Development of a High-Cell-Density Production Process for a Biotherapeutic Yeast, Saccharomyces cerevisiae var. boulardii, for Use as a Human Probiotic
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain Identification and Preservation
2.2. Cultivation for High-Cell-Density Production
2.2.1. S. cerevisiae var. boulardii Inoculum Preparation
2.2.2. 30 L Bioreactor Cultivation
2.2.3. Validation of Cultivation Process at 30 L Scale
2.3. Sampling and Data Analysis
Determination of Key Process Indicators and Statistical Analysis
2.4. Downstream Processing
2.4.1. Cell Harvesting Post Separation and Cell Separation
2.4.2. Lyophilization
2.5. Determining Viability of Freeze-Dried S. cerevisiae var. boulardii in Simulated Gastrointestinal Fluid Conditions
2.5.1. Assessment of Freeze-Dried Material Pre-Exposure
2.5.2. Preparation of Simulated Gastric Fluid and Simulated Intestinal Fluid Solutions
2.5.3. Cultivation of Freeze-Dried S. cerevisiae var. boulardii Under SGF Conditions to Assess Viability
2.5.4. Cultivation and Viability of Freeze-Dried S. cerevisiae var. boulardii Under SIF Conditions
2.6. Techno-Economic Feasibility Assessment
3. Results and Discussion
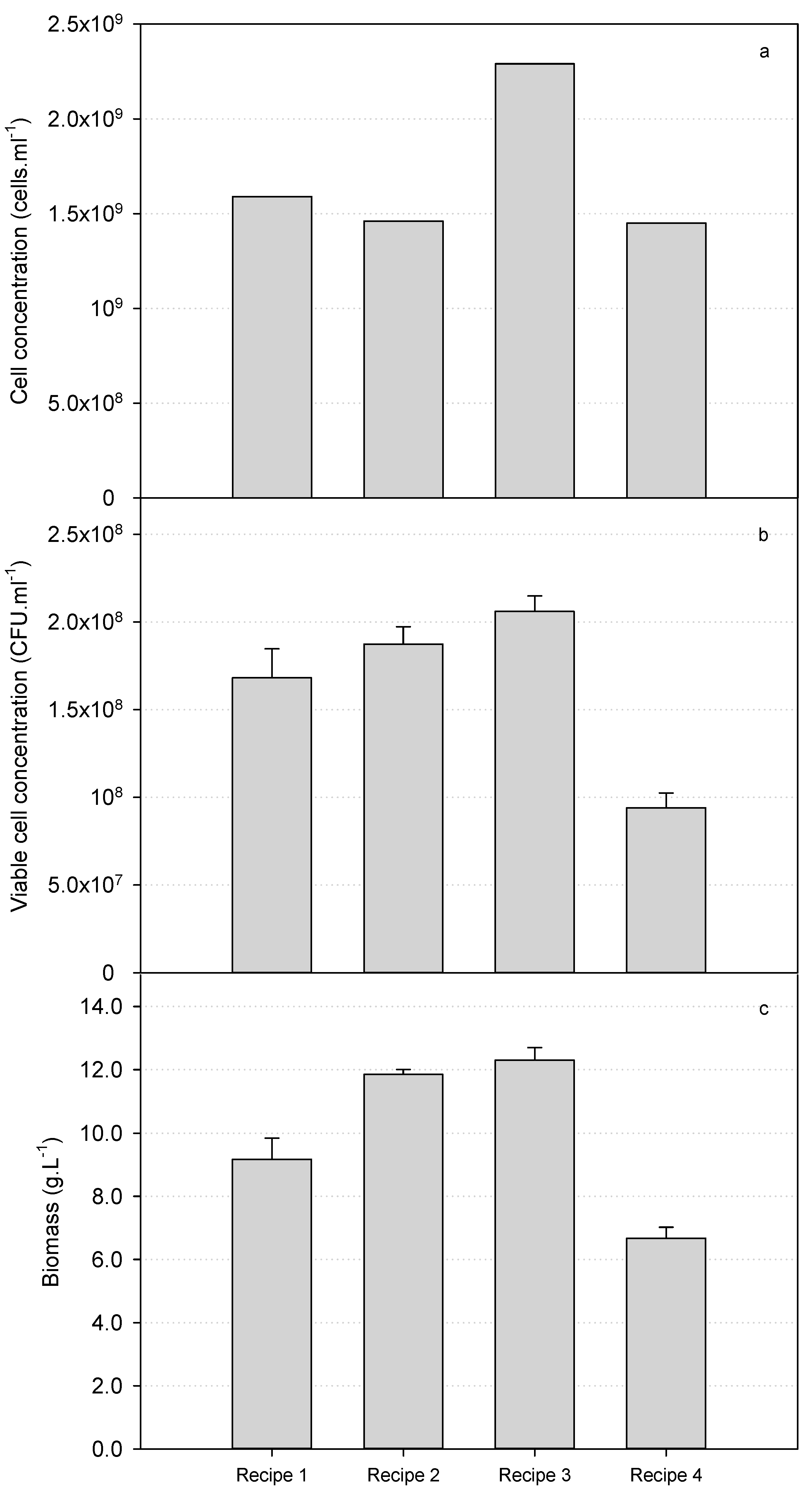
3.1. Production of S. cerevisiae var. boulardii in 30 L Bioreactors
3.2. Validation of a Fed-Batch Fermentation Process for the Cultivation of S. cerevisiae var. boulardii
3.3. Recovery After Centrifugation and Freeze-Drying
3.4. Viability of S. cerevisiae var. boulardii After Exposure to Simulated Gastrointestinal Environment Conditions
3.5. Techno-Economic Assessment and Process Simulations at Manufacturing Scale
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Abulizi, N.; Pither, J.; Hart, M.M.; Gibson, D.L. Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live? Front. Microbiol. 2017, 8, 1935. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, F.; Thirugnanam, S.; Welsh, D.A.; Kim, W.K.; Rappaport, J.; Bittinger, K.; Rout, N. The Role of Gut Dysbiosis in the Loss of Intestinal Immune Cell Functions and Viral Pathogenesis. Microorganisms 2023, 11, 1849. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, Their Prophylactic and Therapeutic Applications in Human Health Development: A Review of the Literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- FAO; WHO. WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2007. [Google Scholar]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Ali, G.M.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces boulardii CNCM I-745 Mechanisms of Action against Intestinal Infections. World J. Gastroenterol. 2019, 25, 2188–2203. [Google Scholar] [PubMed]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and Antioxidant Activities of Saccharomyces cerevisiae IFST062013, a Potential Probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae Strains as Biotherapeutic Tools: Is There Room for Improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef]
- Altmann, M. The Benefits of Saccharomyces boulardii. In The Yeast Role in Medical Applications; InTech: Tipperary, Ireland, 2018; pp. 3–10. [Google Scholar]
- Cascio, V.; Gittings, D.; Merloni, K.; Hurton, M.; Laprade, D.; Austriaco, N. S-Adenosyl-L-Methionine Protects the Probiotic Yeast, Saccharomyces boulardii, from Acid-Induced Cell Death. BMC Microbiol. 2013, 13, 35. [Google Scholar]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential Probiotic Characterization and Effect of Encapsulation of Probiotic Yeast Strains on Survival in Simulated Gastrointestinal Tract Condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar]
- Gao, H.; Li, Y.; Sun, J.; Xu, H.; Wang, M.; Zuo, X.; Fu, Q.; Guo, Y.; Chen, Z.; Zhang, P.; et al. Saccharomyces boulardii Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating NF-ΚB and Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 1622375. [Google Scholar] [CrossRef]
- Girard, P.; Coppé, M.-C.; Pansart, Y.; Gillardin, J.-M. Gastroprotective Effect of Saccharomyces boulardii in a Rat Model of Ibuprofen-Induced Gastric Ulcer. Pharmacology 2010, 85, 188–193. [Google Scholar] [CrossRef]
- Oh, G.M.; Moon, W.; Seo, K.I.; Jung, K.; Kim, J.H.; Kim, S.E.; Park, M.I.; Park, S.J. Changes in the Crohn’s Disease Activity Index and Safety of Administering Saccharomyces boulardii in Patients with Crohn’s Disease in Clinical Remission: A Single Hospital-Based Retrospective Cohort Study. Korean J. Gastroenterol. 2020, 76, 314–321. [Google Scholar]
- Dalmasso, G.; Cottrez, F.; Imbert, V.; Lagadec, P.; Peyron, J.F.; Rampal, P.; Czerucka, D.; Groux, H. Saccharomyces boulardii Inhibits Inflammatory Bowel Disease by Trapping T Cells in Mesenteric Lymph Nodes. Gastroenterology 2006, 131, 1812–1825. [Google Scholar]
- Billoo, A.G.; Memon, M.A.; Khaskheli, S.A.; Murtaza, G.; Iqbal, K.; Shekhani, M.S.; Siddiqi, A.Q. Role of a Probiotic (Saccharomyces boulardii) in Management and Prevention of Diarrhoea. World J. Gastroenterol. 2006, 12, 4557–4560. [Google Scholar] [PubMed]
- Zhang, J.; Wan, S.; Gui, Q. Comparison of Safety, Effectiveness and Serum Inflammatory Factor Indexes of Saccharomyces boulardii versus Bifidobacterium Triple Viable in Treating Children with Chronic Diarrhea: A Randomized Trial. Transl. Pediatr. 2021, 10, 1677–1685. [Google Scholar] [PubMed]
- Justino, P.F.C.; Melo, L.F.M.; Nogueira, A.F.; Costa, J.V.G.; Silva, L.M.N.; Santos, C.M.; Mendes, W.O.; Costa, M.R.; Franco, A.X.; Lima, A.A.; et al. Treatment with Saccharomyces boulardii Reduces the Inflammation and Dysfunction of the Gastrointestinal Tract in 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Br. J. Nutr. 2014, 111, 1611–1621. [Google Scholar] [PubMed]
- Ling, H.; Liu, R.; Sam, Q.H.; Shen, H.; Chai, L.Y.A.; Chang, M.W. Engineering of a Probiotic Yeast for the Production and Secretion of Medium-Chain Fatty Acids Antagonistic to an Opportunistic Pathogen Candida albicans. Front. Bioeng. Biotechnol. 2023, 11, 1090501. [Google Scholar]
- He, X.J.; Wang, X.L.; Sun, D.J.; Huang, X.Y.; Liu, G.; Li, D.Z.; Lin, H.L.; Zeng, X.P.; Li, D.L.; Wang, W. The Efficacy and Safety of Saccharomyces boulardii in Addition to Antofloxacin-Based Bismuth Quadruple Therapy for Helicobacter pylori Eradication: A Single-Center, Prospective Randomized-Control Study. Ther. Adv. Gastroenterol. 2023, 16, 17562848221147763. [Google Scholar]
- Hun, C.H.; Sueb, M.M.S.; Malek, A.R.; Othman, Z.; Elsayed, E.A.; Ramili, S.; Elmarzugi, N.A.; Sarmidi, M.R.; Aziz, R.; El Enshasy, H.A. Bioprocess Development for High Cell Mass Production of the Probiotic Yeast-Kluyveromyces lactis. IOSR J. Pharm. Biol. Sci. 2013, 8, 49–59. [Google Scholar]
- Malairuang, K.; Krajang, M.; Sukna, J.; Rattanapradit, K.; Chamsart, S. High Cell Density Cultivation of Saccharomyces cerevisiae with Intensive Multiple Sequential Batches Together with a Novel Technique of Fed-Batch at Cell Level (FBC). Processes 2020, 8, 1321. [Google Scholar] [CrossRef]
- Zinco, A.G.; Lazo, R.E.L.; Guimarães, T.M.; Brand, D.; Bonfim, T.M.B.; Murakami, F.S. Obtention of Probiotic Cells of Saccharomyces boulardii in Unsupplemented Sugarcane Juice. Open Sci. Res. V. 2022, 5, 1158–1166. [Google Scholar]
- Chin, T.S.; Othman, Z.; Malek, R.A.; Elmarzugi, N.; Leng, O.M.; Ramli, S.; Fashya Musa, N.; Aziz, R.; Enshasy, H. El Bioprocess Optimization for Biomass Production of Probiotics Yeast Saccharomyces boulardii in Semi-Industrial Scale. J. Chem. Pharm. Res. 2015, 7, 122–132. [Google Scholar]
- Fu, T.J.; Abbott, U.R.; Hatzos, C. Digestibility of Food Allergens and Nonallergenic Proteins in Simulated Gastric Fluid and Simulated Intestinal Fluid—A Comparative Study. J. Agric. Food Chem. 2002, 50, 7154–7160. [Google Scholar]
- Thantsha, M.S.; Cloete, T.E.; Moolman, F.S.; Labuschagne, P.W. Supercritical Carbon Dioxide Interpolymer Complexes Improve Survival of B. Longum Bb-46 in Simulated Gastrointestinal Fluids. Int. J. Food Microbiol. 2009, 129, 88–92. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Media for Industrial Fermentations. In Principles of Fermentation Technology; Stanbury, P.F., Whitaker, A., Hall, S.J., Eds.; Pergamon Press: Oxford, UK, 1995; pp. 93–121. [Google Scholar]
- Atkinson, B.; Sainter, P. Development of Downstream Processing. J. Chem. Technol. Biotechnol. 1982, 32, 100–108. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Ter Riet, B.; Vink, E.; Blad, S.; De Nobel, H.; Van Den Ende, H.; Klis, F.M. Low External pH Induces HOG1-dependent Changes in the Organization of the Saccharomyces cerevisiae Cell Wall. Mol. Microbiol. 2001, 39, 469–479. [Google Scholar] [CrossRef]
- Lucena, R.M.; Dolz-Edo, L.; Brul, S.; de Morais, M.A.; Smits, G. Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes 2020, 11, 656. [Google Scholar] [CrossRef]
- Chen, A.K.-L.; Gelling, C.; Rogers, P.L.; Dawes, I.W.; Rosche, B. Response of Saccharomyces cerevisiae to Stress-Free Acidification. J. Microbiol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- de Lucena, R.M.; Elsztein, C.; de Barros Pita, W.; de Souza, R.B.; de Sá Leitão Paiva Júnior, S.; de Morais Junior, M.A. Transcriptomic Response of Saccharomyces cerevisiae for Its Adaptation to Sulphuric Acid-Induced Stress. Antonie Leeuwenhoek 2015, 108, 1147–1160. [Google Scholar]
- Levin, D.E. Regulation of Cell Wall Biogenesis in Saccharomyces cerevisiae: The Cell Wall Integrity Signaling Pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Lin, X.; Qi, Y.; Yan, D.; Liu, H.; Chen, X.; Liu, L. CgMED3 Changes Membrane Sterol Composition to Help Candida glabrata Tolerate Low-PH Stress. Appl. Environ. Microbiol. 2017, 83, e00972-17. [Google Scholar] [CrossRef]
- Fletcher, E.; Feizi, A.; Bisschops, M.M.M.; Hallström, B.M.; Khoomrung, S.; Siewers, V.; Nielsen, J. Evolutionary Engineering Reveals Divergent Paths When Yeast Is Adapted to Different Acidic Environments. Metab. Eng. 2017, 39, 19–28. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival during Gastric Transit. Hayati 2017, 24, 1–5. [Google Scholar]
- ISO 19344|IDF 232:2015; Milk, Milk. Products—Starter Cultures, Probiotics and Fermented Products—Quantification of Lactic Acid. Bacteria by Flow. Cytometry. ISO: Geneva, Switzerland; IDF: Tel Aviv, Israel, 2015.
- Ayama, H.; Sumpavapol, P.; Chanthachum, S. Effect of Encapsulation of Selected Probiotic Cell on Survival in Simulated Gastrointestinal Tract Condition. Songklanakarin J. Sci. Technol. 2014, 36, 291–299. [Google Scholar]
- Ragavan, M.L.; Das, N. Nanoencapsulation of Saccharomycopsis fibuligera VIT-MN04 Using Electrospinning Technique for Easy Gastrointestinal Transit. In Proceedings of the 2nd International Conference on Nanoscience and Nanotechnology (ICNAN2019), Vellore, India, 29 November–1 December 2019; Institution of Engineering and Technology: Perth, Australia, 2020; Volume 14, pp. 766–773. [Google Scholar]
- Ragavan, M.L.; Das, N. Process Optimization for Microencapsulation of Probiotic Yeasts. Front. Biol. 2018, 13, 197–207. [Google Scholar]
- Petrides, D.; Carmichael, D.; Siletti, C.; Koulouris, A. Bioprocess Simulation and Economics. In Essentials in Fermentation Technology; Berenjian, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 273–305. [Google Scholar]



| Measured Variable | Unit | Mean | Std Dev | Coefficient of Variance | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|
| Growth age in fermenter | h | 25.33 | 1.16 | 4.56 | 24.03 | 26.64 |
| Final biomass concentration | g.L−1 | 12.70 | 0.50 | 3.94 | 12.13 | 13.27 |
| YPS | g.g−1 | 0.20 | 0.01 | 4.47 | 0.19 | 0.21 |
| YPP | g.g−1 | 23.31 | 0.79 | 3.39 | 22.42 | 24.20 |
| Cell Productivity | g.L−1.h−1 | 0.42 | 0.02 | 3.93 | 0.40 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moonsamy, G.; Singh, S.; Roets-Dlamini, Y.; Baikgaki, K.K.; Ramchuran, S.O. Development of a High-Cell-Density Production Process for a Biotherapeutic Yeast, Saccharomyces cerevisiae var. boulardii, for Use as a Human Probiotic. Fermentation 2025, 11, 186. https://doi.org/10.3390/fermentation11040186
Moonsamy G, Singh S, Roets-Dlamini Y, Baikgaki KK, Ramchuran SO. Development of a High-Cell-Density Production Process for a Biotherapeutic Yeast, Saccharomyces cerevisiae var. boulardii, for Use as a Human Probiotic. Fermentation. 2025; 11(4):186. https://doi.org/10.3390/fermentation11040186
Chicago/Turabian StyleMoonsamy, Ghaneshree, Sarisha Singh, Yrielle Roets-Dlamini, Koketso Kenneth Baikgaki, and Santosh Omrajah Ramchuran. 2025. "Development of a High-Cell-Density Production Process for a Biotherapeutic Yeast, Saccharomyces cerevisiae var. boulardii, for Use as a Human Probiotic" Fermentation 11, no. 4: 186. https://doi.org/10.3390/fermentation11040186
APA StyleMoonsamy, G., Singh, S., Roets-Dlamini, Y., Baikgaki, K. K., & Ramchuran, S. O. (2025). Development of a High-Cell-Density Production Process for a Biotherapeutic Yeast, Saccharomyces cerevisiae var. boulardii, for Use as a Human Probiotic. Fermentation, 11(4), 186. https://doi.org/10.3390/fermentation11040186







