Abstract
Probiotics improve the quality of silage during the planting and fermentation processes. This study was designed to investigate the accumulation of nutritional components in the fresh corn variety Jingkenuo 2000 waxy maize under different fertilization conditions and its effectiveness as silage feed. The nutrient accumulation trends of dry matter (DM), starch, neutral detergent fiber (NDF), acid detergent fiber (ADF), crude protein (CP), and ether extract (EE) in the stems, leaves, grains, and whole plant of Jingkenuo 2000 waxy maize were evaluated during different growth cycles. The relative feed value (RFV) was also assessed, with the results showing that the whole plant of Jingkenuo 2000 waxy maize at the wax stage was most suitable for use as silage. The experiment conducted in the present study was carried out in a completely random block design with two additives and three fertilizer application conditions: no-additive + conventional fertilization treatment (CKCK); no-additive + conventional fertilization + drip irrigation of bacterial solution treatment (CKJJ); no-additive + conventional fertilization + microbial organic fertilizer treatment (CKYJ); additive + conventional fertilization treatment (FJCK); additive + conventional fertilization + drip irrigation of bacterial solution treatment (FJJJ); and additive + conventional fertilization + microbial organic fertilizer treatment (YJFJ). Additionally, the nutrient composition, fermentation quality, and bacterial community structure of the silage fermentation treatments were analyzed. The results indicate that there was significant interaction between the additive and fertilization treatments, with them significantly influencing the parameters CP, EE, NDF, ADF, and RFV (p < 0.01). In particular, the treatment combining additives, conventional fertilization, and drip irrigation of bacterial solution exhibited the highest CP, EE, and starch (p < 0.01) among all the tested treatments, while also displaying the lowest NDF and ADF contents (p < 0.01). Furthermore, this treatment reduced the pH value (p < 0.01), decreased bacterial diversity, and fostered the growth of Lactobacillus. Overall, the findings presented herein demonstrate that, through precise nutritional accumulation monitoring and scientific biological pretreatment methods, Jingkenuo 2000 waxy maize has the potential to become a high-quality silage feed.
1. Introduction
In countries with well-developed animal husbandry practices, the cultivation area of silage maize exceeds 40% [1]. In China, for example, the animal husbandry industry has experienced rapid growth, with the scale of livestock breeding continuously expanding [2]. However, the Chinese feed industry still lags behind the highly intensive animal husbandry industry [3]. Notably, the feed crop cultivation sector is particularly underdeveloped, with both natural pastures and cultivated forages being insufficient in meeting the growing demands of the animal husbandry industry. Whole-plant corn silage (WPCS) has emerged as a crucial coarse feed for ruminants owing to its excellent palatability and high protein and energy content [4].
Waxy maize is one of the main coarse food grains grown in China; however, the development of the waxy corn processing industry is relatively slow. Jingkenuo 2000 waxy maize was the first such variety to achieve international certification in China, characterized by high yield and high quality and having widespread application [5]. Researchers have highlighted that the silage feed indices of waxy corn, such as its crude protein and crude fat content, are superior to those of regular corn [6]. Waxy maize starch, which contains almost 100% amylopectin content in its endosperm, is characterized by higher digestibility and palatability [7]. Notably, waxy maize whole-plant corn silage could also be used as roughage for ruminants.
Owing to the high cellulose content in its stems and the abundance of starch in its grains, the nutritional profile of whole-plant corn, characterized by both high fiber and high energy content, renders it an ideal cultivated feed crop [8]. Numerous studies on corn silage have been conducted to date; however, the silage characteristics of different tissues of the corn plant have yet to be elucidated. The accumulation of nutrients in feed plays a vital role during the plant growth stage. Furthermore, the improvement of maize silage quality is not limited to the post-processing of feed materials; the early planting process is also vital in improving the feeding quality of forage grass. In maize, a number of microbial fertilizers have been studied, such as single microbial fertilizers, compound microbial fertilizers, and bio-organic fertilizers. At present, there are a substantial number of studies on the growth promotion and disease prevention effects of microorganisms and microbial organic fertilizers on corn. The beneficial bacteria in these fertilizers primarily promote plant growth through different metabolic activities [9,10]. For example, bacterial fertilizers dominated by Bacillus subtilis not only increase the yield of corn but also enhance its protein and cellulose content [11].
The principle of silage is to create anaerobic conditions that promote the growth of lactic acid bacteria (LAB) by compacting and sealing the feed. During this process, oxygen levels are reduced and lactic acid (LA) accumulates, effectively inhibiting aerobic and harmful microorganisms [12]. However, in general, the LA content in silage is below 1%; it is therefore necessary to add LAB to enhance fermentation [13]. Supplementing silage with LAB such as Lactobacillus plantarum can expedite the fermentation process, increase LA production, and reduce the pH level [14]. Across different types of silage, LAB inoculants modulate the microbial community composition through various mechanisms, with them being influenced by the epiphytic microorganisms present in fresh forage. These inoculants streamline the interactions among bacterial populations to enhance fermentation quality [15]. The LAB utilized as silage inoculants not only enhance the quality and aerobic stability of silage but also play a probiotic role in the animal digestive system [16]. After microbial fermentation, the feed will produce a particularly pleasant smelling wine fragrance, which stimulates animals’ appetite and increases feed intake [17]. By using microbial feed additives in forage feed, macromolecular substances can be degraded and transformed to a certain extent [1]. As a result, the biological feed ultimately contains a large number of bacterial proteins, active peptides, and other nutrients, in addition to active enzymes and some unknown growth factors. Consequently, acid detergent fiber (ADF) [18,19] and neutral detergent fiber (NDF), which are not easily digestible, can also be degraded to a certain extent, thereby effectively improving the nutritional value of the feed [20].
While some researchers have explored the effects of specific planting methods on crop yields, silage quality, and economic benefits, a comprehensive and integrated approach that combines these aspects is still in the early stages of development [21]. The authors of existing studies primarily concentrate on enhancing single elements, such as specific cultivation techniques or the use of certain additives, rather than fully evaluating the overall influence of probiotic-based cultivation and additive application in silage production. Moreover, there is limited research on improving silage fermentation quality throughout the entire process of growth, fertilization, and fermentation. In this study, we examined the nutrient accumulation and feed value of each tissue and the whole plant of Jingkenuo 2000 waxy maize at different stages of growth. We also evaluated the quality and dynamic changes of the bacterial community of whole-plant corn silage. We aimed to explore the influence of probiotics on the quality improvement of whole-plant corn feed from the planting stage to the fermentation stage. We explore the feasibility of using Jingkenuo 2000 waxy maize as silage and provide scientific data for its further development and utilization as a potential silage feed.
2. Materialsand Methods
2.1. Planting Experimental Design
The experimental material Jingkenuo 2000 waxy maize was cultivated by the Corn Research Center of the Beijing Academy of Agriculture and Forestry Sciences (Beijing, China). It is a fresh corn variety with an average growth period of 90 days. Microbial organic fertilizers were made of Bacillus subtilis (application of 1 × 1012 cfu/g), straw, mushroom residue, and other raw materials. The liquid microbial agents uesed Bacillus subtilis (application of 1 × 1012 cfu/g). The preparations were developed and provided by the Institute of Applied Microbiology, Xinjiang Academy of Agricultural Sciences.
From 29 April 2023 to 28 July 2023, the field trial was was carried out in the National Agricultural Urumqi Observation and Experiment Station (Comprehensive test Ground of Xinjiang Academy of Agricultural Sciences in the northern suburbs of Urumqi City), which is located at 87°28′ E, 45°56′ N and 590 m above sea level. The climate at the trial site was temperate continental climate, with maximum and minimum temperatures ranging between 40.4 °C and 10.50 °C. The total rainfall was between 55.8 mm and 180.2 mm, and the relative humidity was between 30.9% and 75.3%. In the experiment, three different fertilization treatments were set up, with 3 replications for each treatment, resulting in a total of 9 plots. Each plot area was 60 m2, measuring 12 m × 5 m. The row spacing was 40 cm, the plant spacing was 20 cm, one seed was planted in each hole, and the sowing depth was 3–4 cm. To avoid interference from nutrient diffusion, all plots were spaced within 2 m of each other. Maize was sown on 28 April 2023 at a seed rate of about 67,500 plants ha−1. According to different fertilization conditions, the experiment was divided into three treatments:
- (1)
- Conventional fertilization treatment (CK)
During the experiment, fertilization and drip irrigation were carried out according to the local conventional planting technology. The application rates were 1.67 kg ha−1 of diammonium phosphate and 0.67 kg ha−1 of compound fertilizer. A total of 60 days after sowing, the soil was watered seven or eight times, with an interval of 10–15 days between each watering. Nitrogen fertilizer was applied with water in the first three waterings.
- (2)
- Conventional fertilization + drip irrigation bacterial solution treatment (JJ)
Based on the application of conventional fertilizers, 60 days after sowing, the soil was watered seven or eight times, at an interval of 10–15 days. Nitrogen fertilizer was applied with water in the first three waterings. Additionally, a liquid microbial agent was added with water at a rate of 0.1 L ha−1.
- (3)
- Conventional fertilization + microbial organic fertilizer treatment (YJ)
Based on the application of conventional fertilizers, the amount of microbial fertilizer was 4 kg ha−1, calculated according to the nitrogen content.
2.2. Tissue Sample Collection
Collection of whole plant, stem and leaf tissue samples. Samples were collected at the vegetative stage (V3), the large tassel stage (V12), the milk stage (R3), and the waxy stage (R5) stage. Whole corn plants above 20 cm from the ground were collected. To ensure the statistical significance of the samples, at least 30 samples were collected for each treatment.
Collection of grain samples. Samples were collected at the R3 stage (divided into three periods, with samples collected from 5 to 10 days, namely, R31, R32, and R33 (corresponding to the time of leaf and stem collection at the R3 stage)) and R5 stage. To ensure statistical significance of the samples, at least 10 samples were collected for each treatment. The samples were brought back to the laboratory at 4 °C. Fresh tissues were stripped, cut into small pieces, mixed evenly, and naturally dried in the sun. They were then ground finely through a 60-mesh sieve and thoroughly mixed; 200 g was taken, and the quartered method was used to test the relevant indexes.
The test samples were categorized based on the fertilization treatment groups as follows: conventional fertilization treatment (CK), conventional fertilization + drip irrigation bacterial liquid treatment (JJ), and conventional fertilization + microbial organic fertilizer treatment (YJ).
2.3. Fermentation Sample Collection
The whole plant samples were collected at the wax ripening stage (R5), and the stalks above 20 cm from the ground were collected. The entire corn plant was thoroughly ground to ensure that the whole-plant pieces were 2–3 cm in length and then fermented for use as whole-plant sliage.
The silage compound bacteria agent was prepared by combining of Lactobacillus plantarum, Lactobacillus buchneri, Bacillus coagulans, Lactobacillus casei, and cellulose enzyme preparations, which was provided by the Institute of Microbiology, Xinjiang Academy of Agricultural Sciences. In the stage of maize wax ripening (R5), the whole-plant samples were harvested under three fertilization treatments. The two silage additive levels: (1) no-additive control (CK); (2) addition of silage compound bacteria agent (FJ, the viable number of probiotics reached 1012 CFU/g, and one gram of the agent can treat one ton of silage). The whole-plant corn silage materials were divided into 6 treatments according to different fertilization and fermentation treatments, which were as follows: no-additive + conventional fertilization treatment (CKCK); no-additive + conventional fertilization + drip irrigation bacterial solution treatment (CKJJ); no-additive + conventional fertilization + microbial organic fertilizer treatment (CKYJ); additive + conventional fertilization treatment (FJCK); additive + conventional fertilization + drip irrigation bacterial solution treatment (FJJJ); additive + conventional fertilization + microbial organic fertilizer treatment (YJFJ). The fermentation conditions of whole plant silage were 60% moisture content, materiel length no more than 2 cm, 15 samples were prepared for each treatment using a space cup, and anaerobic solid fermentation was carried out at room temperature for 30 days. Nutrient composition sample collection. Six treatments were performed. On day 30, random samples were taken for each treatment and repeated three times.
2.4. Chemical Composition
A total of 200 g of both fresh and ensiled materials were collected, subjected to heating at 105 °C for 30 min, and subsequently dried at 65 °C until reaching a constant weight. The dried samples were then moved to a desiccator, cooled to room temperature, weighed, and their DM content was calculated. These samples were further processed by grinding and sieving through a 40-mesh sieve (with a pore size of 0.425 mm). CP was assessed using the Kjeldahl nitrogen method. NDF and ADF were evaluated using van Soest’s fiber analysis technique [22]. EE was determined via the Soxhlet fat extraction method [23]. Starch content was quantified using the acid hydrolysis-DNS method. Based on these parameters, the relative feed value (RFV) of the forage was calculated [24] using the following formulas.
Here, DMI represents the random consumption of dry matter from roughage, and DDM refers to the digestible dry matter.
A 10 g sample was collected from each bag of silage after thorough manual mixing within the bag. The sample was then combined with 90 mL of sterilized water and vigorously shaken at 180 rpm for 2 h at 4°C. The mixture was filtered through a 0.45 µm membrane [25]. The pH value of the resulting silage extract was determined using a calibrated glass electrode pH meter (Mettler Toledo Co., Ltd., Shanghai, China). The organic acid contents (lactic acid (LA), acetic acid (AA), propionic acid (PA) and butyric acid (BA)) were determined using high-performance liquid chromatography (Prominence LC-20AT, SHIMADZU, Kyoto, Japan).
2.5. Analysis of Bacterial Communities in Fermented Feed
For bacterial diversity sample collection, 150 mL of phosphate buffer solution was combined with the sample and mixed at 30 °C for 20 min at a speed of 150 rpm. The mixture was then filtered through a four-layer gauze. The filtrate was subjected to centrifugation at 10,000 rpm for 10 min at 4 °C. The resulting sediment was transferred to 1.5 mL sterile centrifuge tubes and stored at −80 °C for subsequent DNA extraction. DNA extraction was carried out using the Tiangen Genomic DNA Extraction Kit, and the quality of the extracted DNA was assessed via 1% agarose gel electrophoresis. The full-length 16S rRNA gene was amplified using the forward primer 27F (5′-AGRGTTTGATYNTGGCTCAG-3′) and the reverse primer 1492R (5′-TASGGHTACCTTGTTASGACTT-3′). Sequencing of the bacterial DNA fragments was performed bidirectionally on the Illumina NovaSeq platform. Non-redundant sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity using the QIIME2 software. A QIIME-based OTU abundance matrix was constructed, and four commonly used alpha diversity indices (Ace, Chao1, Shannon, and Simpson) were calculated. Principal component analysis (PCA) was performed on each sample group to study similarities and differences in community composition. Linear discriminant analysis effect size (LEfSe) was used to compare differences between the six groups at the genus level on day 30.
2.6. Statistical Analyses
Utilize Microsoft Excel 2019 for the collection, organization, and analysis of experimental data. To assess the impact of fertilization conditions and the addition of silage compound bacteria agents on the nutritional and fermentation quality of whole-plant silage, a two-way ANOVA was employed, considering the interaction effects between these factors. Post hoc comparisons between treatments were conducted using the LSD test at a significance level of p < 0.05. All statistical analyses were performed using IBM SPSS 27.0.
3. Results
3.1. Nutrient Accumulation in the Tissues and Whole Plant
The effects of different fertilization conditions on the nutrient accumulation in each tissue and the whole plant were evaluated. The DM content in the stems, leaves, and grains was the lowest at the V3 stage (p < 0.05) (Figure 1A). At the R5 stage, the DM content in the stems under JJ treatment and YJ treatment was higher than that under CK treatment (p < 0.05) (Figure 1A). The DM content in the leaves under JJ treatment and YJ treatment reached its peak at the V12 stage (p < 0.05) and was higher than that under CK treatment (p < 0.05) (Figure 1A). From the V12 stage to the R5 stage, the DM content accumulation in the leaves under the three treatment conditions was relatively stable, with no significant increase (Figure 1A). The accumulation of DM in the grains and the whole plant gradually increased at different stages of growth, peaking at the R5 stage (p < 0.05) (Figure 1A). The DM content of the whole plant under JJ treatment and YJ treatment conditions was significantly higher than that under CK treatment conditions (p < 0.05) (Figure 1A). Throughout the entire growth period, the starch content in the stems, leaves, grains, and whole plant across each treatment condition showed an upward trend, peaking at the R5 stage (p < 0.05) (Figure 1B). There was no significant difference in starch content among the treatments at the different stages of growth (p > 0.05) (Figure 1B). At the R5 stage, the starch content in the whole plant across each treatment condition was 30.37%, 30.28%, and 30.84%, respectively (Figure 1B). The NDF content of the stems, grains, and the whole plant across the three fertilization treatments exhibited an increase; in comparison, the NDF content of the leaves showed a decrease in all treatment conditions (Figure 1C). Despite the increase in NDF content in the grains, it remained relatively lower compared to that of the stems and leaves (Figure 1C). At the R5 stage, the NDF content of the whole plant across each treatment condition was 53.30%, 54.10%, and 54.80%, respectively (Figure 1C), with no significant difference observed among all treatments (p > 0.05) (Figure 1C). The ADF content of the stems, leaves, and the whole plant under the three treatments showed an upward trend in all cases (Figure 1D). The ADF content of the grains was relatively lower than that of the stems and leaves (Figure 1D). The cumulative trend of ADF content in the whole plant was CK > JJ > YJ (Figure 1D). The CP content in the stems and leaves gradually decreased from the V3 to the R5 stage, reaching the lowest level at the R5 stage (p < 0.05) (Figure 1E). The CP content of the grains was relatively stable throughout the R3 period and significantly decreased at the R5 stage (p < 0.05) (Figure 1E). The CP content of the whole plant also showed a decreasing trend throughout the entire growth period, reaching the lowest level at the R5 stage (p < 0.05) (Figure 1E). The CP content across the three fertilization treatments was 8.02%, 8.05%, and 8.12%, respectively (Figure 1E), and the CP content under JJ treatment and YJ treatment was significantly higher than that under CK treatment (p < 0.05) (Figure 1E). Throughout the entire growth period, the EE content in the stems, leaves, grains, and the whole plant across each treatment condition showed an upward trend, reaching a peak at the R5 stage (p < 0.05) (Figure 1F). There was no significant difference in the EE content of the whole plant among all treatment conditions (p > 0.05), amounting to 5.54%, 5.62%, and 5.69%, respectively (Figure 1F).
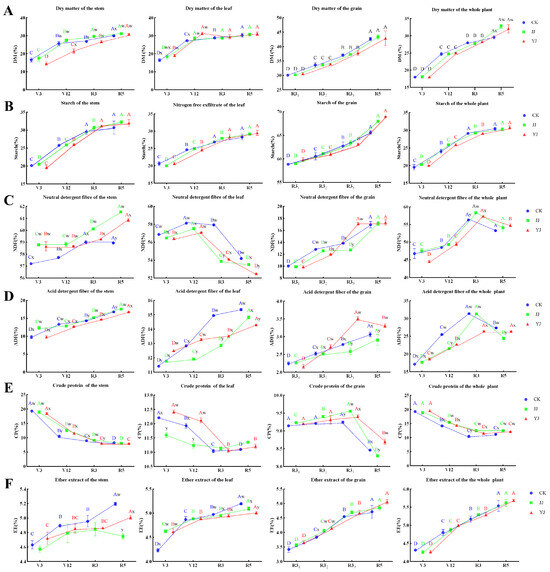
Figure 1.
The accumulation of dry matter in different fertilization treatments of Jingkenuo 2000 across various tissues and the whole plant: (A) dry matter; (B) starch; (C) neutral detergent fiber; (D) acid detergent fiber; (E) crude protein; (F) ether extract. CK represents conventional fertilization treatment; JJ represents conventional fertilization + drip irrigation bacterial liquid treatment; YJ represents conventional fertilization + microbial organic fertilizer treatment. V3 represents the vegetative stage; V12 represents the large tassel stage; R31 represents the pre-milk stage; R32 represents the mid-milk ripening stage; R3 (R33) represents the milk stage; and R5 represents the waxy stage. A, B, C, and D represent significant differences in data among different growth stages under the same fertilization treatment to the level of significance of p < 0.05. w, x, and y represent significant differences in data of different fertilization treatments in the same growth period to the level of significance of p < 0.05.
As shown in Table 1, the relative feed value (RFV)—an important indicator for measuring the intake and energy value of forage—of the different tissues and the whole plant under different fertilization conditions and at different growth stages was analyzed. The RFV of the stems, grains, and whole plant gradually decreased with progression of the growth stage; however, the RFV of the leaves first decreased and then increased (Table 1). At the R5 stage, the RFV of the stems was the highest under CK treatment (p < 0.01), and the RFV of the leaves was highest under YJ treatment (p < 0.01) (Table 1). There was no significant difference in the RFV of the whole plant among the treatments at the R5 stage (p > 0.05) (Table 1), and the RFV varied within the range of 116.00 to 120.21 (Table 1). In addition, the DM and starch content of the whole plants also reached 30% at the R5 stage (Figure 1A,B). The whole-plant samples at the R5 stage were therefore collected for subsequent fermentation experiments. By analyzing the accumulation patterns of nutritional components, the optimal harvest time for the subsequent fermentation test of Jing-kenuo 2000 waxy maize was determined. The above findings will aid in the optimization of fertilization conditions at the planting stage of Jingkenuo 2000 waxy maize for use as silage feed.

Table 1.
The RFV of various tissues and the whole plant.
3.2. Nutrient Accumulation in the Tissues and Whole Plant
The effects of different fertilization conditions and silage compound bacterial agent additives on silage nutritional quality were examined (Table 2). The silage compound bacterial agent additives did not significantly (p > 0.05) affect the nutritional quality and feed value of the silage (Table 2). In terms of fertilization conditions, the different fertilization conditions had significant (p < 0.05) effects on the starch content and RFV of the silage (Table 2). In addition, there was a significant interaction between NDF, ADF, CP, EE, starch, and the RFV of the silage in F (fertilization conditions) × A (addition of silage compound bacterial agent) (p < 0.05) (Table 2). Upon the addition of the compound bacterial agent to the silage, the CK fertilization treatment exhibited the highest DM content (p < 0.05) (Table 2). The whole plant materials from the JJ treatment group were employed as the raw materials. Compared with the treatment with the omission of the silage compound bacteria (CK), the CP content of the treatments with the addition of the silage compound bacterial agent increased by 9.15% and 8.49%, respectively (Table 2). Similarly, the EE content of the additive compound bacterial agent treatments also, respectively, increased (Table 2). The additives also resulted in the starch content exceeding 30% in all treatments (Table 2). The silage compound bacterial agent was able to digest the NDF and ADF. Specifically, the NDF and ADF contents in the FJ fermentation + JJ fertilization treatment were reduced to the lowest levels among all treatments, amounting to 30.62% and 19.94%, respectively (Table 2). The RFV of the silage varied within the range of 138.05% to 222.99%, with the FJ fermentation + JJ fertilization treatment achieving the highest RFV of 222.99% (p < 0.01) (Table 2). The RFV of the whole plant harvested at the R5 stage under each fertilization treatment was notably elevated post-fermentation, irrespective of whether the silage compound bacterial agent was added or omitted (Figure 2). Notably, the enhancement in the RFV was more significant when the silage compound bacterial agent was incorporated as opposed to when it was omitted. Among the treatments, FJJJ treatment demonstrated the most substantial increase in the RFV, resulting in an improvement of 102.78% (Figure 2). The above findings indicate that the synergistic interaction between the FJ fermentation treatment and the JJ fertilization treatment markedly augments the feeding value of the silage.

Table 2.
Effects of additives on the nutritional quality (DM-basis) of silage under different fertilization conditions.
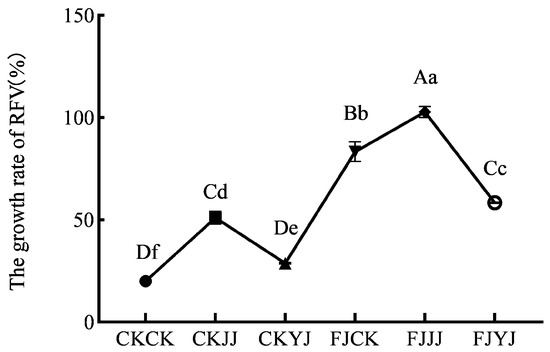
Figure 2.
The growth rate of RFV. CK, no-additive control; FJ, application of silage compound bacteria agent. CK, JJ, and YJ represent different fertilization conditions. Capital letters indicate significant differences between varieties under the same treatment (p < 0.01). Lowercase letters indicate significant differences among different treatments of the same variety (p < 0.05).
3.3. Fermentation Quality of the Silage
The effects of different fertilization conditions and silage compound bacterial agent additives on silage fermentation quality were evaluated (Table 3). In terms of the addition of silage compound bacterial agents and fertilization conditions, each one had no significant impact on the fermentation quality of the silage (p > 0.05) (Table 3). There was a highly significant interaction between the LA of the silage in F × A treatment (p < 0.01) (Table 3). After fermentation, the pH values of all treatments were lower than 4.0, with the pH value of the JJ fertilization treatment including the compound bacterial agent being the lowest (p < 0.01) (Table 3). When adding the compound bacterial agent to the silage, the LA content of each fertilization treatment increased by 13.06%,13.79%, and 1.40% (Table 3). These results provide evidence that the quality of silage fermentation was optimized under the combined influence of silage compound bacterial agent treatment and fertilization treatment.

Table 3.
Effects of additives on the fermentation quality (DM-basis) of silage under different fertilization conditions.
3.4. Correlation Analysis Between Silage Nutritional Quality and Fermentation Quality
Pearson correlation analyses were performed to determine the relationship between the silage nutritional quality and fermentation quality (Figure 3). The LA content of the silage showed a significant positive correlation with the CP and starch content (Figure 3). The NDF content of the silage showed a significant positive correlation with the ADF content and a negative correlation with CP, EE, and starch content (Figure 3). Similarly, the ADF content of the silage showed a negative correlation with CP, EE, and starch content (Figure 3). CP content was positively correlated with EE and starch content (Figure 3), and EE content was positively correlated with starch content (Figure 3).
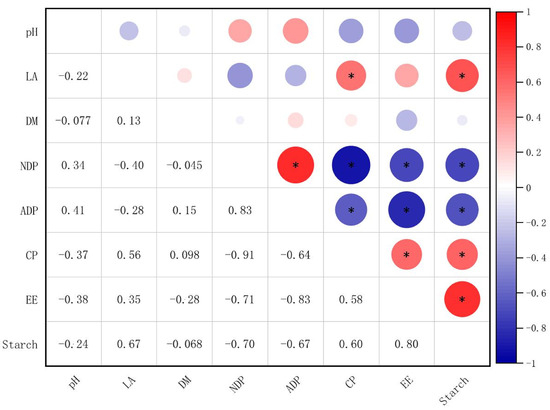
Figure 3.
Correlation between silage nutritional quality and fermentation quality. The corresponding values of the heatmap are the Pearson correlation coefficient r (−1 to 1), with a value below 0 representing a negative correlation (blue) and a value over 0 representing a positive correlation (red). (*) indicates p < 0.05.
3.5. Bacterial Diversity and Community Composition of the Whole-Plant Corn Silage
The alpha diversity of dynamic whole-plant corn silage bacterial communities was analyzed (Table 4). The sequencing coverage for all samples exceeded 99%, indicating sufficient sequencing depth to enable comprehensive characterization of the bacterial community and supporting effective analysis of its diversity (Table 4). Similarly, the ace index value of the FJCK treatment was lower than that of the FJJJ and FJYJ treatments (p < 0.01). The ace index value of the CKCK treatment was lower than that of the CKJJ and CKYJ treatments (p < 0.05) (Table 4). The chao1 index value of the CKJJ treatment was found to be lower than that observed in the other fertilization treatments (p < 0.05). However, no significant difference was detected between the CKCK treatment and the CKYJ treatment (Table 4). There was also no significant difference between FJJJ treatment and FJYJ treatment; however, the chao1 index value of FJCK treatment was the lowest among the different fertilization treatments (p < 0.05) (Table 4). The Shannon index value of the CKJJ treatment was lower than that of the CKCK and CKYJ treatments (p < 0.05), with the Shannon index value of the FJJJ treatment being the lowest (p < 0.01) among the three fermentation treatments (Table 4). The Simpson index value of the CKJJ treatment was significantly higher than that of the CKCK and CKYJ treatments (p < 0.01), with the Simpson index value of the FJJJ treatment being the highest (p < 0.01) among the three fermentation treatments (Table 4).

Table 4.
Alpha diversity index of bacterial community during whole-plant silage fermentation.
The alterations in the microbial community of whole-plant corn silage were further substantiated through beta diversity analysis by utilizing Principal Coordinates Analysis (PCoA) (Figure 4A). The difference interpretation of the PC1 and PC2 samples was 46.04% and 23.40%, respectively. FJCK treatment produced distinctly separate results compared with the other treatments. Notably, the JJ treatments, both with and without the compound bacterial agent, clustered together, as did the CKCK, CKYJ, and FJYJ treatments (Figure 4A). A similar trend can be seen in Figure 4B.
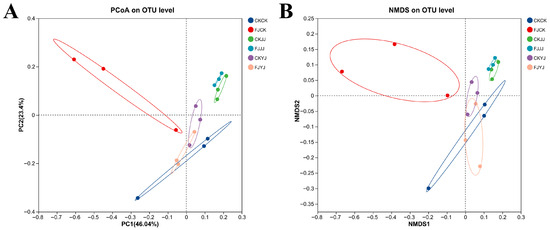
Figure 4.
Analysis of bacterial diversity in whole-plant corn silage under different treatments: (A) differences in bacterial community structure; (B) differences in bacterial community structure. CK, no-additive control; FJ, application of silage compound bacteria agent. CK, JJ, and YJ represent different fertilization conditions.
Through subsequent analyses, we explored the taxonomic profile of bacterial communities within the silage that had undergone various treatments, with particular attention paid to their phylum and genus classifications. The predominant phyla identified were Firmicutes and Proteobacteria (Figure 5A). In particular, the analysis revealed that FJCK treatment resulted in greater abundance of Proteobacteria compared to the other treatment groups, standing at 40.23% (Figure 5A). At the genus level, Lactobacillus was found to be enriched in the FJJJ treatment group; in comparison, FJCK treatment resulted in comparatively lower abundance of Lactobacillus (Figure 5B). Weissella, a common bacterium found during fermentation, was enriched in all treatments to some extent (Figure 5B). The abundance of this bacterium in the treatment group without the addition of the silage compound bacterial agent was higher than that in the treatment group with the compound agent added (Figure 5B). Ochrobactrum and Paenibacillus were both significantly enriched in the FJCK treatment group (Figure 5B).
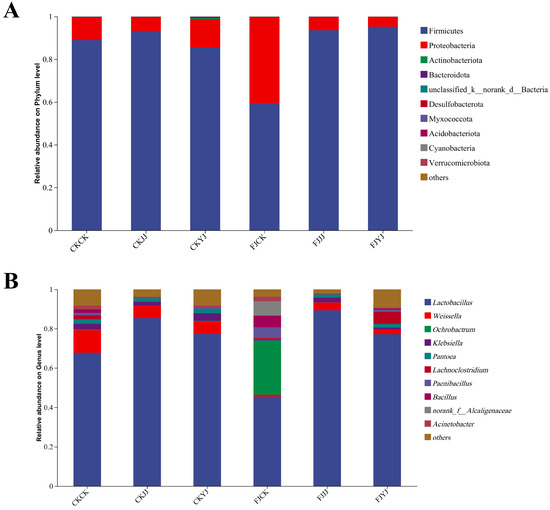
Figure 5.
Bacterial communities in the silage samples: (A) bacterial community at the phylum level; (B) bacterial communities at the genus level. CK, no-additive control; FJ, application of silage compound bacteria agent. CK, JJ, and YJ represent different fertilization conditions.
To further elucidate the microbial community differences induced by the application of additives across varying fertilization regimes, LEfSe analysis was performed to identify the enrichment of bacteria with an LDA score exceeding 3.5 at the genus level (Figure 6). The results showed that Lactobacillus was predominantly enriched in FJJJ treatment across all fertilization treatments. In the CKCK treatment group, Weissella, Lysinibacillus, and Enterobacter were enriched. In addition, Ochrobactrum and Paenibacillus were more abundant in the FJCK treatment group. In the CKJJ treatment group, one bacterial taxon was significantly enriched. Specifically, Leuconostoc obtained the highest LDA score in all treatments. In the CKYJ treatment group, Klebsiella, Rosenbergiella, Lactococcus, Chryseobacterium, and Sphingobacterium were enriched. In the FJYJ treatment group, Lachnoclostridium and Clostridium_sensu_stricto_1 were significantly enriched. These results demonstrate the distinct microbial community structures induced by the different treatments.
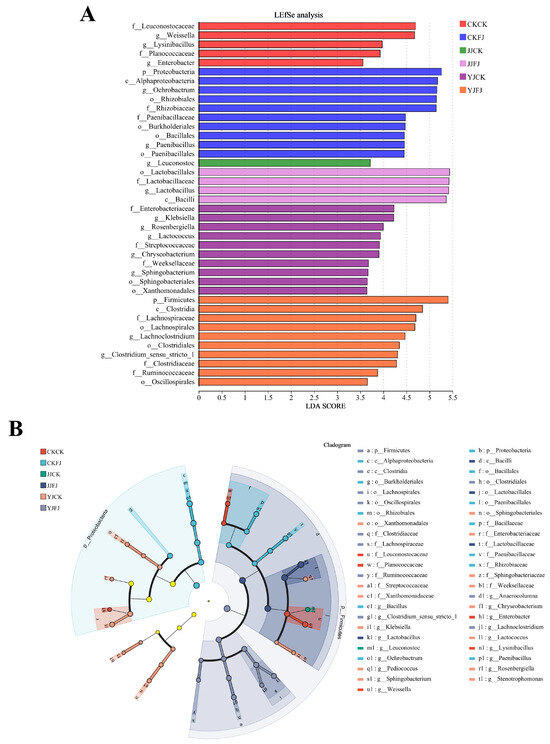
Figure 6.
The microbial community differences are attributable to the use of additives across varying fertilization conditions. (A) The LDA discriminant analysis. CK, no-additive control; FJ, application of silage compound bacteria agent. CK, JJ, and YJ represent different fertilization conditions. (B) The cladogram generated using LEfSe to identify specific microbial communities within silage. Each color-coded node signifies a microbial taxon that shows significant enrichment in its respective group and plays a crucial role in distinguishing between groups.
3.6. Correlation Between Fermentation Parameters and Bacterial Communities
A correlation analysis was performed to provide a visual depiction of the connections among chemical constituents, fermentation conditions, and primary fermentation outcomes at the genus level. The results highlighted several notable relationships. Specifically, CP displayed a negative correlation with Weissella, with LA and starch also being negatively correlated with this bacterium (Figure 7). Furthermore, Acinetobacter and Ochrobactrum were found to be negatively correlated with EE (Figure 7). A significantly negative correlation between DM and Pantoea was found (Figure 7). Moreover, NDF was found to have a negative correlation with Acinetobacter, Weissella, and Klebsiella (Figure 7). pH exhibited a positive correlation with Ochrobactrum, Paenibacillus, and Bacillus but displayed a negative correlation with Lactobacillus (Figure 7). Additionally, ADF was found to have a positive correlation with Acinetobacter and Ochrobactrum, while also displaying a negative correlation with Lactobacillus (Figure 7). Starch demonstrated a positive correlation with Lachnoclostridium (Figure 7). These findings provide substantial insights into the connections between various factors and microbial communities, shedding light on the complex correlation patterns and dynamic shifts that occur during fermentation.
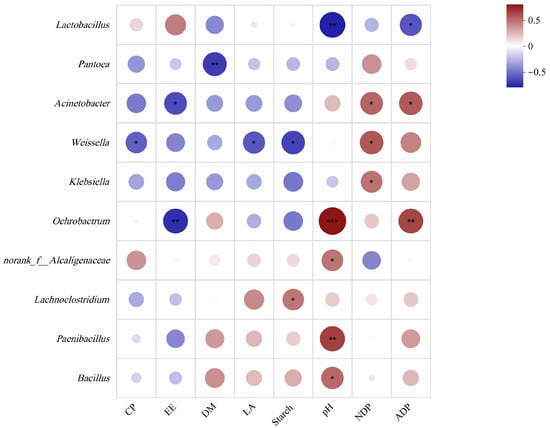
Figure 7.
Correlation between the bacterial genera and fermentation parameters of different silage groups. Heat map based on Spearman correlation coefficients. Blue indicates a negative correlation, and red indicates a positive correlation. (*) indicates p < 0.05; (**) indicates p < 0.01; (***) indicates p < 0.001.
4. Discussion
In the present study, we have departed from the traditional research paradigm that either concentrates solely on enhancing the yield of silage corn through cultivation practices or focuses exclusively on improving silage quality by optimizing fermentation conditions. Instead, we have innovatively integrated these two approaches. The results presented herein confirm our hypothesis that the concurrent application of microbial fertilizer during the planting phase and the addition of a silage compound bacterial agent during the fermentation phase significantly enhance the feed quality of Jingkenuo 2000 waxy maize when used as silage. These findings provide a robust scientific basis for the combined use of fertilization and fermentation techniques in the production of Jingkenuo 2000 waxy maize silage.
With the rapid advancement of the livestock industry in China, corn silage has garnered increasing attention from researchers. Although researchers have made significant efforts in breeding different corn silage varieties, the demand for corn silage is still not fully met. Fresh waxy corn is rich in nutrients [26] and can be ensiled, thus providing a valuable reference for the diversified and efficient development of the fresh waxy corn industry.
Analyzing the forage quality of the different tissues of corn from a whole-plant perspective provides a valuable reference for future efforts aimed at screening varieties with appropriate source–sink ratios and superior nutritional quality, in addition to breeding high-quality silage corn varieties [19]. In the present study, we found that the accumulation trends of NDF and CP content in the leaves exhibited the opposite trend to those in other plant tissues. This finding implies the possible existence of a source–sink relationship in corn, wherein the leaves may act as nutrient suppliers to the grains or other tissues, as previously reported by Hahnen et al. [27], who demonstrated in their study that leaves and bracts are the primary sources of dry matter for the whole plant and serve as temporary storage sites for nutrients for the grains. We also found that the accumulation of dry matter (DM) in the leaves reached a plateau from the V12 stage to the R5 stage, whereas DM accumulation in the grains continued to increase from the R3 stage to the R5 stage (Figure 1A). Further research is required to determine whether the leaves provide DM for the grains, as there exists very little research in this particular field. Furthermore, we evaluated the accumulation of nutritional components and the feeding value of each tissue and the whole plant of Jingkenuo 2000 waxy maize, which offers a reference for its application in silage production. By assessing the DM and starch content and the RFV of the whole plant, we determined that the optimal harvest period is the R5 stage.
The authors of most recent studies predominantly concentrate on the yield of corn and forage, with comparatively little focus on nutrient accumulation throughout the entire growth period. In our study, neutral detergent fiber (NDF) and acid detergent fiber (ADF) reached their highest levels at the R3 stage and subsequently remained relatively stable or experienced a slight decrease at the R5 stage (Figure 1C,D). This increase could potentially contribute more effectively to enhancing corn’s lodging resistance [28]. As significant indicators for evaluating silage quality [29], lower NDF and ADF levels generally indicate a higher feed nutritional value [30,31]. At the R5 stage, there was no significant difference in NDF among the three fertilization treatments. However, the ADF level was relatively lower in the treatment involving conventional fertilization combined with drip irrigation of liquid microbial agents. In contrast with our findings, some researchers have demonstrated that the ADF content of corn increases with a prolonged harvest period [32]. This finding suggests that our microbial fertilizers may exert a regulatory effect on ADF accumulation. However, further in-depth research is warranted to confirm this hypothesis. The results of our subsequent fermentation experiment showed that, with the addition of the silage compound microbial agent under the three fertilization conditions, the NDF and ADF contents of WPCS both decreased, and FJJJ treatment resulted in the lowest content. Moreover, the interaction between the addition of the silage compound microbial agent and the fertilization treatment significantly affected the reduction in NDF and ADF contents. This finding indicates that the authors of future studies should not only focus on improving feed quality during fermentation but also consider enhancing the quality of ensiled feed by employing different planting or fertilization methods. The production of high-quality corn silage is dependent on nutrient content, including high dry matter yield, high starch levels, substantial levels of crude protein, and low fiber content [33]. The authors of the majority of existing studies generally conduct separate research on the cultivation and silage production of feed corn. In our study, during the cultivation phase, we applied drip irrigation of microbial agents and microbial fertilizer to the corn plants. We subsequently added a silage composite agent to the whole-plant silage production process. Our results showed that during the growth of Jingknuo 2000, key nutrients such as DM [34], starch, EE, and CP [35] began to accumulate gradually, reaching their highest levels in the treatment groups involving liquid bacterial fertilizer and microbial organic fertilizer at the R5 stage. This indicates that the liquid bacterial fertilizer and microbial organic fertilizer, primarily based on Bacillus subtilis, can promote the cellulose content [11]. We also analyzed our results and found that under the interaction of fertilization treatment and the addition of the silage compound bacterial agent, the nutritional factors of silage and the RFV significantly improved. Under FJJJ treatment, in particular, the contents of CP, EE, and starch were relatively high, and the RFV reached a maximum of 222.99% (Table 2). Under microbial organic fertilizer treatment, the RFV of Mexican corn ranges from 118.88% to 127.19% [36]. Our research findings provide a new approach for the utilization of sweet corn for fresh consumption. Sweet corn can be harvested as young stalks for fresh consumption during the milk stage, as silage corn during the dough stage, or as corn for starch processing when it matures. The specific utilization method can be adaptively adjusted according to local production conditions and requirements in the same year.
The use of additives during silage processing can effectively influence the silage fermentation process, thus improving the quality of the silage and facilitating greater control of the silage fermentation process [37]. In this study, the whole-plant silage from the three fertilization treatments was divided into a treatment group with either the addition or omission of silage bacteria. The pH values of the whole-plant corn silage feed in each treatment were maintained at around 3.8, with pH being one of the key indicators for evaluating high-quality silage [38]. However, it is important to note that while natural fermentation can produce effective results, the use of specific types of LAB as additives can improve silage quality further [39]. In this study, the pH value of the whole-plant silage treated with the silage compound bacterial agent was lower than that in which it was omitted. In our study, the pH value was found to exhibit a negative correlation with the abundance of Lactobacillus, a finding that aligns with the study conducted by Mu, et al. [40]. Concurrently, this finding can be attributed to the ability of Lactobacillus species to out-compete other microorganisms for nutrients during the fermentation process, thus efficiently enhancing the production of LA, as reported by Tucak et al. and Dunière et al. [23,41]. As highlighted by Lv et al. [42], maintaining an optimal pH level through the action of LAB is a key factor in ensuring the long-term stability and nutritional value of silage. This factor is particularly important in agricultural practices where silage is used as a feed source for livestock, as it directly impacts both animal health and productivity.
The above results were corroborated by the analysis of the dominant flora during the fermentation process. In the present study, we identified Firmicutes and Proteobacteria as the primary bacterial phyla in the silage, with Firmicutes being significantly more abundant than Proteobacteria (Figure 5A). This finding is consistent with the results of the study by Hu et al. [43]. This shift may potentially be attributed to the increased prevalence of Lactobacillus, which is recognized as being able to flourish in the anaerobic conditions typical of silage fermentation [44]. In this study, the predominant bacterial community in both the FJJJ and FJYJ treatments was Lactobacillus of the Firmicutes phylum. Lactobacillus is recognized for its ability to enhance fermentation quality [45,46] and inhibit the growth of miscellaneous bacteria [46]. We also verified through microbial diversity analysis that the chao1 and ace indices of the treatments with the addition of the silage compound bacterial agent were lower than those of CK, with a marked decrease being observed in bacterial diversity post-fermentation [47]. Overall, FJJJ treatment exhibited the highest proportion of Lactobacillus in the silage, indicating that incorporating the silage compound formulation may amplify the beneficial effects of Lactobacillus on feed fermentation conditions and quality when used in conjunction with conventional fertilization and drip irrigation of microbial solution. The results of other studies have shown that Lactobacillus is recognized for its ability to degrade cellulose and xyloglucan, the essential constituents of plant cell walls [48]. Lactobacillus therefore plays an important role in the degradation and transformation of plant materials. In this study, Lactobacillus exhibited a negative correlation with ADF, with it exhibiting a positive correlation with Acinetobacter and Ochrobactrum, whose mold production led to the failure of silage fermentation [49]. In our experiment, the abundance of Ochrobactrum was relatively high in the FJCK treatment group, and a significant distinction was found between FJCK treatment and the other treatments during PCoA and NMDS analysis. The above results indicate that different fertilization and fermentation treatments affect the quality of feed to varying degrees, findings that may be related to different microbial community structures.
This research breaks through previous research on high-quality silage production that focuses solely on the optimization of conditions during the fermentation stage. By adding microbial fertilizer during the growth stage and silage fermentation additive during the fermentation stage, FJJJ treatment produced the best effects, demonstrating a significant synergistic effect between fertilization and the addition of the silage fermentation additive. However, further validation studies are necessary to confirm these findings, including increasing the amount of fertilizer applied, exploring different types of fermentation treatments, and clarifying the metabolic mechanisms involved in the silage preparation process, to thoroughly assess the impact of planting and fermentation conditions on silage quality improvement. Such efforts will provide more detailed data to support research in related fields.
5. Conclusions
In this study, we examined the nutritional components of various tissues and the whole plant of Jingkenuo 2000 waxy maize at key growth stages. Furthermore, the application of a compound silage additive enhanced the diversity of the microbial community during the silage fermentation process, thereby improving the quality of whole-plant silage of Jingkenuo 2000 waxy maize. Specifically, the combination of the compound silage additive and fertilization treatment stimulated the growth of beneficial bacteria such as Lactobacillus, reduced the pH level, and effectively suppressed the proliferation of harmful microorganisms. Herein, we propose a scientific approach for the effective storage and utilization of the whole-plant silage of waxy maize. However, further research is still necessary in order to elucidate the underlying mechanisms of the fermentation process. The authors of future studies should focus on exploring metabolic pathways and elucidating the mechanisms involved in the silage preparation process.
Author Contributions
M.H., F.Y., W.C., X.H., and D.D. performed the experiments; M.H., Y.L., F.Y., W.C., D.D., L.D., and B.Z. analyzed the results; M.H., W.C., L.D., and B.Z. designed the project and experiments; M.H., Y.L., and F.Y. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project of the post scientist of the National Forage Industry Technology System (grant no. CARS-34), the Key Research and Development Task Special Project of Xinjiang Uygur Autonomous Region (grant no. 2022B02042), and the Project of Fund for Stable Support to Agricultural Sci-Tech Renovation (grant no. xjnkywdzc-2023005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.I.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, H.; Sun, P.; Zhao, B.; Dong, S. Research Progress on Harmless Treatment of Dead Livestock and Poultry with Alkaline Hydrolysis in China. Anim. Husb. Feed. Sci. 2017, 9, 346–354. [Google Scholar]
- Guan, H.; Ran, Q.; Li, H.; Zhang, X. Succession of Microbial Communities of Corn Silage Inoculated with Heterofermentative Lactic Acid Bacteria from Ensiling to Aerobic Exposure. Fermentation 2021, 7, 258. [Google Scholar] [CrossRef]
- Lu, B.-S.; Xu, L.; Zhao, J.-R.; Shi, Y.-X.; Fan, Y.-L.; Xi, S.-L.; Zhang, C.-F.; Shi, Y.-M.; Zhang, X.-J. Breeding and Application of Jingkenuo2000 Series of Waxy Corn Varieties. J. Maize Sci. 2019, 27, 1. [Google Scholar] [CrossRef]
- Barlow, J.; Bernard, J.; Mullis, N. Production response to corn silage produced from normal, brown midrib, or waxy corn hybrids. J. Dairy Sci. 2012, 95, 4550–4555. [Google Scholar] [CrossRef]
- Gao, H.; Gadlage, M.J.; Lafitte, H.R.; Lenderts, B.; Yang, M.; Schroder, M.; Farrell, J.; Snopek, K.; Peterson, D.; Feigenbutz, L.; et al. Superior field performance of waxy corn engineered using CRISPR–Cas9. Nat. Biotechnol. 2020, 38, 579–581. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Z.Z.; Zuo, Y.C.; Wang, H.L.; Wang, Q.F.; Li, Y.; Kou, J.; Du, Z.H. Silage Characteristics of Different Corn Plant Parts and Strategies for Improving Their Silage Quality. Acta Agrestia Sin. 2023, 31, 2275–2286. [Google Scholar] [CrossRef]
- Amanullah, A.; Khan, A. Phosphorus and Compost Management Influence Maize (Zea mays) Productivity Under Semiarid Condition with and without Phosphate Solubilizing Bacteria. Front. Plant Sci. 2015, 6, 1083. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Farooq, H.M.; Hussain, M. Bacteria in combination with fertilizers promote root and shoot growth of maize in saline-sodic soil. Braz. J. Microbiol. 2015, 46, 97–102. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Kasimatis, C.N.; Kakabouki, I.; Leonidakis, D.; Danalatos, N.; et al. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 2022, 12, 11598. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Jiang, Y.; Wang, L.; Wang, S.; Zhang, Z.; Tong, X.; Wang, S. Effects of Different Soybean and Maize Mixed Proportions in a Strip Intercropping System on Silage Fermentation Quality. Fermentation 2022, 8, 696. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2022, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, K.D.; Choi, K.C. Role of lab in silage fermentation: Effect on nutritional quality and organic acid production—An overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Chauhan, N.; Kumari, N.; Mani, V.; Pradhan, D.; Gowane, G.R.; Kumar, S.; Tyagi, N. Effects of Lactiplantibacillus plantarum, Limosilactobacillus fermentum, and Propionic Acid on the Fermentation Process Of Sugarcane Tops Silages Along with Variations in pH, Yeast and Mould Count After Aerobic Exposure. Waste Biomass Valorization 2024, 15, 2215–2230. [Google Scholar] [CrossRef]
- Momota Rani, D.; Brigitta, W.; Annette, L. Method development to reduce the fiber content of wheat bran and rice bran through anaerobic fermentation with rumen liquor for use in poultry feed. Asian-Australas. J. Anim. Sci. 2019, 32, 395–404. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanaguri, Y.; Smith, R.L. Hydrothermal separation of lignin from bark of Japanese cedar. J. Supercrit. Fluids 2018, 133, 696–703. [Google Scholar] [CrossRef]
- Wang, Y. Study on the correlation of the different organs and the yield and quality in silage maize. Chin. Agric. Sci. Bull. 2015, 31, 22–27. [Google Scholar] [CrossRef]
- Cherdthong, A.; Khonkhaeng, B.; Seankamsorn, A.; Supapong, C.; Wanapat, M.; Gunun, N.; Gunun, P.; Chanjula, P.; Polyorach, S. Effects of feeding fresh cassava root with high-sulfur feed block on feed utilization, rumen fermentation, and blood metabolites in Thai native cattle. Trop. Anim. Health Prod. 2018, 50, 1365–1371. [Google Scholar] [CrossRef]
- Edson, C.; Takarwirwa, N.N.; Kuziwa, N.L.; Stella, N.; Maasdorp, B. Effect of mixed maize-legume silages on milk quality and quantity from lactating smallholder dairy cows. Trop. Anim. Health Prod. 2018, 50, 1255–1260. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Tucak, M.; Ravlić, M.; Horvat, D.; Čupić, T. Improvement of Forage Nutritive Quality of Alfalfa and Red Clover through Plant Breeding. Agronomy 2021, 11, 2176. [Google Scholar] [CrossRef]
- Mohd, A.; Arvind, K.; Pourouchottamane, R.; Gupta, D.L.; Singh, M.K.; Rai, B. Effect of intercropping row ratios on yield and nutritive value of maize and cowpea fodder. Range Manag. Agrofor. 2022, 43, 292–298. [Google Scholar]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. Part Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef]
- Hahnen, S.; Joeris, T.; Kreuzaler, F.; Peterhänsel, C. Quantification of photosynthetic gene expression in maize C(3) and C(4) tissues by real-time PCR. Photosynth. Res. 2003, 75, 183–192. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wang, H.; Wu, X.; Xu, J.; Liu, T.; Ding, R.; Han, Q. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crop. Res. 2018, 224, 148–159. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Yusuf, H.A.; Ma, T.; Diao, Q.; Kong, L.; Kang, L.; Tu, Y. Evaluating Fermentation Quality, Aerobic Stability, and Rumen-Degradation (In Situ) Characteristics of Various Protein-Based Total Mixed Rations. Animals 2023, 13, 2730. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela Saldinger, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Lu, W.; Li, F.; Ma, C. Improved Quality of Corn Silage When Combining Cellulose-Decomposing Bacteria and Lactobacillus buchneri during Silage Fermentation. Biomed Res. Int. 2019, 2019, 4361358. [Google Scholar] [CrossRef]
- Nogoy, K.M.; Zhang, Y.; Lee, Y.H.; Li, X.Z.; Seong, H.A.; Choi, S.H. Nutrient composition and in vitro fermentability of corn grain and stover harvested at different periods in Goesan, a mountainous area. J. Anim. Sci. Technol. 2019, 61, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Undersander, D. Relative forage quality: An alternative to relative feed value and quality index. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 11–12 January 2002. [Google Scholar]
- Zhai, J.; Zhang, G.; Zhang, Y.; Xu, W.; Xie, R.; Ming, B.; Hou, P.; Wang, K.; Xue, J.; Li, S. Effect of the Rate of Nitrogen Application on Dry Matter Accumulation and Yield Formation of Densely Planted Maize. Sustainability 2022, 14, 14940. [Google Scholar] [CrossRef]
- Bolsen, K.K.; Ashbell, G.; Weinberg, Z.G. Silage fermentation and silage additives—Review. Asian Australas. J. Anim. Sci. 1996, 9, 483–494. [Google Scholar] [CrossRef]
- Wen-zhan, M. Effects of biological organic fertilizer on feeding value of Mexico corn. Prataculturalscience 2013, 30, 763r766. [Google Scholar]
- Jiang, H.; Wang, H.; Bao, B.; Qu, H.; Wang, J.; Sun, L.; Liu, B.; Gao, F. Effect of compound additives on nutritional composition, fermentation quality, and bacterial community of high-moisture alfalfa silage. Fermentation 2023, 9, 453. [Google Scholar] [CrossRef]
- Queiroz, O.C.; Arriola, K.G.; Daniel, J.L.; Adesogan, A.T. Effects of 8 chemical and bacterial additives on the quality of corn silage. J. Dairy Sci. 2013, 96, 5836–5843. [Google Scholar] [CrossRef]
- Jatkauskas, J.; Vrotniakiene, V.; Eisner, I.; Witt, K.L.; Copani, G. Effect of different conditions to forage before ensiling and use of a lactic acid bacteria-based additive on the fermentation and aerobic stability of maize silage. Zemdirb.-Agric. 2023, 110, 183–190. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.; Chen, G.; Zhang, Z. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Lv, J.; Fang, X.; Feng, G.; Zhang, G.; Zhao, C.; Zhang, Y.; Li, Y. Effects of Sodium Formate and Calcium Propionate Additives on the Fermentation Quality and Microbial Community of Wet Brewers Grains after Short-Term Storage. Animals 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chang, J.; Yu, J.; Li, S.; Niu, H. Diversity of bacterial community during ensiling and subsequent exposure to air in whole-plant maize silage. Asian-Australas. J. Anim. Sci. 2018, 31, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of Applying Lactic Acid Bacteria Isolated from Forage Crops on Fermentation Characteristics and Aerobic Deterioration of Silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Si, Q.; Sun, L.; Wang, Z.; Liu, M.; Du, S.; Ge, G.; Jia, Y. Effects of Cellulase and Xylanase Addition on Fermentation Quality, Aerobic Stability, and Bacteria Composition of Low Water-Soluble Carbohydrates Oat Silage. Fermentation 2023, 9, 638. [Google Scholar] [CrossRef]
- Santos, E.M.; Da Silva, T.C.; Macedo, C.H.O.; Campos, F.S. Lactic Acid Bacteria in Tropical Grass Silages. In Lactic Acid Bacteria-R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013. [Google Scholar]
- Ali, N.; Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Microbial diversity and fermentation profile of red clover silage inoculated with reconstituted indigenous and exogenous epiphytic microbiota. Bioresour. Technol. 2020, 314, 123606. [Google Scholar] [CrossRef]
- Dewar, W.A.; McDonald, P.; Whittenbury, R. The hydrolysis of grass hemicelluloses during ensilage. J. Sci. Food Agric. 2010, 14, 411–417. [Google Scholar] [CrossRef]
- Yang, C.; Huang, B.; Lin, J.; Yang, Q.; Guo, Y.; Liu, D.; Sun, B. Isolation and screening of high biofilm producing lactic acid bacteria, and exploration of its effects on the microbial hazard in corn straw silage. J. Hazard. Mater. 2024, 480, 136009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).