Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Chlamydospores
2.2. SMS and Inoculum
2.3. Experimental Design
2.4. Chemical-Physical Analysis
2.5. Microbial Analysis
2.6. Statistical Analysis
2.6.1. Kinetic Model
2.6.2. Correlation Network Analysis
2.6.3. LEfSe Analysis
2.6.4. Data Analysis
3. Results
3.1. Phenotypes, Enzyme Activities, and SMS Composition Changes During Pretreatment
3.2. Methanogenic Performance of SMS After Tv Chlamydospores Pretreatment
3.3. Dynamic Changes of pH, VFAs, TAN, and FAN During Anaerobic Digestion
3.4. Effect of Tv Chlamydospores. Pretreatment on Microbial Community Structures
3.4.1. Alpha Diversity Analysis of Fungal, Bacterial, and Archaeal Communities
3.4.2. Beta Diversity and Composition Analysis of Fungal, Bacterial, and Archaeal Communities
3.4.3. LEfSe Analysis of Fungal, Bacterial, and Archaeal Communities
3.4.4. Correlation Network Analysis of Fungal, Bacterial, and Archaeal Communities
3.5. Production Recommendation and Future Prospects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baptista, F.; Campos, J.; Costa-Silva, V.; Pinto, A.R.; Saavedra, M.J.; Ferreira, L.M. Nutraceutical potential of lentinula edodes’ spent mushroom substrate: A comprehensive study on phenolic composition, antioxidant activity, and antibacterial effects. J. Fungi 2023, 9, 1200. [Google Scholar] [CrossRef]
- Kousar, A.; Khan, H.A.; Farid, S.; Zhao, Q.; Zeb, I. Recent advances on environmentally sustainable valorization of spent mushroom substrate: A review. Biofuels Bioprod. Biorefining 2024, 18, 639–651. [Google Scholar] [CrossRef]
- Lam, S.S.; Lee, X.Y.; Nam, W.L.; Phang, X.Y.; Liew, R.K.; Yek, P.N.; Rosli, M.H. Microwave vacuum pyrolysis conversion of waste mushroom substrate into biochar for use as growth medium in mushroom cultivation. J. Chem. Technol. Biotechnol. 2019, 94, 1406–1415. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Stanley, J.T.; Thanarasu, A.; Kumar, P.S.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential pre-treatment of lignocellulosic biomass for the enhancement of biomethane production through anaerobic digestion—A review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Dahadha, S.; Amin, Z.; Bazyar Lakeh, A.A.; Elbeshbishy, E. Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuels 2017, 31, 10335–10347. [Google Scholar] [CrossRef]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Kainthola, J.; Podder, A.; Fechner, M.; Goel, R. An overview of fungal pretreatment processes for anaerobic digestion: Applications, bottlenecks and future needs. Bioresour. Technol. 2021, 321, 124397. [Google Scholar] [CrossRef]
- Brémond, U.; de Buyer, R.; Steyer, J.-P.; Bernet, N.; Carrere, H. Biological pretreatments of biomass for improving biogas production: An overview from lab scale to full-scale. Renew. Sustain. Energy Rev. 2018, 90, 583–604. [Google Scholar] [CrossRef]
- Prasad, R.K.; Sharma, A.; Mazumder, P.B.; Dhussa, A. A comprehensive pre-treatment strategy evaluation of ligno-hemicellulosic biomass to enhance biogas potential in the anaerobic digestion process. RSC Sustain. 2024, 2, 2444–2467. [Google Scholar] [CrossRef]
- Ali, S.S.; Sun, J. Physico-chemical pretreatment and fungal biotreatment for park wastes and cattle dung for biogas production. SpringerPlus 2015, 4, 712. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, C.; Li, G.; Ding, P.; Lan, M.; Gao, Z.; Jiao, Y. Biological pretreatment of corn straw for enhancing degradation efficiency and biogas production. Bioengineered 2020, 11, 251–260. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Elshikh, M.S.; Aljowaie, R.M.; Hussein, J.M.; Dufossé, L.; Wu, C.; Lu, J. Competitive antagonistic action of laccase between Trichoderma species and the newly identified wood pathogenic Ganoderma camelum. Front. Microbiol. 2024, 15, 1408521. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.A.; Șesan, T.E.; Oancea, A.; Craciunescu, O.; Ghiurea, M.; Răut, I.; Oancea, F. Influence of Foliar Treatment with Suspensions Rich in Trichoderma Chlamydospores on Momordica charantia Physiology, Yield, and Quality. Horticulturae 2024, 10, 371. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Z.; Xu, Y.; Shi, Q.; Ma, Y.; Aung, M.; Cheng, Y.; Zhu, W. Interactions between Anaerobic Fungi and Methanogens in the Rumen and Their Biotechnological Potential in Biogas Production from Lignocellulosic Materials. Microorganisms 2021, 9, 190. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. A comprehensive review on anaerobic fungi applications in biofuels production. Sci. Total Environ. 2022, 829, 154521. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Yuan, X.; Hong, S.; Xiong, W.; Raza, W.; Shen, Z.; Wang, B.; Li, R.; Ruan, Y.; Shen, Q.; Dini-Andreote, F. Development of fungal-mediated soil suppressiveness against Fusarium wilt disease via plant residue manipulation. Microbiome 2021, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Sui, Y.; Li, Z.; Huang, Y.; Zhang, H.; Wang, W. Colonization of Trichoderma viride Tv-1511 in peppermint (Mentha x piperita L.) roots promotes essential oil production by triggering ROS-mediated MAPK activation. Plant Physiol. Biochem. 2020, 151, 705–718. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Wierinck, I. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Cai, Y.; Wang, J.; Zhao, Y.; Zhao, X.; Zheng, Z.; Wen, B.; Cui, Z.; Wang, X. A new perspective of using sequential extraction: To predict the deficiency of trace elements during anaerobic digestion. Water Res. 2018, 140, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Yuan, X.; Cao, Y.; Liu, Y.; Wang, X.; Cui, Z. Optimization of liquid fermentation of microbial consortium WSD-5 followed by saccharification and acidification of wheat straw. Bioresour. Technol. 2012, 118, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the methane yield of maize straw: Focus on the effects of pretreatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour. Technol. 2018, 250, 204–213. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10-25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Sun, J.; Li, Y.; Chen, J. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef]
- Li, Z.Y.; Inoue, D.; Ike, M. Mitigating ammonia-inhibition in anaerobic digestion by bioaugmentation: A review. J. Water Process Eng. 2023, 52, 103506. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Wang, X. Obstacles faced by methanogenic archaea originating from substrate-driven toxicants in anaerobic digestion. J. Hazard. Mater. 2021, 403, 123938. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, Q.M.; Goker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Boekhout, T.; et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Mitsui, R.; Kusano, Y.; Yurimoto, H.; Sakai, Y.; Kato, N.; Tanaka, M. Formaldehyde fixation contributes to detoxification for growth of a nonmethylotroph, Burkholderia cepacia TM1, on vanillic acid. Appl. Environ. Microbiol. 2003, 69, 6128–6132. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Bernet, N.; Steyer, J.P.; Delgenes, J.P.; Escudie, R. Methanosarcina plays a main role during methanogenesis of high-solids food waste and cardboard. Waste Manag. 2018, 76, 423–430. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Y.; Wu, H.; Meng, Q.; Liu, S.; Chai, S.; Hao, L.; Zhou, Z. Dynamic changes in the yak rumen eukaryotic community and metabolome characteristics in response to feed type. Front. Vet. Sci. 2022, 9, 1027967. [Google Scholar] [CrossRef]
- Wang, N.; Huang, D.; Shao, M.; Sun, R.; Xu, Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 2022, 347, 126701. [Google Scholar] [CrossRef]
- Jin, R. Identification and characterization of a fungal strain with lignin and cellulose hydrolysis activities. Afr. J. Microbiol. Res. 2012, 6, 6545–6550. [Google Scholar] [CrossRef]
- Badalato, N.; Guillot, A.; Sabarly, V.; Dubois, M.; Pourette, N.; Pontoire, B.; Robert, P.; Bridier, A.; Monnet, V.; Sousa, D.Z.; et al. Whole proteome analyses on ruminiclostridium cellulolyticum show a modulation of the cellulolysis machinery in response to cellulosic materials with subtle differences in chemical and structural properties. PLoS ONE 2017, 12, e0170524. [Google Scholar] [CrossRef]
- Haddad, P.G.; Mura, J.; Casteran, F.; Guignard, M.; Ranchou-Peyruse, M.; Senechal, P.; Larregieu, M.; Isaure, M.P.; Svahn, I.; Moonen, P.; et al. Biological, geological and chemical effects of oxygen injection in underground gas storage aquifers in the setting of biomethane deployment. Sci. Total Environ. 2022, 806, 150690. [Google Scholar] [CrossRef]
- Cheng, Z.; Wei, Y.; Zhang, Q.; Zhang, J.; Lu, T.; Pei, Y. Enhancement of surfactant biodegradation with an anaerobic membrane bioreactor by introducing microaeration. Chemosphere 2018, 208, 343–351. [Google Scholar] [CrossRef]
- Peng, W.; Lu, F.; Duan, H.; Zhang, H.; Shao, L.; He, P. Biological denitrification potential as an indicator for measuring digestate stability. Sci. Total Environ. 2021, 752, 142211. [Google Scholar] [CrossRef]
- Maus, I.; Bremges, A.; Stolze, Y.; Hahnke, S.; Cibis, K.G.; Koeck, D.E.; Kim, Y.S.; Kreubel, J.; Hassa, J.; Wibberg, D.; et al. Genomics and prevalence of bacterial and archaeal isolates from biogas-producing microbiomes. Biotechnol. Biofuels 2017, 10, 264. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Dede, A.; Guven, K.; Sahi, N.N. Isolation, plant growth-promoting traits, antagonistic effects on clinical and plant pathogenic organisms and identification of actinomycetes from olive rhizosphere. Microb. Pathog. 2020, 143, 104134. [Google Scholar] [CrossRef]
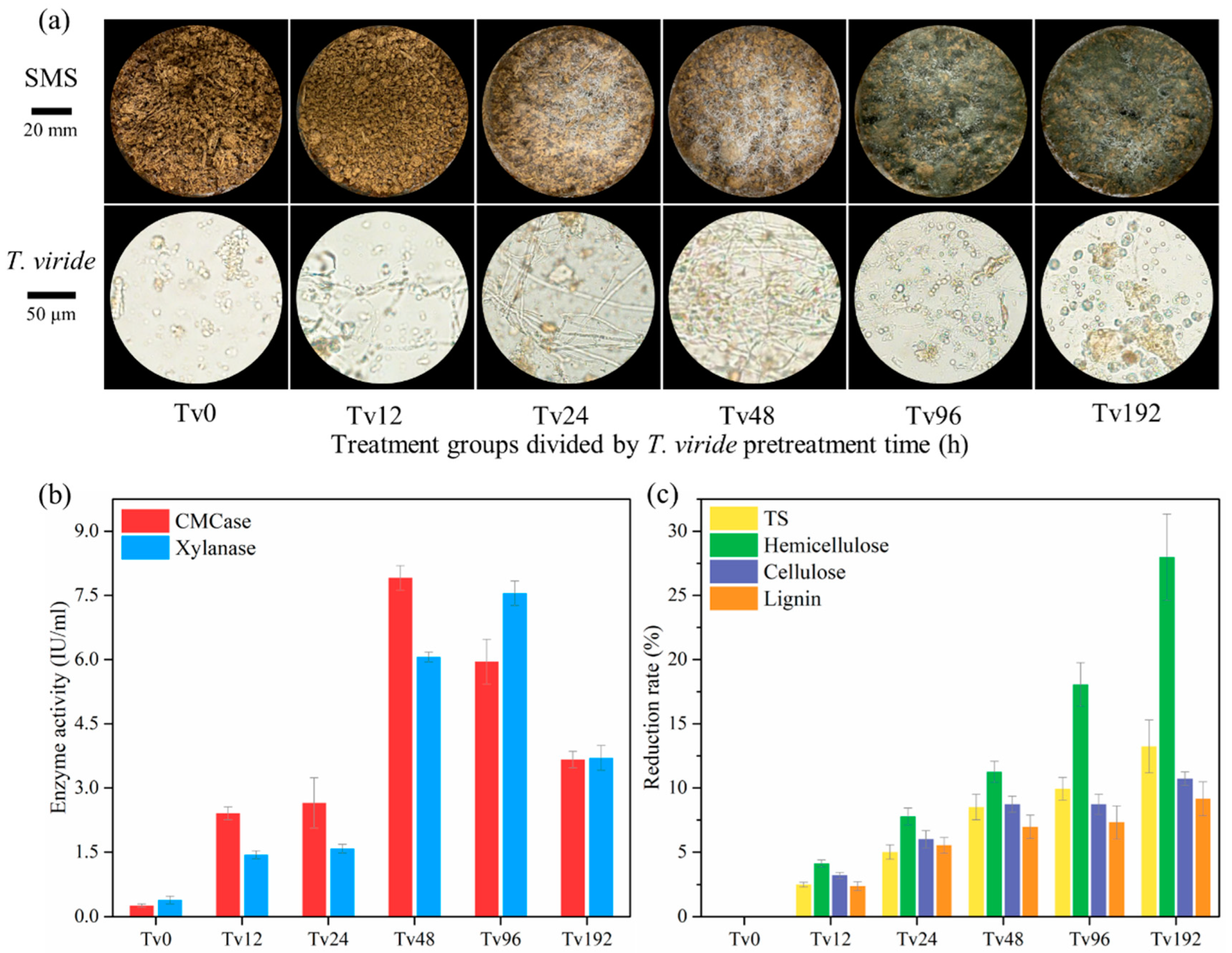
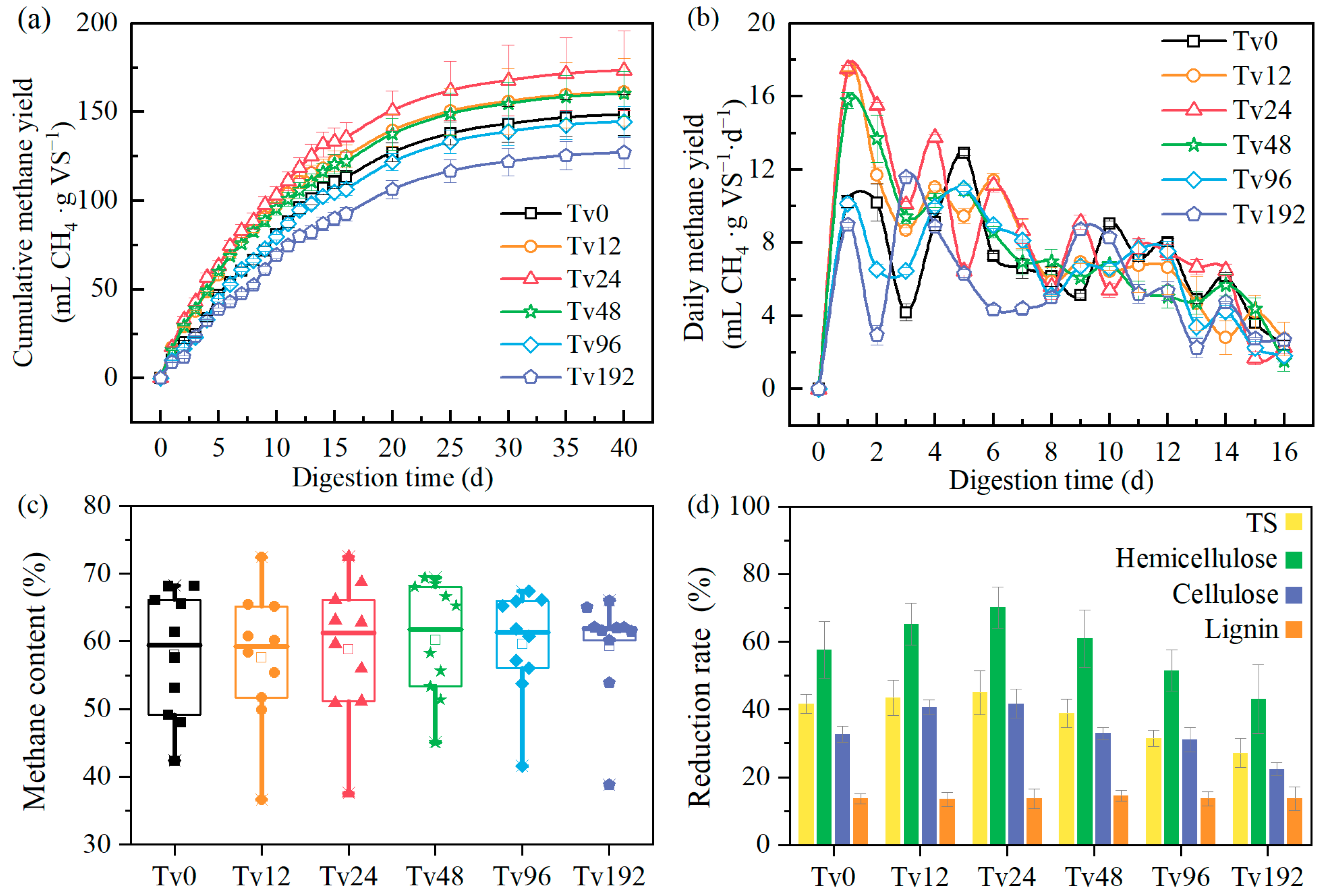
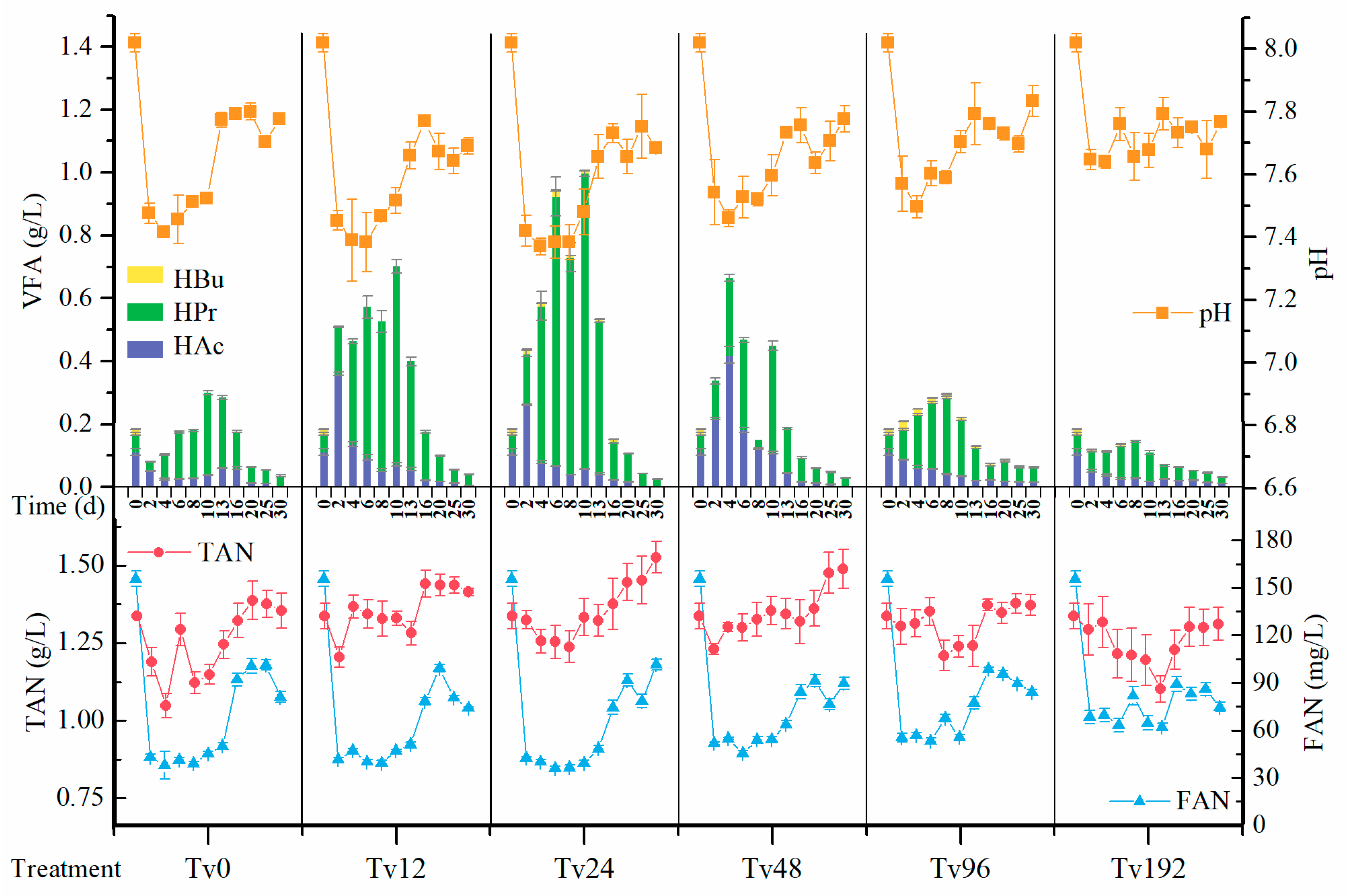

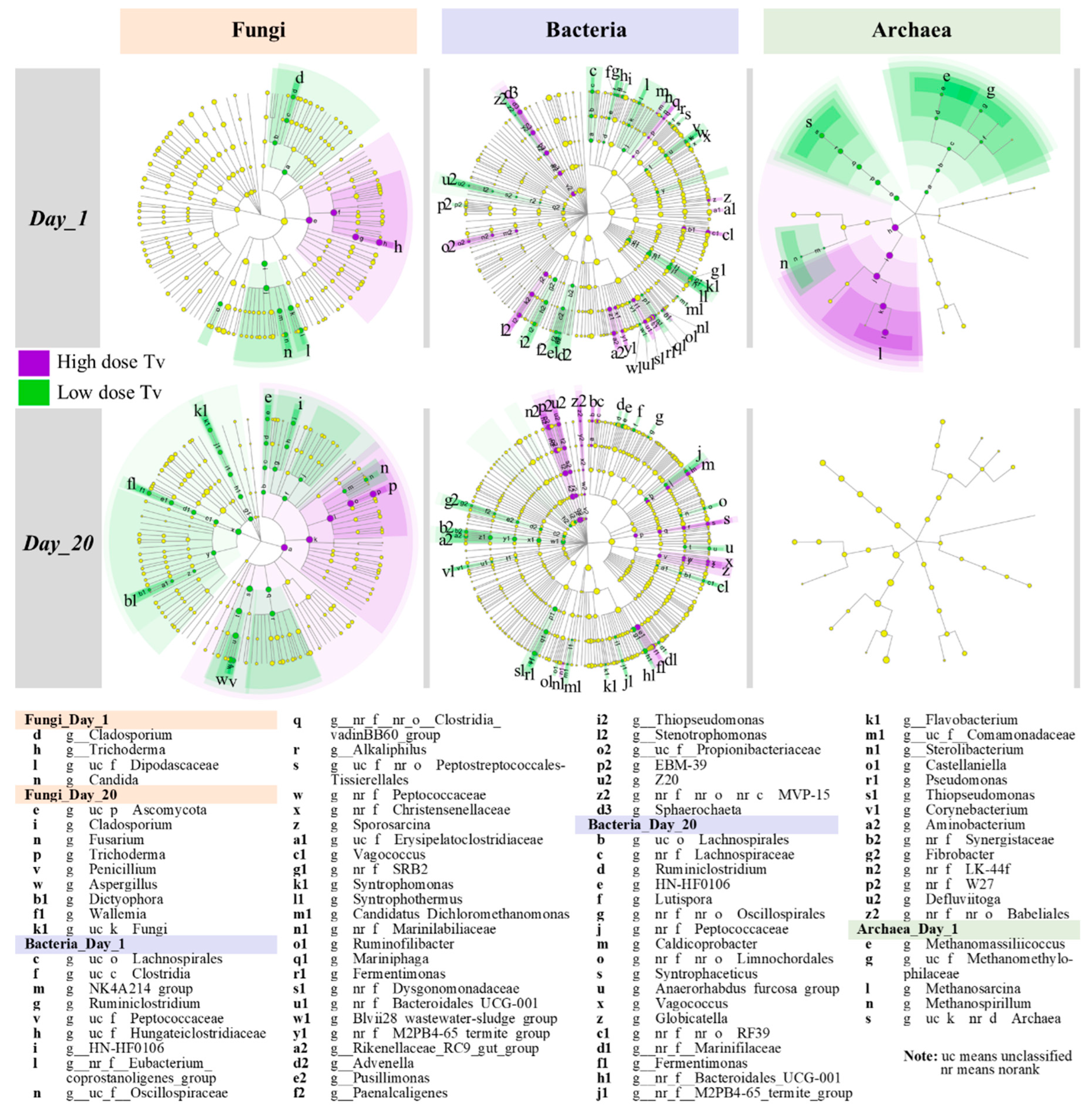

| Character (Unit) | SMS | Inoculum |
|---|---|---|
| TS (%) | 93.87 ± 0.00 | 1.06 ± 0.00 |
| VS (%TS) | 90.14 ± 0.10 | 49.63 ± 0.10 |
| C (%TS) | 34.79 ± 0.11 | 18.38 ± 0.09 |
| N (%TS) | 2.04 ± 0.04 | 2.81 ± 0.08 |
| C/N | 17.05 | 6.55 |
| Hemicellulose (%TS) | 19.83 ± 0.25 | n.d. |
| Cellulose (%TS) | 29.30 ± 0.24 | n.d. |
| Lignin (%TS) | 19.81 ± 0.28 | n.d. |
| Ash (%TS) | 9.86 ± 0.10 | 50.37 ± 0.10 |
| pH | n.d. | 8.02 ± 0.03 |
| Formic acid (mg/mL) | n.d. | 0.000 ± 0.000 |
| Acetic acid (mg/mL) | n.d. | 0.112 ± 0.002 |
| Propionic acid (mg/mL) | n.d. | 0.058 ± 0.000 |
| Butyric acid (mg/mL) | n.d. | 0.013 ± 0.000 |
| CH4 Yield (mL/gVS) | First-Order Model | Modified Gompertz Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K (d−1) | Mmax (mL/gVS) | DIFF * | R2 | Rmax (mL/gVS·d) | λ (d) | Mmax (mL/gVS) | DIFF * | ||
| Tv0 | 148.5 ± 11.6 b | 0.9938 | 0.70 ± 0.02 | 163.4 ± 3.3 | 10.0% | 0.9952 | 8.3 ± 0.2 | 0.04 ± 0.25 | 146.9 ± 1.8 | −1.0% |
| Tv12 | 161.2 ± 18.9 ab | 0.9993 | 0.88 ± 0.01 | 167.2 ± 0.9 | 3.7% | 0.9895 | 9.1 ± 0.3 | −0.98 ± 0.36 | 157.6 ± 2.7 | −2.3% |
| Tv24 | 173.4 ± 22.6 a | 0.9986 | 0.89 ± 0.01 | 179.8 ± 1.3 | 3.7% | 0.9892 | 9.8 ± 0.4 | −1.06 ± 0.37 | 170.1 ± 2.9 | −1.9% |
| Tv48 | 160.2 ± 12.8 ab | 0.9991 | 0.86 ± 0.01 | 166.1 ± 1.0 | 3.7% | 0.9870 | 8.6 ± 0.4 | −1.22 ± 0.41 | 157.0 ± 3.1 | −2.0% |
| Tv96 | 144.6 ± 8.5 b | 0.9952 | 0.70 ± 0.02 | 157.7 ± 2.8 | 9.0% | 0.9928 | 8.0 ± 0.2 | 0.01 ± 0.30 | 141.6 ± 2.2 | −2.0% |
| Tv192 | 127.0 ± 8.6 c | 0.9950 | 0.65 ± 0.02 | 141.1 ± 2.7 | 11.1% | 0.9927 | 6.6 ± 0.2 | −0.07 ± 0.32 | 125.8 ± 2.0 | −1.0% |
| Fungi | Bacteria | Archaea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Chao | Heip | Shannon | Chao | Heip | Shannon | Chao | Heip | Shannon | |
| Inoculum | 46 | 0.466 | 3.09 | 365 | 0.077 | 3.19 | 46 | 0.012 | 0.25 |
| Day_1 | |||||||||
| Tv0_1 | 144 | 0.362 | 3.96 | 433 | 0.096 | 3.53 | 61 | 0.015 | 0.56 |
| Tv12_1 | 44 | 0.682 | 3.41 | 422 | 0.081 | 3.35 | 29 | 0.032 | 0.63 |
| Tv24_1 | 23 | 0.390 | 2.26 | 394 | 0.084 | 3.31 | 57 | 0.017 | 0.60 |
| Tv48_1 | 29 | 0.246 | 2.06 | 372 | 0.091 | 3.38 | 47 | 0.017 | 0.56 |
| Tv96_1 | 5 | 0.005 | 0.02 | 428 | 0.083 | 3.33 | 46 | 0.015 | 0.47 |
| Tv192_1 | 5 | 0.042 | 0.16 | 370 | 0.090 | 3.33 | 52 | 0.010 | 0.38 |
| Day_20 | |||||||||
| Tv0_20 | 23 | 0.186 | 1.59 | 571 | 0.115 | 3.89 | 80 | 0.031 | 1.17 |
| Tv12_20 | 109 | 0.407 | 3.81 | 548 | 0.115 | 4.04 | 74 | 0.018 | 0.48 |
| Tv24_20 | 107 | 0.125 | 2.65 | 555 | 0.126 | 4.15 | 63 | 0.019 | 0.69 |
| Tv48_20 | 31 | 0.000 | 0.01 | 555 | 0.090 | 3.79 | 82 | 0.047 | 1.52 |
| Tv96_20 | 7 | 0.000 | 0.00 | 547 | 0.086 | 3.75 | 86 | 0.017 | 0.87 |
| Tv192_20 | 7 | 0.005 | 0.02 | 521 | 0.081 | 3.63 | 73 | 0.019 | 0.74 |
| Germi- Nation Rate (%) | Plant Height (mm) | Root Length (mm) | Fresh Weight (mg) | Dry Weight (mg) | Index of Seeding Quality | |
|---|---|---|---|---|---|---|
| BL | 66.7 ± 7.2 a | 17.0 ± 2.9 d | 23.9 ± 7.9 c | 48.5 ± 5.3 d | 3.0 ± 0.3 d | 0.200 |
| Tv0 | 60.4 ± 9.1 a | 18.3 ± 1.8 cd | 43.3 ± 9.8 b | 70.6 ± 5.9 c | 4.5 ± 0.5 c | 0.547 |
| Tv12 | 58.2 ± 13.3 a | 19.8 ± 2.6 bc | 44.3 ± 8.6 ab | 73.2 ± 6.4 bc | 4.8 ± 0.5 bc | 0.610 |
| Tv24 | 56.8 ± 6.4 a | 20.3 ± 1.9 bc | 46.8 ± 7.7 ab | 75.6 ± 7.8 abc | 4.8 ± 0.6 bc | 0.636 |
| Tv48 | 61.5 ± 4.5 a | 20.9 ± 2.8 ab | 47.8 ± 6.8 ab | 76.2 ± 3.7 ab | 5.1 ± 0.7 ab | 0.772 |
| Tv96 | 56.6 ± 5.7 a | 21.8 ± 2.5 a | 49.2 ± 8.7 a | 77.9 ± 8.9 a | 5.4 ± 0.5 a | 0.828 |
| Tv192 | 54.9 ± 9.1 a | 22.7 ± 3.4 ab | 50.6 ± 6.9 ab | 79.5 ± 4.4 ab | 5.5 ± 0.6 a | 0.739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Lai, X.; Liu, C.; Wu, X.; Bai, X.; Cai, Y.; Zhao, X.; Li, Z.; Hao, Y.; Huang, Y.; et al. Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride. Fermentation 2025, 11, 152. https://doi.org/10.3390/fermentation11030152
Zhu W, Lai X, Liu C, Wu X, Bai X, Cai Y, Zhao X, Li Z, Hao Y, Huang Y, et al. Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride. Fermentation. 2025; 11(3):152. https://doi.org/10.3390/fermentation11030152
Chicago/Turabian StyleZhu, Wentao, Xianzhi Lai, Changfa Liu, Xiao Wu, Xiaochen Bai, Yafan Cai, Xiaoling Zhao, Zhe Li, Yongren Hao, Yanhua Huang, and et al. 2025. "Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride" Fermentation 11, no. 3: 152. https://doi.org/10.3390/fermentation11030152
APA StyleZhu, W., Lai, X., Liu, C., Wu, X., Bai, X., Cai, Y., Zhao, X., Li, Z., Hao, Y., Huang, Y., Zheng, Z., & Chu, J. (2025). Enhancement of Biomethane Yield from Spent Mushroom Substrate: Biological Pretreatment with the Chlamydospores of Trichoderma viride. Fermentation, 11(3), 152. https://doi.org/10.3390/fermentation11030152








