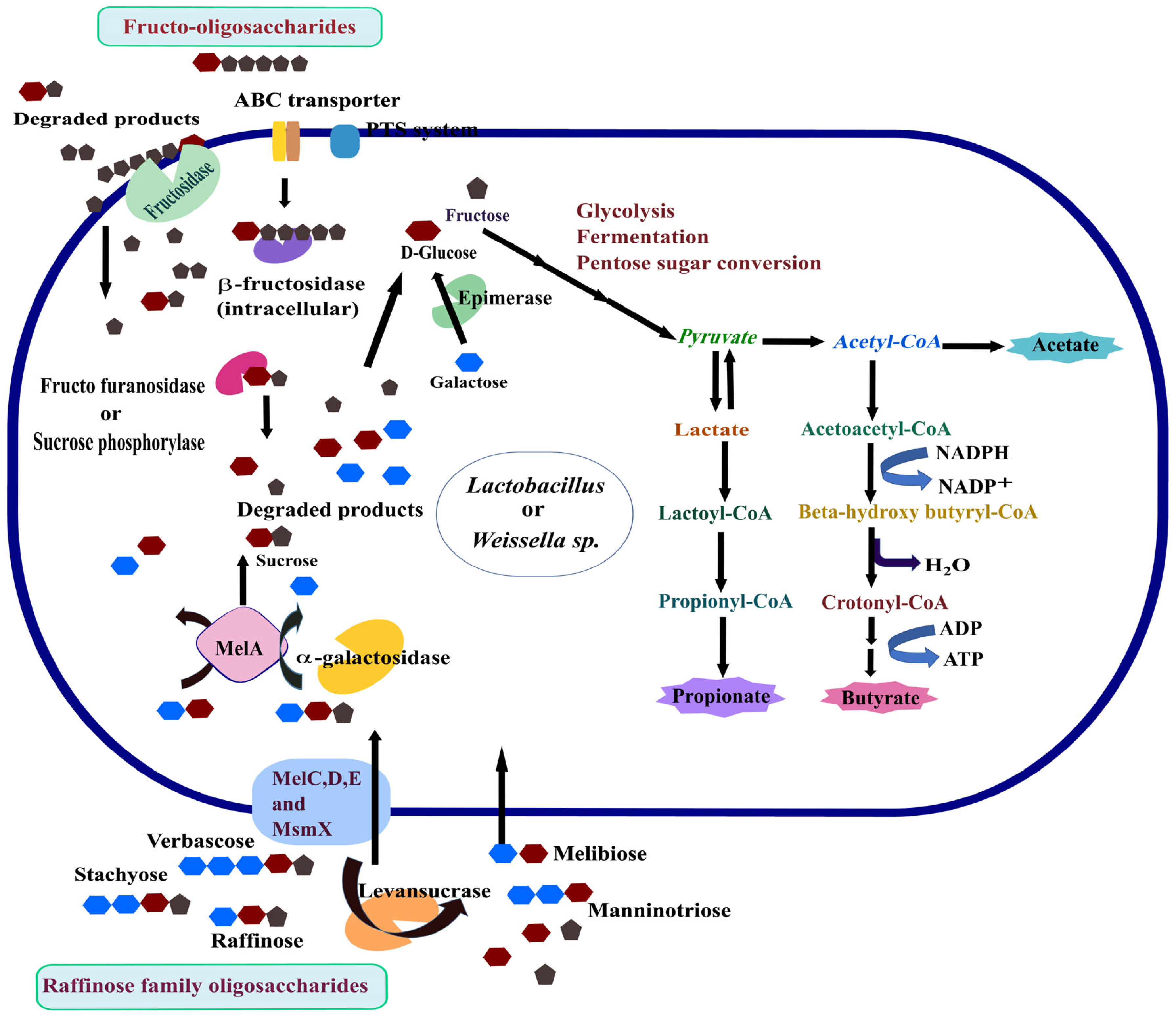
3.2. Utilisation of Prebiotic Oligosaccharides—RFOs and FOS
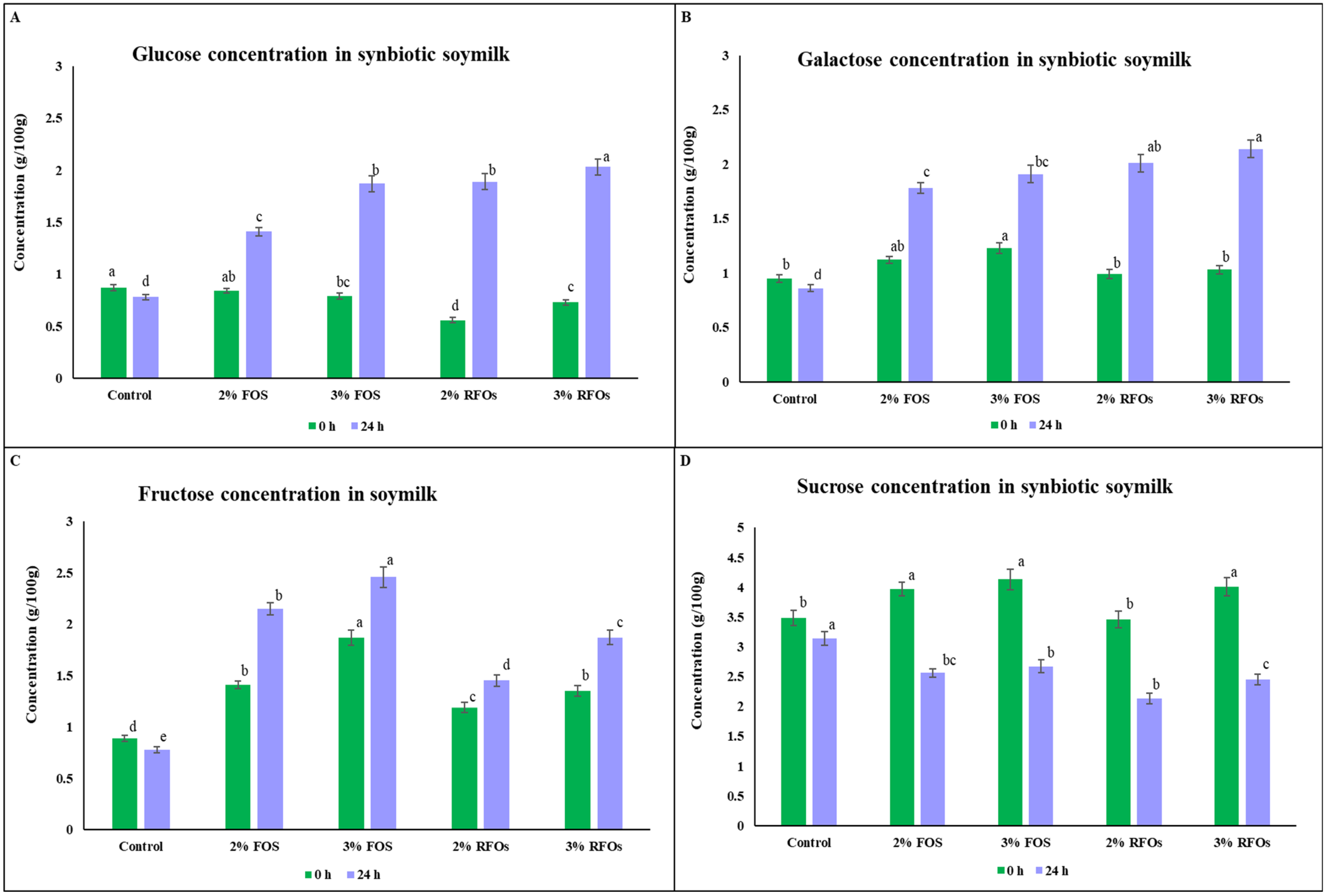
3.3. Effect of Supplementation and Fermentation on Monosaccharides and Sucrose Concentrations
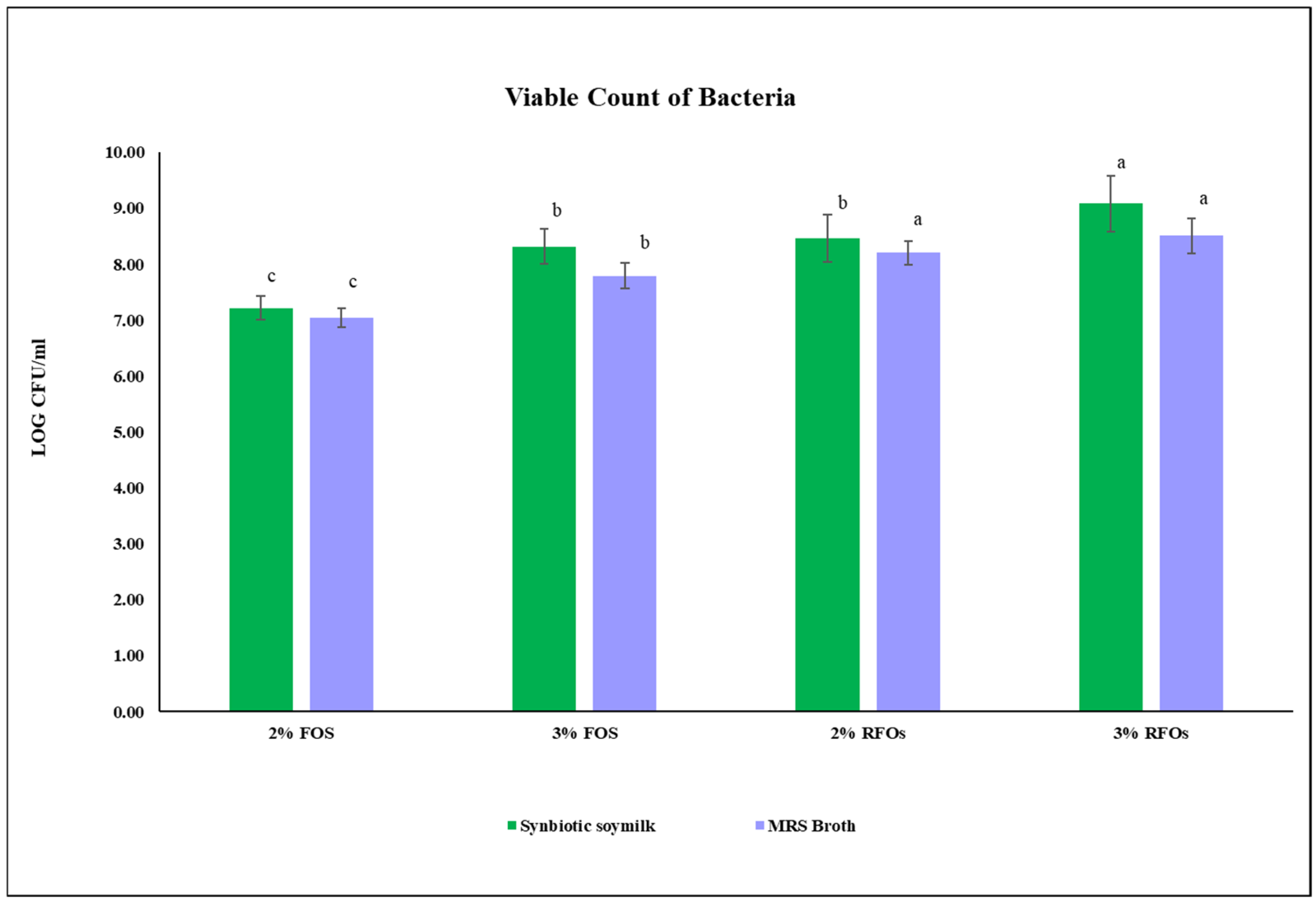
3.4. Measurement of Viable Count of Bacteria in Synbiotic Soymilk
3.5. Growth Curve Analysis
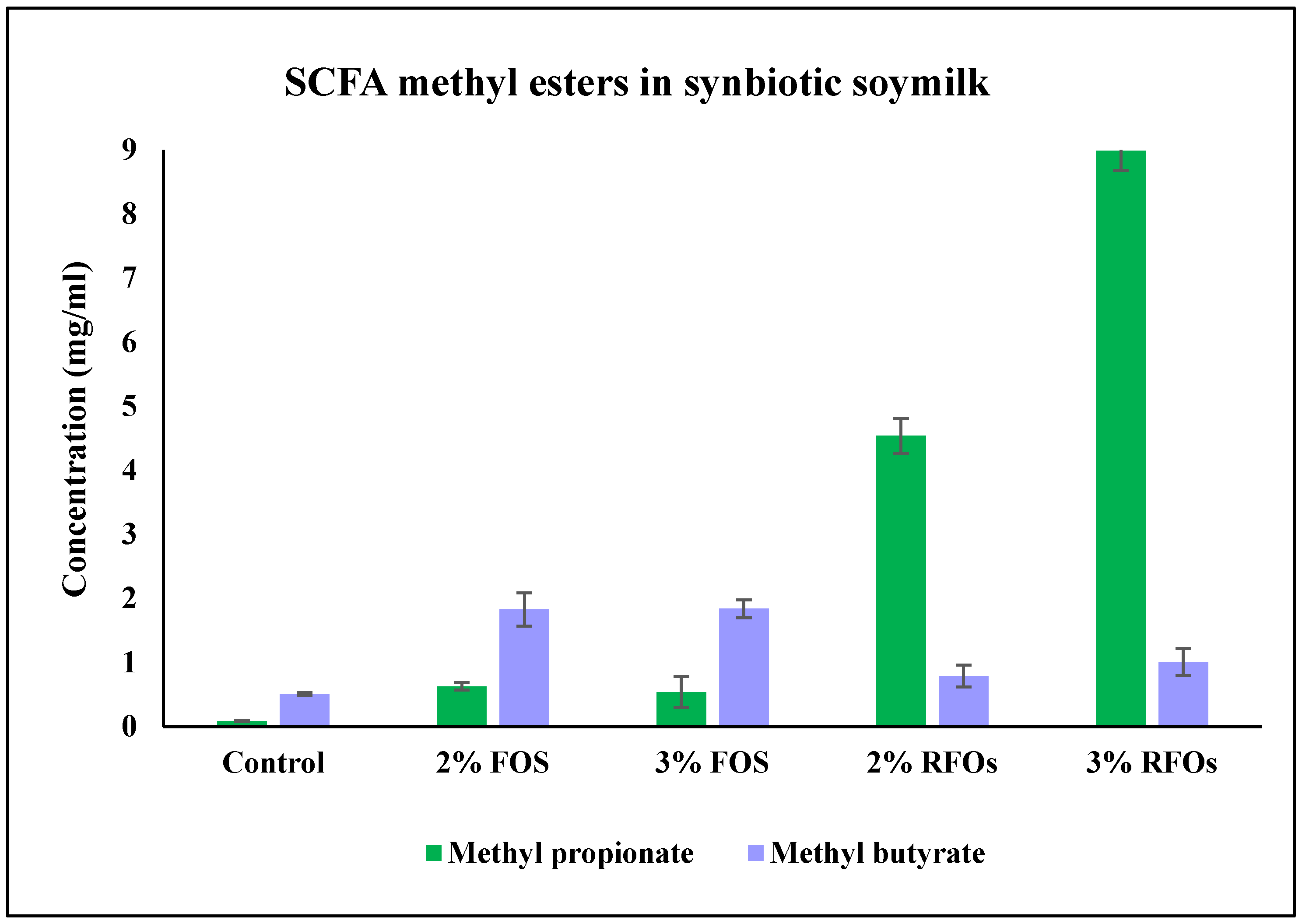
3.6. Change in SCFA Methyl Esters
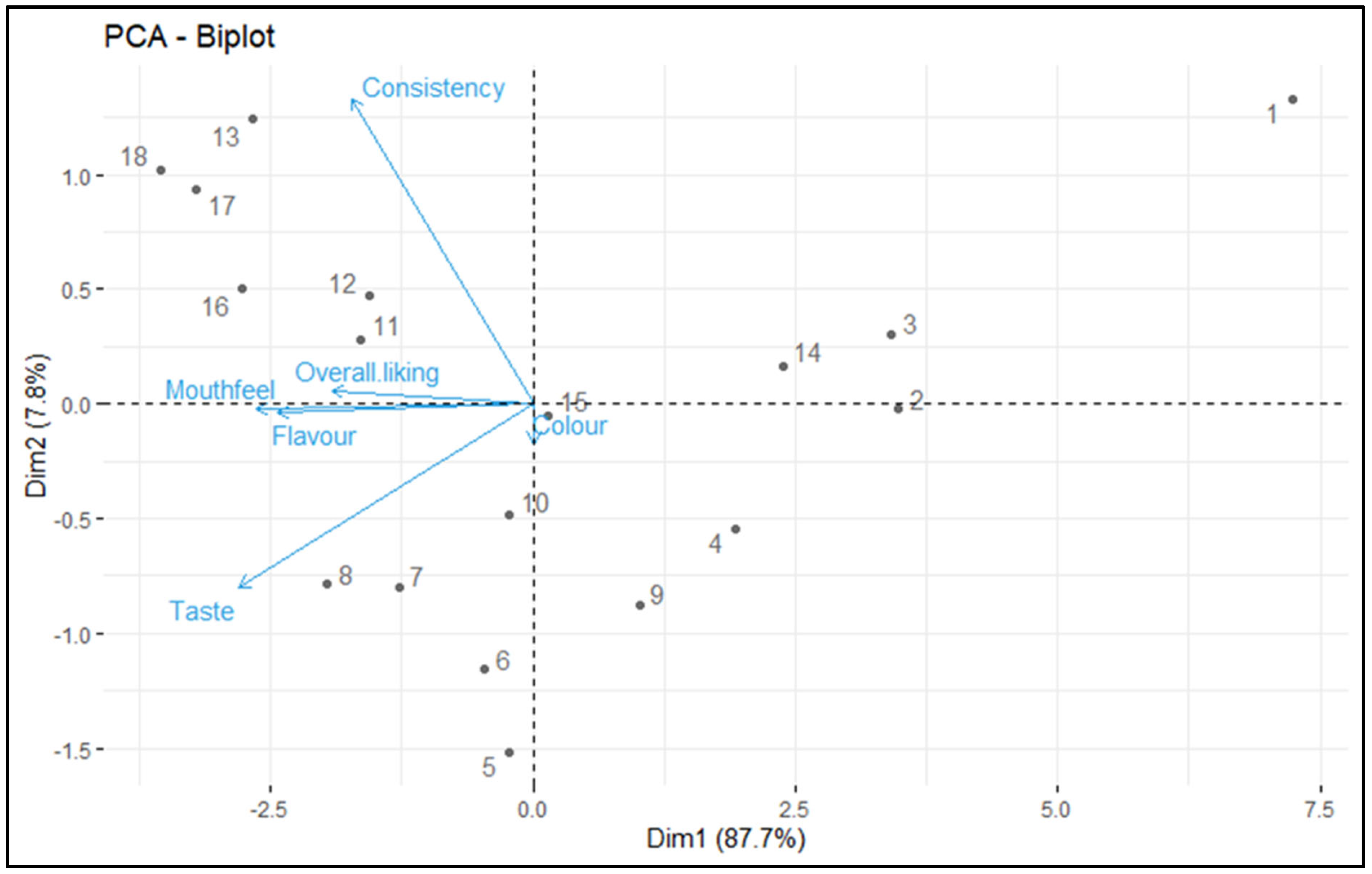
3.7. Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic acid bacteria |
| FOS | Fructooligosaccharide |
| RFOs | Raffinose family oligosaccharides |
| SCFAs | Short-chain fatty acids |
| ANF | Anti-nutritional factor |
| TAO | Total antioxidant activity |
| CFU | Colony-forming unit |
| MRS | De Man, Rogosa and Sharpe medium |
| TA | Titratable acidity |
| GSO | Galactosyl-sucrose oligosaccharide |
| GOPOD | Glucose oxidase/peroxidase |
| FID | Flame ionisation detector |
| MeP | Methyl propionate |
| MeB | Methyl butyrate |
| PCA | Principal component analysis |
| TBA | 2-thiobarbituric acid |
| DMRT | Duncan multiple range test |
References
- Das, S.; Das, M.K.; Kesharwani, R.K. Recent Advances of Nutraceutical and Functional Foods in Immune Health. In Nutraceuticals and Functional Foods in Immunomodulators, 1st ed.; Springer: Singapore, 2022; pp. 313–330. [Google Scholar] [CrossRef]
- Hassan, M.; Tripathi, K.; Harun, M.; Krishnan, V.; Kaushik, R.; Chawla, G.; Shakil, N.A.; Kumar, M. Unravelling the effect of extraction on anthocyanin functionality and prebiotic potential. Heliyon 2024, 10, e31780. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Zhang, X.; Zeng, J.; Zou, J.; Zhang, L.; Gong, P. Texture analysis and physicochemical characteristics of fermented soymilk gel by different lactic acid bacteria. Food Hydrocoll. 2023, 136, 108252. [Google Scholar] [CrossRef]
- Madjirebaye, P.; Peng, Z.; Mueed, A.; Huang, T.; Peng, F.; Allasra, Y.; Benar, M.E.; Hu, Z.; Xie, M.; Xiong, T. Promising probiotic-fermented soymilk for alleviating acute diarrhea: Insights into the microbiome and metabolomics. Food Funct. 2024, 15, 4462–4474. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Filho, M.L.; Busanello, M.; Prudencio, S.H.; Garcia, S. Soymilk with okara flour fermented by Lactobacillus acidophilus: Simplex-centroid mixture design applied in the elaboration of probiotic creamy sauce and storage stability. LWT 2018, 93, 339–345. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Hasan, M.; Garcia-Gutierrez, E.; Kumari, S.; Prakash, O.; Nain, L.; Sachdev, A.; Dahuja, A. Current trends in the development of soy-based foods containing probiotics and paving the path for soy-synbiotics. Crit. Rev. Food Sci. Nutr. 2022, 63, 9995–10013. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Kazemi, A.; UPBartolomaeus, T.; Martami, F.; Noormohammadi, M.; Salari, A.; Löber, U.; Balou, H.A.; KForslund, S.; Mahdavi-Roshan, M. The effect of probiotic and synbiotic supplementation on lipid parameters among patients with cardiometabolic risk factors: A systematic review and meta-analysis of clinical trials. Cardiovasc. Res. 2023, 119, 933–956. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.; Zhao, Q.; Lei, Y.; Gao, C.; Yuan, Q.; Sun, X.; Liu, X.; Ma, T. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Zayed, A.; Farag, M.A. Gut homeostasis and microbiota under attack: Impact of the different types of food contaminants on gut health. Crit. Rev. Food Sci. Nutr. 2022, 62, 738–763. [Google Scholar] [CrossRef]
- Jeong, S.; Kwon, A.; Jeong, H.; Park, Y.S. Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce. Microorganisms 2023, 11, 1354. [Google Scholar] [CrossRef]
- Babot, J.D.; Argañaraz-Martínez, E.; Apella, M.C.; Perez Chaia, A. Microencapsulation of Probiotics with Soy Protein Isolate and Alginate for the Poultry Industry. Food Bioprocess. Technol. 2023, 16, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose family oligosaccharides: Friend or foe for human and plant health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Zhu, M.J. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef]
- Cai, J.S.; Feng, J.Y.; Ni, Z.J.; Ma, R.H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.G.; Wei, Z.J. An update on the nutritional, functional, sensory characteristics of soy products, and applications of new processing strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- Bharathi, S. Functional Effects of Soy-Raffinose on the Quality Parameters of Yogurt. Ph.D. Thesis, University of Central Oklahoma, Edmond, OK, USA, 2019. [Google Scholar]
- Association of Official Analytical Chemists; Association of Official Agricultural Chemists (US). Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Rockville, MD, USA, 1931. [Google Scholar]
- Assaker, K.; Rima, J. A New Spectrophometric Method for The Analysis of Fructose, Glucose and Sucrose, Using 2-Thiobarbituric Acid and Zero-Valent Iron Powder (ZVIP). J. Food Res. 2019, 8, 48–55. [Google Scholar] [CrossRef]
- Rana, V.S.; Das, M. Fatty Acid and Non-Fatty Acid Components of the Seed Oil of Celastrus paniculatus wild. Int. J. Fruit. Sci. 2017, 17, 407–414. [Google Scholar] [CrossRef]
- Fatima, S.M.; Hekmat, S. Microbial and Sensory Analysis of Soy and Cow Milk-Based Yogurt as a Probiotic Matrix for Lactobacillus rhamnosus GR-1. Fermentation 2020, 6, 74. [Google Scholar] [CrossRef]
- Zong, L.; Lu, M.; Wang, W. The Quality and Flavor Changes of Different Soymilk and Milk Mixtures Fermented Products during Storage. Fermentation 2022, 8, 668. [Google Scholar] [CrossRef]
- Du, L.; Ro, K.S.; Zhang, Y.; Tang, Y.J.; Li, W.; Xie, J.; Wei, D. Effects of Lactiplantibacillus plantarum X7021 on physicochemical properties, purines, isoflavones and volatile compounds of fermented soymilk. Process Biochem. 2022, 113, 150–157. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, X. Genetic mechanisms of prebiotic carbohydrate metabolism in lactic acid bacteria: Emphasis on Lacticaseibacillus casei and Lacticaseibacillus paracasei as flexible, diverse and outstanding prebiotic carbohydrate starters. Trends Food Sci. Technol. 2021, 115, 486–499. [Google Scholar] [CrossRef]
- Choudhary, S.; Singh, M.; Sharma, D.; Attri, S.; Sharma, K.; Goel, G. Principal component analysis of stimulatory effect of synbiotic combination of indigenous probiotic and inulin on antioxidant activity of soymilk. Probiotics Antimicrob. Proteins 2019, 11, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Tsangalis, D.; Shah, N.P. Metabolism of oligosaccharides and aldehydes and production of organic acids in soymilk by probiotic bifidobacteria. Int. J. Food Sci. Technol. 2004, 39, 541–554. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Zhou, X.L.; Kong, X.F.; Lian, G.Q. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr. Res. 2014, 34, 780–788. [Google Scholar] [CrossRef]
- Kumari, M.; Patel, H.K.; Kokkiligadda, A.; Bhushan, B.; Tomar, S.K. Characterization of probiotic lactobacilli and development of fermented soymilk with improved technological properties. LWT 2022, 154, 112827. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, H.N. Comparative study of the synbiotic effect of inulin and fructooligosaccharides with probiotics with regard to the various properties of fermented soy milk. Food Sci. Technol. Int. 2018, 24, 564–575. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, H.N. Effect of synbiotic interaction of fructooligosaccharides and probiotics on the acidification profile, textural and rheological characteristics of fermented soy milk. Food Bioprocess. Technol. 2013, 6, 3166–3176. [Google Scholar] [CrossRef]
| Treatment No. | Samples | Colour | Mouth Feel | Consistency | Taste | Flavour | Overall Liking |
|---|---|---|---|---|---|---|---|
| T1 | Control (unfermented soymilk) | 6.17 ± 0.27 de | 3.23 ± 0.11 n | 4.37 ± 0.17 l | 2.12 ± 0.05 m | 3.62 ± 0.22 n | 3.9 ± 0.06 n |
| T2 | Unfermented + 2% table sugar | 6.21 ± 0.31 de | 4.32 ± 0.12 l | 4.71 ± 0.1 jk | 5.28 ± 0.1 l | 5.61 ± 0.25 m | 5.23 ± 0.24 m |
| T3 | Unfermented + 1% vanillin | 6.22 ± 0.27 de | 3.71 ± 0.09 m | 5.13 ± 0.16 h | 5.33 ± 0.21 k | 6.01 ± 0.31 l | 5.28 ± 0.27 l |
| T4 | Unfermented with 3% FOS | 6.51 ± 0.13 bc | 5.12 ± 0.18 j | 4.93 ± 0.08 i | 6.61 ± 0.31 i | 5.92 ± 0.26 l | 5.82 ± 0.51 j |
| T5 | Fermented with 3% FOS | 6.27 ± 0.34 d | 6.7 ± 0.31 e | 4.62 ± 0.31 k | 8 ± 0.42 d | 6.91 ± 0.11 ij | 6.5 ± 0.26 h |
| T6 | Fermented with 3% FOS + 2% table sugar | 6.32 ± 0.45 cd | 7.29 ± 0.45 d | 4.74 ± 0.71 jk | 7.45 ± 0.37 g | 7.23 ± 0.52 gh | 6.61 ± 0.5 f |
| T7 | Fermented with 3% FOS + 1% vanillin | 6.81 ± 0.41 a | 6.73 ± 0.32 e | 5.51 ± 0.21 g | 7.92 ± 0.21 e | 8.19 ± 0.71 e | 7.03 ± 0.61 e |
| T8 | Fermented with 3% FOS + 2% table sugar + 1% vanillin | 6.57 ± 0.23 b | 7.23 ± 0.47 d | 5.63 ± 0.27 fg | 8.12 ± 0.43 c | 8.64 ± 0.27 ab | 7.24 ± 0.27 d |
| T9 | Unfermented with 3% RFOs | 5.91 ± 0.11 fg | 5.22 ± 0.21 i | 4.81 ± 0.61 ij | 7.13 ± 0.32 h | 7.09 ± 0.52 hi | 6.03 ± 0.34 i |
| T10 | Fermented with 3% RFOs | 6.23 ± 0.56 g | 6.18 ± 0.61 g | 5.75 ± 0.53 ef | 7.89 ± 0.26 e | 6.75 ± 0.61 j | 6.56 ± 0.45 g |
| T11 | Fermented with 3% RFOs + 2% table sugar | 5.91 ± 0.36 fg | 6.53 ± 0.26 f | 6.82 ± 0.76 d | 8.21 ± 0.52 b | 7.89 ± 0.42 f | 7.07 ± 0.52 e |
| T12 | Fermented with 3% RFOs + 1% vanillin | 5.84 ± 0.21 g | 6.57 ± 0.39 f | 6.81 ± 0.54 d | 7.82 ± 0.35 f | 8.16 ± 0.26 e | 7.04 ± 0.26 e |
| T13 | Fermented with 3% RFOs + 2% table sugar + 1% vanillin | 6.11 ± 0.65 def | 7.52 ± 0.41 c | 7.81 ± 0.32 a | 7.92 ± 0.61 e | 8.31 ± 0.51 b | 7.53 ± 0.42 c |
| T14 | Unfermented with 2% RFOs + 2% FOS | 6.28 ± 0.27 d | 4.89 ± 0.11 k | 5.19 ± 0.18 h | 5.67 ± 0.41 j | 6.23 ± 0.62 k | 5.65 ± 0.52 f |
| T15 | Fermented with 2% RFOs + 2% FOS | 6.31 ± 0.23 cd | 5.72 ± 0.17 h | 5.82 ± 0.16 e | 7.11 ± 0.27 h | 7.38 ± 0.41 g | 6.47 ± 0.21 h |
| T16 | Fermented with 2% RFOs + 2% FOS + 2% table sugar | 6.01 ± 0.43 efg | 7.77 ± 0.32 b | 7.12 ± 0.28 c | 8.23 ± 0.21 b | 8.41 ± 0.62 cd | 7.51 ± 0.31 c |
| T17 | Fermented with 2% RFOs + 2% FOS + 1% vanillin | 6.18 ± 0.25 de | 8.09 ± 0.53 a | 7.63 ± 0.87 b | 8.19 ± 0.36 b | 8.52 ± 0.71 bc | 7.72 ± 0.42 b |
| T18 | Fermented with 2% RFOs + 2% FOS + 2% table sugar + 1% vanillin | 6.31 ± 0.12 cd | 8.16 ± 0.53 a | 7.84 ± 0.65 a | 8.34 ± 0.51 a | 8.72 ± 0.24 a | 7.87 ± 0.52 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasi, M.; Kumar, S.; Prakash, O.; Krishnan, V.; Vinayaka; Tomar, G.S.; Mishra, J.; R, A.S.; Kaushik, P.; Rana, V.S.; et al. Evaluation of Sensory Properties and Short-Chain Fatty Acid Production in Fermented Soymilk on Addition of Fructooligosaccharides and Raffinose Family of Oligosaccharides. Fermentation 2025, 11, 194. https://doi.org/10.3390/fermentation11040194
Sasi M, Kumar S, Prakash O, Krishnan V, Vinayaka, Tomar GS, Mishra J, R AS, Kaushik P, Rana VS, et al. Evaluation of Sensory Properties and Short-Chain Fatty Acid Production in Fermented Soymilk on Addition of Fructooligosaccharides and Raffinose Family of Oligosaccharides. Fermentation. 2025; 11(4):194. https://doi.org/10.3390/fermentation11040194
Chicago/Turabian StyleSasi, Minnu, Sandeep Kumar, Om Prakash, Veda Krishnan, Vinayaka, Govind Singh Tomar, Jigni Mishra, Arpitha S R, Parshant Kaushik, Virendra Singh Rana, and et al. 2025. "Evaluation of Sensory Properties and Short-Chain Fatty Acid Production in Fermented Soymilk on Addition of Fructooligosaccharides and Raffinose Family of Oligosaccharides" Fermentation 11, no. 4: 194. https://doi.org/10.3390/fermentation11040194
APA StyleSasi, M., Kumar, S., Prakash, O., Krishnan, V., Vinayaka, Tomar, G. S., Mishra, J., R, A. S., Kaushik, P., Rana, V. S., & Dahuja, A. (2025). Evaluation of Sensory Properties and Short-Chain Fatty Acid Production in Fermented Soymilk on Addition of Fructooligosaccharides and Raffinose Family of Oligosaccharides. Fermentation, 11(4), 194. https://doi.org/10.3390/fermentation11040194







