Strategies for Reducing Purine Accumulation in Beer: From Metabolic Mechanisms to Brewing Technology Innovations
Abstract
1. Introduction
2. Overview of Purines
2.1. Characterisation of Purines
2.2. Purines and Gout
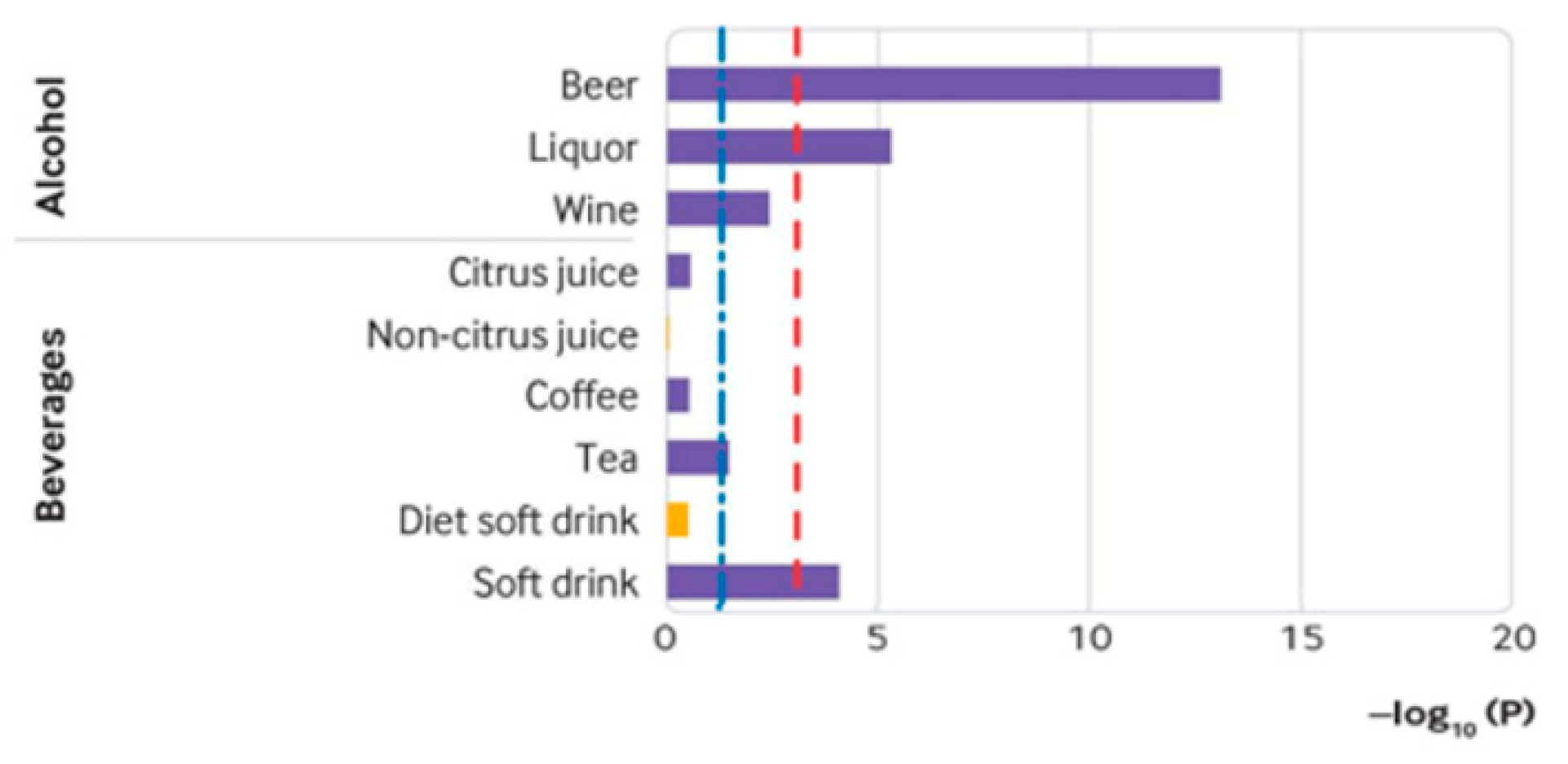
2.3. Purine Content in Alcoholic Beverages and Corresponding Limits
3. Sources of Purines in Beer and Features of the Brewing Process
3.1. Purine Sources
3.2. Changes in Purine Analogues During the Brewing Process
4. Measures for Regulating Purine Levels in Beer
4.1. Physical and Chemical Methods
4.2. Enzymatic Methods
4.3. Biological Methods
4.4. Brewing Process Optimisation Methods
4.4.1. Selection and Handling of Raw Materials
4.4.2. Optimization of the Mashing Process
4.4.3. Optimization of the Fermentation Process
4.5. Modern Bio-Breeding Methods
| Strains | Characterization | Reference |
|---|---|---|
| S. pastorianusFY-2 | Decrease in diacetyl precursors | [84] |
| S. cerevisiae QY5, QY31 | Decrease in diacetyl and increase in glutathione | [85] |
| S. cerevisiae Y1 | Decreased acetaldehyde and increased glutathione | [86] |
| S. cerevisiae YSF-5 | Increased glutathione, more stable foam | [87] |
| S. cerevisiae 396-9-6V | Increased flocculation | [88] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nishioka, K.; Sumida, T.; Iwatani, M.; Kusumoto, A.; Ishikura, Y.; Hatanaka, H.; Yomo, H.; Kohda, H.; Ashikari, T.; Shibano, Y.; et al. Influence of moderate drinking on purine and carbohydrate metabolism. Alcohol. Clin. Exp. Res. 2002, 26, 20S–25S. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [PubMed]
- Almeida, C.; Neves, M.C.; Freire, M.G. Towards the use of adsorption methods for the removal of purines from beer. Molecules 2021, 26, 6460. [Google Scholar] [CrossRef] [PubMed]
- Somers, T.C.; Ziemelis, G. Interpretation of ultraviolet absorption in wort and beer. the major role of nucleic acid derivatives. J. Inst. Brew. 1972, 78, 233–236. [Google Scholar]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The association between purine-rich food intake and hyperuricemia: A cross-sectional study in Chinese adult residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- Feng, S.M.; Wu, S.J.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar]
- Gulcin, I.; Oktay, M.; Koksal, E.; Serbetci, H.; Beydemir, S.; Kufrevioglu, O.I. Antioxidant and radical scavenging activities of uric acid. Asian J. Chem. 2008, 20, 2079–2090. [Google Scholar]
- Liu, Z.; Sun, X.; Liu, Z.; Zhang, T.; Zhang, L.; Wu, C. Phytochemicals in traditional Chinese medicine can treat gout by regulating intestinal flora through inactivating NLRP3 and inhibiting XOD activity. J. Pharm. Pharmacol. 2022, 74, 919–929. [Google Scholar]
- Pascart, T.; Lioté, F. Gout: State of the art after a decade of developments. Rheumatology 2018, 58, 27–44. [Google Scholar]
- Cicero, A.F.G.; Fogacci, F.; Kuwabara, M.; Borghi, C. Therapeutic Strategies for the Treatment of Chronic Hyperuricemia: An Evidence-Based Update. Medicina 2021, 57, 58. [Google Scholar] [CrossRef]
- Brulé, D.; Sarwar, G.; Savoiet, L. Purine content of selected canadian food products. J. Food Compos. Anal. 1988, 1, 130–138. [Google Scholar]
- Torralba, K.D.; De Jesus, E.; Rachabattula, S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: Current and future directions. Int. J. Rheum. Dis. 2012, 15, 499–506. [Google Scholar] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef]
- Major, T.J.; Topless, R.K.; Dalbeth, N.; Merriman, T.R. Evaluation of the diet wide contribution to serum urate levels: Meta-analysis of population based cohorts. BMJ 2018, 363, k3951. [Google Scholar] [PubMed]
- Mikuls, T.R.; Saag, K.G. New insights into gout epidemiology. Curr. Opin. Rheumatol. 2006, 18, 199–203. [Google Scholar]
- Clifford, A.J.; Riumallo, J.A.; Young, V.R.; Scrimshaw, N.S. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J. Nutr. 1976, 106, 428–434. [Google Scholar]
- Yamamoto, T.; Moriwaki, Y. Purines in Beer. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 285–290. [Google Scholar]
- Kaneko, K.; Yamanobe, T.; Fujimori, S. Determination of purine contents of alcoholic beverages using high performance liquid chromatography. Biomed. Chromatogr. 2009, 23, 858–864. [Google Scholar] [PubMed]
- Kaneko, K.; Takayanagi, F.; Fukuuchi, T.; Yamaoka, N.; Yasuda, M.; Mawatari, K.-i.; Fujimori, S. Determination of total purine and purine base content of 80 food products to aid nutritional therapy for gout and hyperuricemia. Nucleosides Nucleotides Nucl. Acids 2020, 39, 1449–1457. [Google Scholar]
- Kaneko, K.; Kudo, Y.; Yamanobe, T.; Mawatari, K.; Yasuda, M.; Nakagomi, K.; Fujimori, S. Purine contents of soybean-derived foods and selected japanese vegetables and mushrooms. Nucleosides Nucleotides Nucl. Acids 2008, 27, 628–630. [Google Scholar]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Hao, J.; Liu, C. Determination of purines in beer by HPLC using a simple and rapid sample pretreatment. J. Am. Soc. Brew. Chem. 2015, 73, 137–142. [Google Scholar]
- Fukuuchi, T.; Yasuda, M.; Inazawa, K.; Ota, T.; Yamaoka, N.; Mawatari, K.-I.; Nakagomi, K.; Kaneko, K. A simple HPLC method for determining the purine content of beer and beer-like alcoholic beverages. Anal. Sci. 2013, 29, 511–517. [Google Scholar]
- Nicholls, A.; Scott, J.T. Effect of weight-loss on plasma and urinary levels of uric acid. Lancet 1972, 300, 1223–1224. [Google Scholar]
- Rodhouse, L.; Carbonero, F. Overview of craft brewing specificities and potentially associated microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 462–473. [Google Scholar]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Brewer’s yeast in controlled and uncontrolled fermentations, with a focus on novel, nonconventional, and superior strains. Food Rev. Int. 2016, 32, 341–363. [Google Scholar]
- Lin, X.J. Study on Low-Purine-Compound Beer. Master’s Dissertation, Jangnan University, Wuxi, China, 2006. [Google Scholar]
- Li, Z.; Zhang, W. Determination of purine in wort, fermenting liquor and beer by HPLC. Food Ferment. Ind. 2006, 32, 73–75. [Google Scholar]
- Shang, Y.; Du, J. Determination of Purine Contents in Beer Brewing. Liquor Mak. 2009, 36, 43–46. [Google Scholar]
- Gasinski, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gasior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Smogrovicova, D.; Mok, C. Recent innovations in the production of selected specialty (non-traditional) beers. Folia Microbiol. 2021, 66, 525–541. [Google Scholar]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Anjos, O.; Zhao, H. Functionality of Special Beer Processes and Potential Health Benefits. Processes 2020, 8, 1613. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piorecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2022, 45, 101495. [Google Scholar]
- Donadini, G.; Porretta, S. Uncovering patterns of consumers’ interest for beer: A case study with craft beers. Food Res. Int. 2017, 91, 183–198. [Google Scholar] [CrossRef]
- Hafez, R.M.; Abdel-Rahman, T.M.; Naguib, R.M. Uric acid in plants and microorganisms: Biological applications and genetics—A review. J. Adv. Res. 2017, 8, 475–486. [Google Scholar] [PubMed]
- Schabo, D.C.; Freire, L.; Sant’Ana, A.S.; Schaffner, D.W.; Magnani, M. Mycotoxins in artisanal beers: An overview of relevant aspects of the raw material, manufacturing steps and regulatory issues involved. Food Res. Int. 2021, 141, 110114. [Google Scholar] [CrossRef]
- Costantini, A.V. The fungal etiology of gout and hyperuricemia: The antifungal mode of action of colchicine. Biomed. Rev. 1992, 1, 47–52. [Google Scholar]
- Loewen, S.K.; Ng, A.M.; Mohabir, N.N.; Baldwin, S.A.; Cass, C.E.; Young, J.D. Functional characterization of a H+/nucleoside co-transporter (CaCNT) from Candida albicans, a fungal member of the concentrative nucleoside transporter (CNT) family of membrane proteins. Yeast 2003, 20, 661–675. [Google Scholar]
- Woods, R.A.; Roberts, D.G.; Friedman, T.; Jolly, D.; Filpula, D. Hypoxanthine: Guanine phosphoribosyltransferase mutants in Saccharomyces cerevisiae. Mol. Gen. Genet. MGG 1983, 191, 407–412. [Google Scholar] [CrossRef]
- Alfonzo, J.D.; Crother, T.R.; Guetsova, M.L.; Daignan-Fornier, B.; Taylor, M.W. APT1, but not APT2, codes for a functional adenine phosphoribosyltransferase in Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 347–352. [Google Scholar]
- Ugbogu, E.A.; Wippler, S.; Euston, M.; Kouwenhoven, E.N.; de Brouwer, A.P.M.; Schweizer, L.M.; Schweizer, M. The contribution of the nonhomologous region of Prs1 to the maintenance of cell wall integrity and cell viability. FEMS Yeast Res. 2013, 13, 291–301. [Google Scholar]
- Carter, A.T.; Narbad, A.; Pearson, B.M.; Beck, K.-F.; Baum, B.; Logghe, M.; Contreras, R.; Schweizer, M. Phosphoribosylpyrophosphate synthetase (PRS): A new gene family in Saccharomyces cerevisiae. Yeast 1994, 10, 1031–1044. [Google Scholar] [CrossRef]
- Wagner, R.; Straub, M.L.; Souciet, J.L.; Potier, S.; de Montigny, J. New plasmid system to select for Saccharomyces cerevisiae purine-cytosine permease affinity mutants. J. Bacteriol. 2001, 183, 4386–4388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelissen, B.; Mordant, P.; Jonniaux, J.L.; De Wachter, R.; Goffeau, A. Phylogenetic classification of the major superfamily of membrane transport facilitators, as deduced from yeast genome sequencing. FEBS Lett. 1995, 377, 232–236. [Google Scholar] [CrossRef]
- Shunichi, F.; Shuzo, S. Method for Producing Fermented Malt Beverage Using Zeolite Treatment. European Patent JP20030086493, 26 March 2003. [Google Scholar]
- Takeshi, F.; Takuya, H.; Koichiro, T.; Kotaro, H. Method for Producing Fermented Malt Beverage and Active Carbon for Removing Purines from Fermented Malt Beverage. European Patent JP20030071780, 17 March 2003. [Google Scholar]
- Li, H.; Liu, C.; Han, X. A Production Method for Low Purine Beer. Chinese Patent CN201310600728, 19 February 2014. [Google Scholar]
- Trautwein-Schult, A.; Jankowska, D.; Cordes, A.; Hoferichter, P.; Klein, C.; Matros, A.; Mock, H.P.; Baronian, K.; Bode, R.; Kunze, G. Arxula adeninivorans recombinant urate oxidase and its application in the production of food with low uric acid content. J. Mol. Microbiol. Biotechnol. 2013, 23, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Shibano, Y.; Yomo, H.; Matsumoto, T.; Koda, H.; Suwa, Y.; Amachi, T.; Hatanaka, H.; Shimizu, S. Process for manufacturing beer. Chinese Patent CA19962188032, 16 February 1996. [Google Scholar]
- Pugmire, M.J.; Ealick, S.E. Structural analyses reveal two distinct families of nucleoside phosphorylases. Biochem. J. 2002, 361, 1–25. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Li, H.; Ren, D. Purine nucleoside phosphorylase from Pseudoalteromonas sp. Bsi590: Molecular cloning, gene expression and characterization of the recombinant protein. Extremophiles 2008, 12, 325–333. [Google Scholar] [CrossRef]
- Cacciapuoti, G.; Marabotti, A.; Fuccio, F.; Porcelli, M. Unraveling the structural and functional differences between purine nucleoside phosphorylase and 5′-deoxy-5′-methylthioadenosine phosphorylase from the archaeon Pyrococcus furiosus. Biochim. Biophys. Acta 2011, 1814, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Breer, K.; Girstun, A.; Wielgus-Kutrowska, B.; Staroń, K.; Bzowska, A. Overexpression, purification and characterization of functional calf purine nucleoside phosphorylase (PNP). Protein Expr. Purif. 2008, 61, 122–130. [Google Scholar] [CrossRef]
- Silva, R.G.; Carvalho, L.P.S.; Oliveira, J.S.; Pinto, C.A.; Mendes, M.A.; Palma, M.S.; Basso, L.A.; Santos, D.S. Cloning, overexpression, and purification of functional human purine nucleoside phosphorylase. Protein Expr. Purif. 2003, 27, 158–164. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef]
- Mahor, D.; Priyanka, A.; Prasad, G.S.; Thakur, K.G. Functional and structural characterization of purine nucleoside phosphorylase from Kluyveromyces lactis and its potential applications in reducing purine content in food. PLoS ONE 2016, 11, e0164279. [Google Scholar]
- Mahor, D.; Prasad, G.S. Biochemical characterization of Kluyveromyces lactis adenine deaminase and guanine deaminase and their potential application in lowering purine content in beer. Front. Bioeng. Biotechnol. 2018, 6, 180. [Google Scholar]
- Jankowska, D.A.; Trautwein-Schult, A.; Cordes, A.; Hoferichter, P.; Klein, C.; Bode, R.; Baronian, K.; Kunze, G. Arxula adeninivorans xanthine oxidoreductase and its application in the production of food with low purine content. J. Appl. Microbiol. 2013, 115, 796–807. [Google Scholar]
- Jankowska, D.A.; Trautwein-Schult, A.; Cordes, A.; Bode, R.; Baronian, K.; Kunze, G. A novel enzymatic approach in the production of food with low purine content using Arxula adeninivorans endogenous and recombinant purine degradative enzymes. Bioengineered 2015, 6, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, R.; Liu, C.X.; Wu, S.X.; Pei, X.D.; Jiang, Y.; Liu, X.L.; Wang, C.H. Computer-assisted in vitro reconstitution of purine degradation pathway to lower the purine content in food. J. Sci. Food Agric. 2022, 102, 7079–7086. [Google Scholar] [PubMed]
- De Windt, F.E.; Wagemaker, M.J.M.; Op den Camp, H.J.M.; van der Drift, C. Purine degradation in the edible mushroom Agaricus bisporus. Folia Microbiol. 2002, 47, 672–676. [Google Scholar] [CrossRef]
- Chen, J.T.; Wen, C.Y.; Fang-Hwa, S.; Meng-Jen, T. Method of Reducing Purine Content of Edible Material. European Patent JP2011083276A, 28 April 2011. [Google Scholar]
- Deng, M.; Li, J.; Xie, J.H. A Kind of Low purine Soya Milk Powder and Its Production Method. Chinese Patent CN201910677421, 25 July 2019. [Google Scholar]
- Li, M.; Yang, D.; Mei, L.; Lin, Y.; Xie, A.; Yuan, J.; Andreas, L.R. Screening andcharacterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PLoS ONE 2014, 9, e105577. [Google Scholar]
- Lu, L.; Liu, T.; Liu, X.; Wang, C. Screening and identification of purine degrading Lactobacillus fermentum 9-4 from Chinese fermented rice-flour noodles. Food Sci. Hum. Wellness 2022, 11, 1402–1408. [Google Scholar]
- Kano, H.; Yamada, N.; Saito, C.; Murayama-Chiba, Y.; Ito, H. Lactobacillus gasseri PA-3, but not L. gasseri OLL2996, reduces the absorption of purine nucleosides in rats. Nucleosides Nucleotides Nuclc Acids 2018, 37, 353–360. [Google Scholar] [CrossRef]
- Cahill, G.; Murray, D.M.; Walsh, P.K.; Donnelly, D. Effect of the concentration of propagation wort on yeast cell volume and fermentation performance. J. Am. Soc. Brew. Chem. 2000, 58, 14–20. [Google Scholar]
- Kitagawa, S.; Mukai, N.; Furukawa, Y.; Adachi, K.; Mizuno, A.; Iefuji, H. Effect of soy peptide on brewing beer. J. Biosci. Bioeng. 2008, 105, 360–366. [Google Scholar]
- Mo, F.; Zhao, H.F.; Lei, H.J.; Zhao, M.M. Effects of nitrogen composition on fermentation performance of brewer’s yeast and the absorption of peptides with different molecular weights. Appl. Biochem. Biotechnol. 2013, 171, 1339–1350. [Google Scholar] [PubMed]
- Kwolek-Mirek, M.; Molon, M.; Kaszycki, P.; Zadrag-Tecza, R. L-carnosine enhanced reproductive potential of the Saccharomyces cerevisiae yeast growing on medium containing glucose as a source of carbon. Biogerontology 2016, 17, 737–747. [Google Scholar] [PubMed]
- Jin, X.; Yang, H.; Chen, M.; Coldea, T.E.; Zhao, H. Improved osmotic stress tolerance in brewer’s yeast induced by wheat gluten peptides. Appl. Microbiol. Biotechnol. 2022, 106, 4995–5006. [Google Scholar] [PubMed]
- Kang, F.; Yan, B.; Lv, N.; Zhou, S. Mutation Breeding of Saccharomyces cerevisiae with Low Purine by Atmospheric and Room Temperature Plasma. Mod. Food Sci. Tech. 2014, 30, 188–191. [Google Scholar]
- Akada, R. Genetically modified industrial yeast ready for application. J. Biosci. Bioeng. 2002, 94, 536–544. [Google Scholar] [CrossRef]
- Fischer, S.; Procopio, S.; Becker, T. Self-cloning brewing yeast: A new dimension in beverage production. Eur. Food Res. Technol. 2013, 237, 851–863. [Google Scholar]
- Dianhui, W.; Xiaomin, L.; Jian, L.; Jian, C.; Liang, Z.; Guangfa, X. Constitutive expression of the DUR1,2 gene in an industrial yeast strain to minimize ethyl carbamate production during Chinese rice wine fermentation. FEMS Microbiol. Lett. 2016, 363, fnv214. [Google Scholar]
- Ogata, T.; Kobayashi, M.; Gibson, B.R. Pilot-scale brewing using self-cloning bottom-fermenting yeast with high SSU1 expression. J. Inst. Brew. 2013, 119, 17–22. [Google Scholar]
- Kusunoki, K.; Ogata, T. Construction of self-cloning bottom-fermenting yeast with low vicinal diketone production by the homo-integration of ILV5. Yeast 2012, 29, 435–442. [Google Scholar]
- Wang, Z.Y.; He, X.P.; Liu, N.; Zhang, B.R. Construction of self-cloning industrial brewing yeast with high-glutathione and low-diacetyl production. Int. J. Food Sci. Tech. 2008, 43, 989–994. [Google Scholar]
- Wang, Z.Y.; Wang, J.J.; Liu, X.F.; He, X.P.; Zhang, B.R. Recombinant industrial brewing yeast strains with ADH2 interruption using self-cloning GSH1+CUP1 cassette. FEMS Yeast Res. 2009, 9, 574–581. [Google Scholar] [PubMed]
- Wang, Z.Y.; He, X.P.; Zhang, B.R. Over-expression of GSH1 gene and disruption of PEP4 gene in self-cloning industrial brewer’s yeast. Int. J. Food Microbiol. 2007, 119, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ishida-Fujii, K.; Goto, S.; Sugiyama, H.; Takagi, Y.; Saiki, T.; Takagi, M. Breeding of flocculent industrial alcohol yeast strains by self-cloning of the flocculation gene FLO1 and repeated-batch fermentation by transformants. J. Gen. Appl. Microbiol. 1998, 44, 347–353. [Google Scholar]
- Liu, J.; Li, X.; Liu, Y.; Xing, C.; Xie, Y.; Cai, G.; Lu, J. Evaluation of genetic diversity and development of core collections of industrial brewing yeast using ISSR markers. Arch. Microbiol. 2020, 203, 1001–1008. [Google Scholar] [PubMed]
- Frankel, O.H. Genetic perspectives of germplasm conservation. In Genetic Manipulation Impact on Man & Society; Arber, W., Illmensee, K., Peacock, W.J., Starlinger, P., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 161–170. [Google Scholar]
- Brown, A.H.D.; Frankel, O.H.; Marshall, D.R.; Williams, J.T. The case for core collections. In The Use of Plant Genetic Resources; Cambridge University Press: Cambridge, UK, 1989; pp. 123–135. [Google Scholar]
- Tian, M.; Li, W.; Luo, P.; He, J.; Zhang, H.; Yan, Q.; Ye, Y. Genetic diversity analysis and core germplasm bank construction in cold resistant germplasm of rubber trees (Hevea brasiliensis). Sci. Rep. 2024, 14, 14533. [Google Scholar]
- Qian, Y.; Jiang, M.; Zou, B.; Li, D. Core Germplasm Construction Based on Genetic and Phenotypic Diversity of Buffalograss (Bouteloua dactyloides (Nutt.) Columbus) from the Great Plains of America. Agronomy 2023, 13, 1382. [Google Scholar] [CrossRef]
- Haupt, M.; Schmid, K. Combining focused identification of germplasm and core collection strategies to identify genebank accessions for central European soybean breeding. Plant Cell Environ. 2020, 43, 1421–1436. [Google Scholar]



| Categories | Purine Content/mg·(100 g)−1 |
|---|---|
| very-low-purine foods | less than 50 |
| low-purine foods | 50–100 |
| medium-purine foods | 100–200 |
| high-purine foods | 200–300 |
| very-high-purine foods | greater than 300 |
| Alcoholic Beverages | Adenine | Guanine | Hypoxanthine | Xanthine | Total Purines |
|---|---|---|---|---|---|
| whiskey | 0 | 0.9 | 0.1 | 0.2 | 1.2 |
| 0.6 | 1.0 | 0.8 | 0.7 | 3.1 | |
| brandy | 0 | 1.5 | 1.8 | 0.5 | 3.8 |
| Sake | 0 | 0.2 | 0.0 | 0.0 | 0.2 |
| 0 | 0.1 | 0.0 | 0.0 | 0.1 | |
| sake | 0.2 | 0.0 | 3.1 | 8.8 | 12.1 |
| grape wine | 0.3 | 0 | 3.1 | 0.5 | 3.9 |
| 0.3 | 1.0 | 1.7 | 13.2 | 16.2 | |
| Beer (regular) | 15.7 | 36.7 | 15.3 | 16.6 | 84.2 |
| Beer (low malt) | 5.3 | 16.4 | 2.1 | 4.6 | 28.4 |
| 7.0 | 16.8 | 5.4 | 9.9 | 39.1 | |
| Beer (low alcohol) | 14.9 | 9.4 | 2.5 | 0.6 | 27.4 |
| 36.0 | 66.6 | 15.0 | 12.3 | 129.9 | |
| Beer (low malt and low purine) | 1.8 | 0 | 0 | 0 | 1.8 |
| Brand | Merchandise | Source | Purine Content mg·L−1 | Alcohol % Vol |
|---|---|---|---|---|
| Kirin | Nodogoshi Strong | Japan | ≤5.0 | 4.0 |
| Sapporo | Goku Zero | Japan | ≤5.0 | 5.0 |
| Kirin | Tanrei Platinum Double | Japan | ≤5.0 | 5.5 |
| Asahi | Off | Japan | ≤5.0 | 3.0–4.0 |
| Suntory | All free | Japan | ≤5.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lu, J. Strategies for Reducing Purine Accumulation in Beer: From Metabolic Mechanisms to Brewing Technology Innovations. Fermentation 2025, 11, 193. https://doi.org/10.3390/fermentation11040193
Liu J, Lu J. Strategies for Reducing Purine Accumulation in Beer: From Metabolic Mechanisms to Brewing Technology Innovations. Fermentation. 2025; 11(4):193. https://doi.org/10.3390/fermentation11040193
Chicago/Turabian StyleLiu, Jun, and Jian Lu. 2025. "Strategies for Reducing Purine Accumulation in Beer: From Metabolic Mechanisms to Brewing Technology Innovations" Fermentation 11, no. 4: 193. https://doi.org/10.3390/fermentation11040193
APA StyleLiu, J., & Lu, J. (2025). Strategies for Reducing Purine Accumulation in Beer: From Metabolic Mechanisms to Brewing Technology Innovations. Fermentation, 11(4), 193. https://doi.org/10.3390/fermentation11040193






