Physiological Function of AtrN in Regulating Intracellular NADPH Levels and the Anti-Reductive Stress Response in Corynebacterium glutamicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmid
2.2. Growth Medium and Culture Conditions
2.3. Intracellular ROS Detection
2.4. Flow Cytometry for Cell Membrane Permeability
2.5. GC-MS Analysis of Cell Membrane Lipid Composition
2.6. ANS Method for Cell Membrane Fluidity
3. Results and Discussion
3.1. Construction of Chassis Cell with High Intracellular NADPH Levels
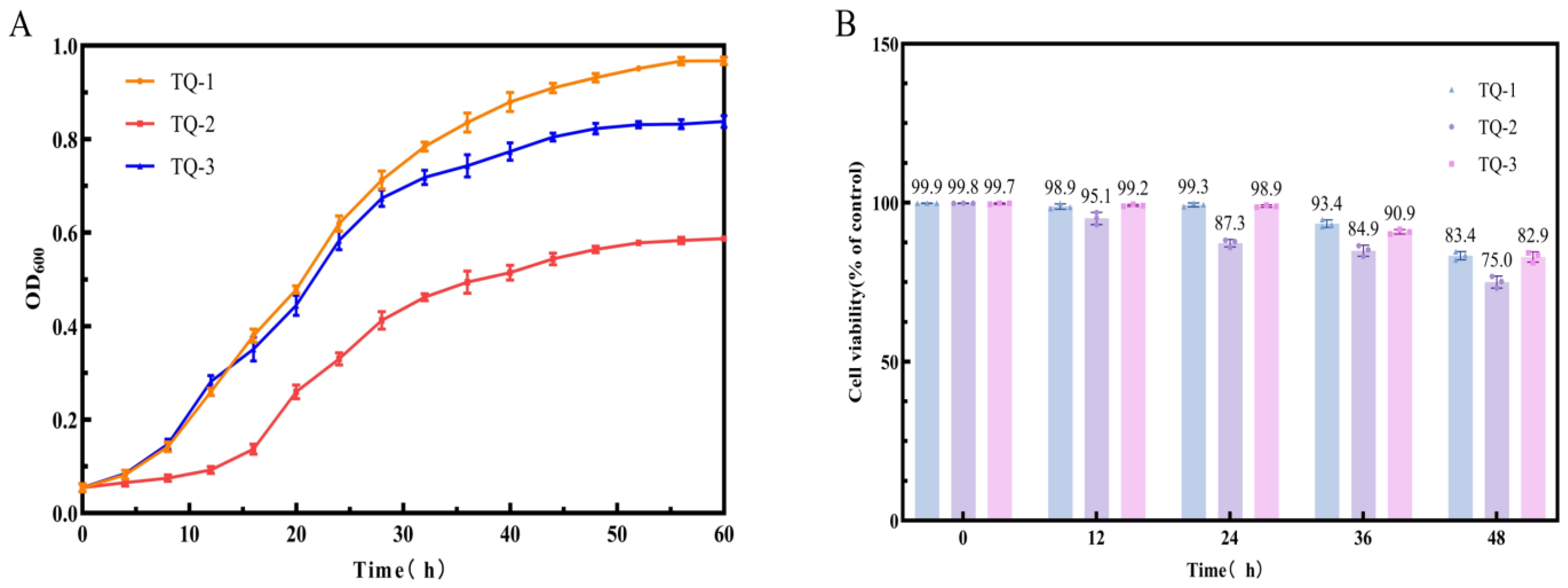
3.2. Determination of Cell Growth and Cell Survival Rate
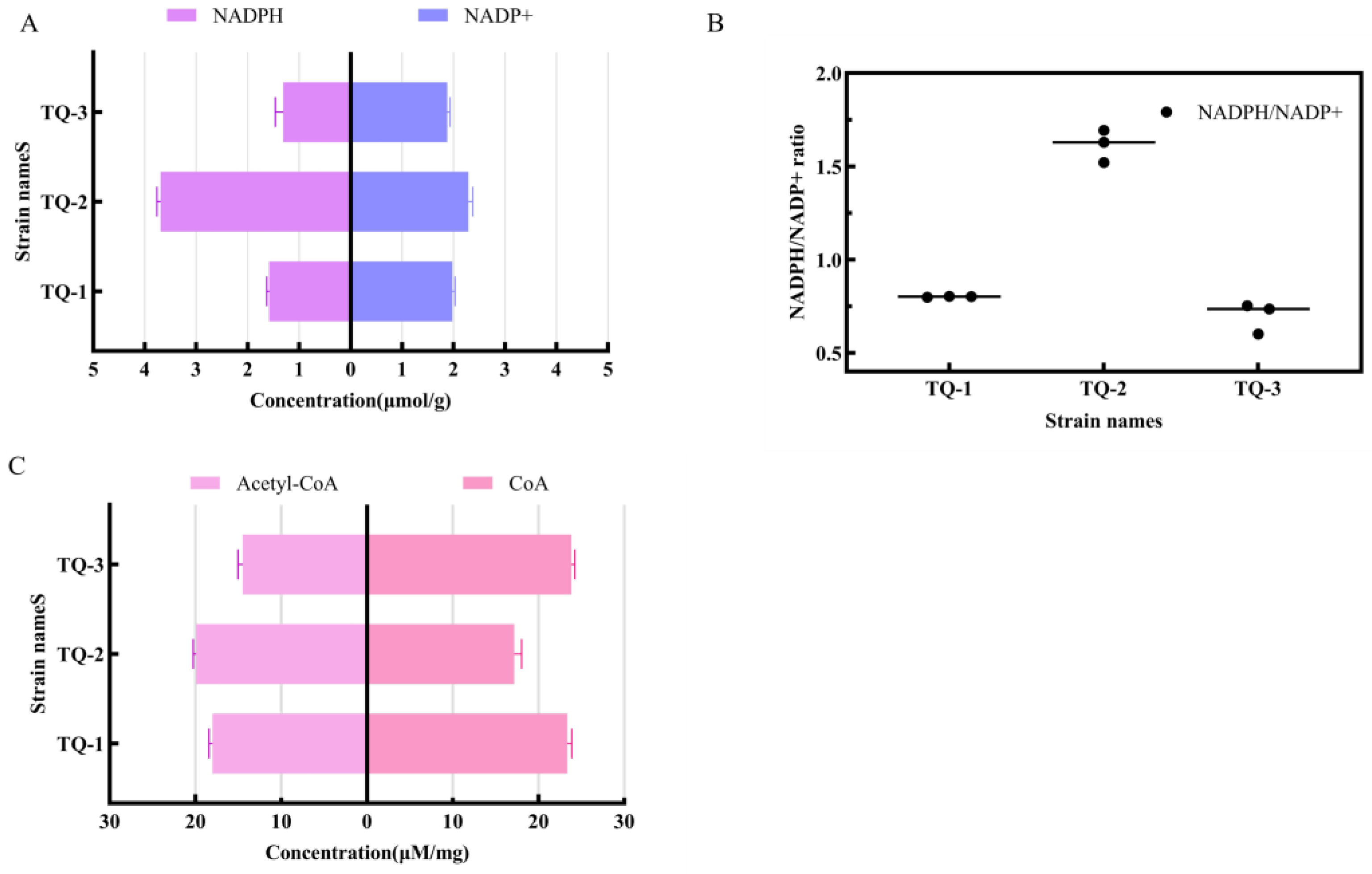
3.3. Detection of Intracellular Redox Molecules
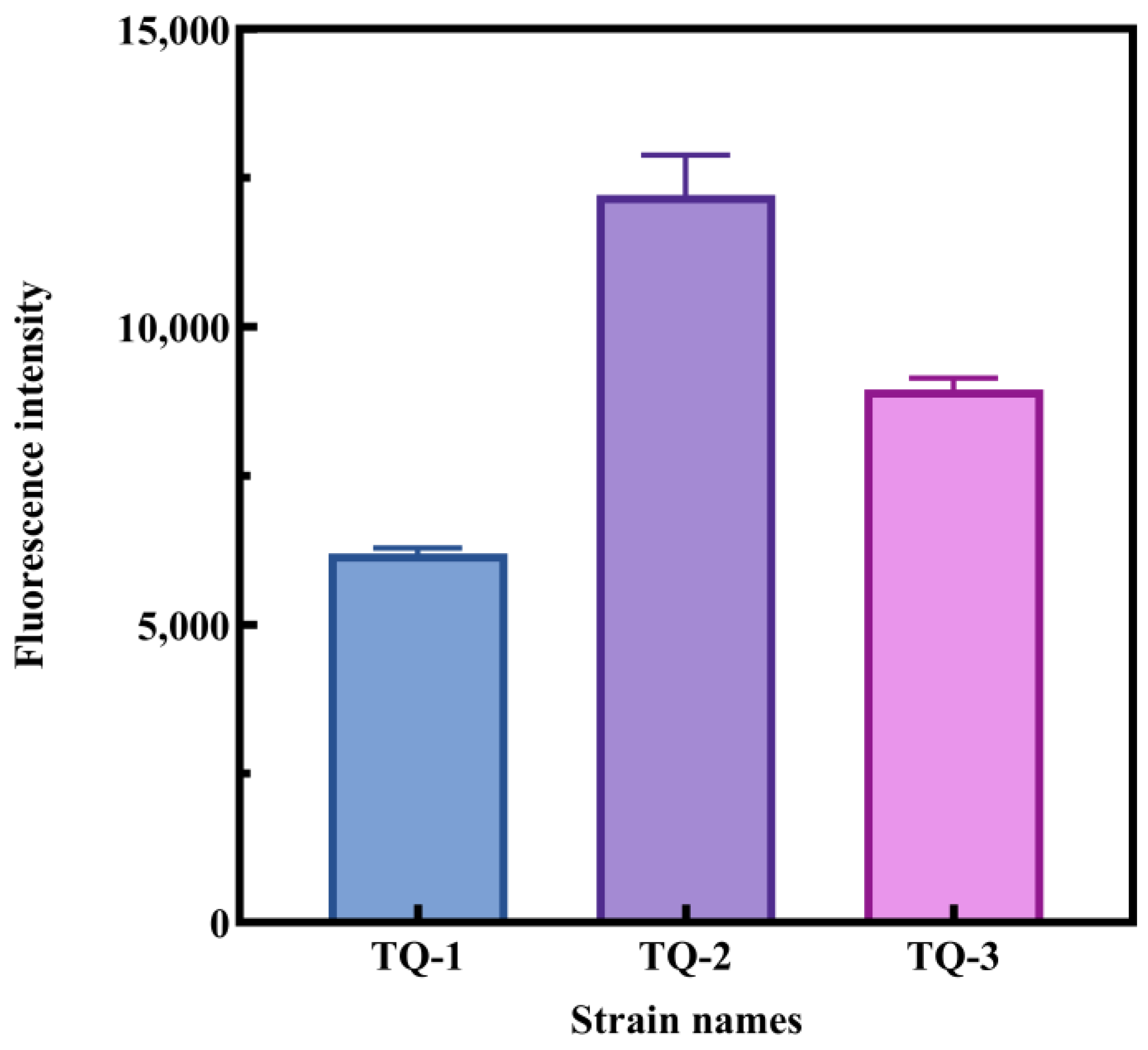
3.4. Detection of Intracellular ROS Levels
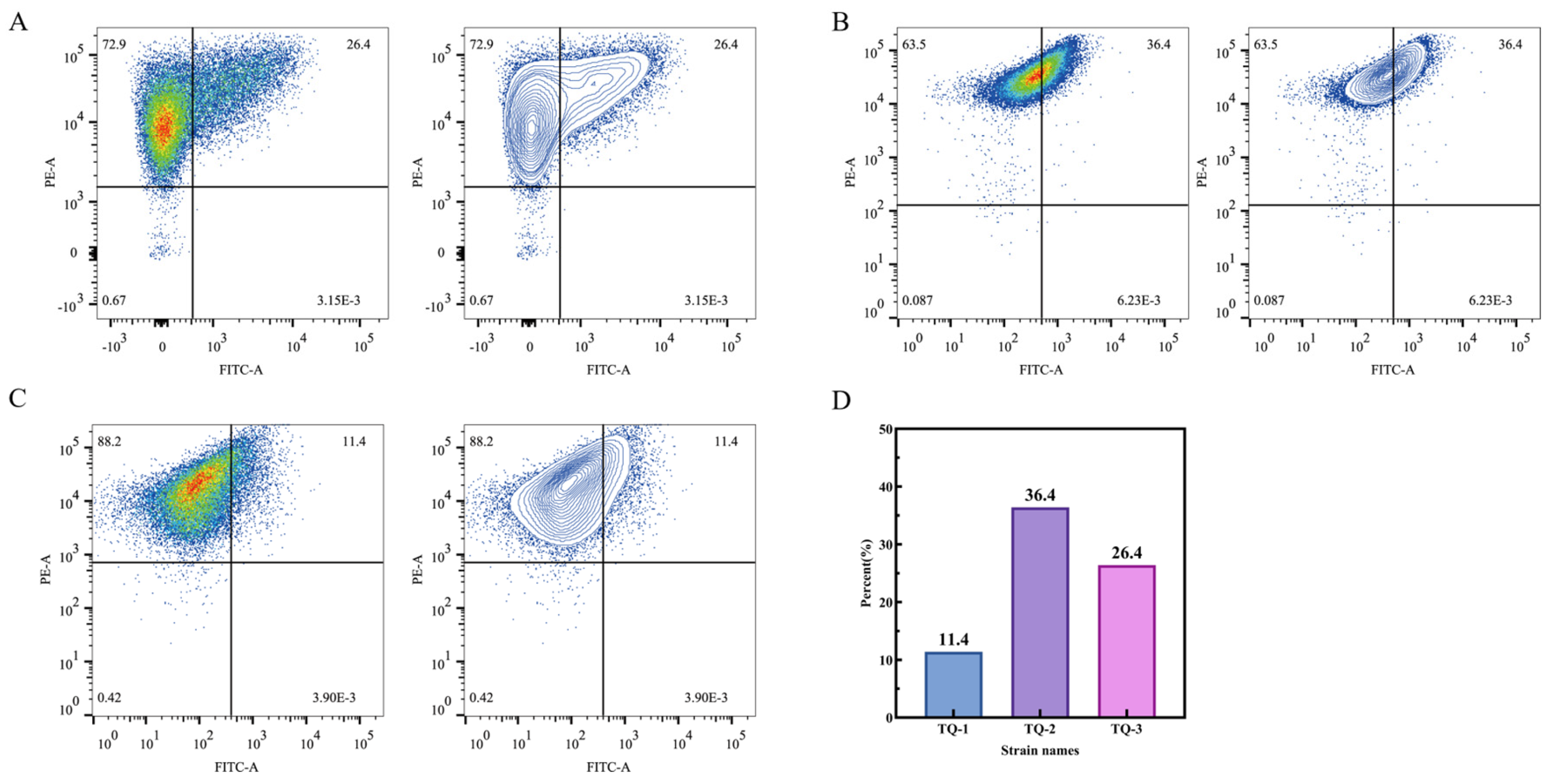
3.5. Detection of Cell Membrane Permeability
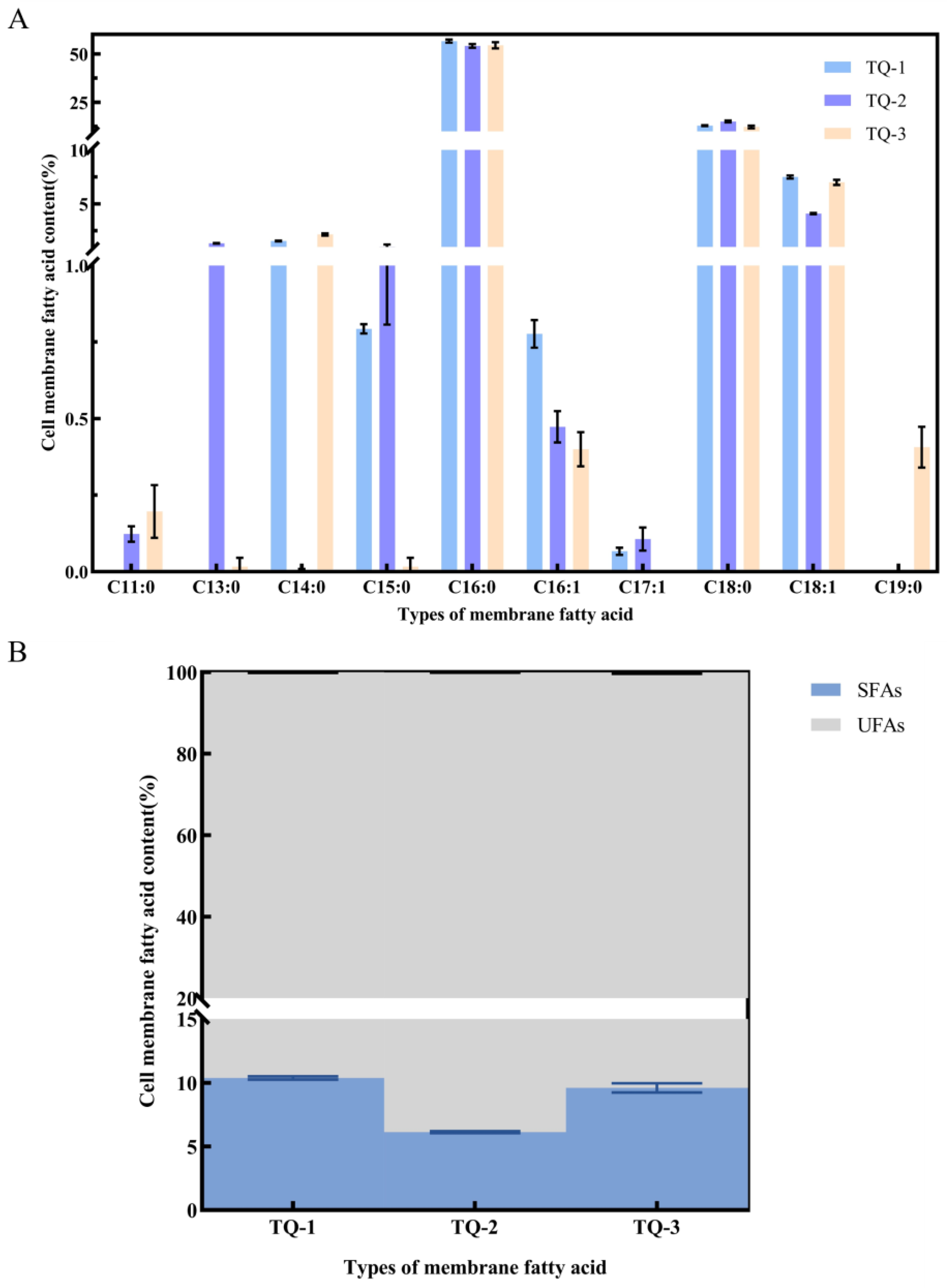
3.6. Detection of Cell Membrane Lipids and Membrane Fluidity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Adaptions to hypoxia and redox stress: Essential concepts confounded by misleading terminology. Circ. Res. 2016, 119, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012, 16, 1323–1367. [Google Scholar] [CrossRef]
- Gores, G.J.; Flarsheim, C.E.; Dawson, T.L.; Nieminen, A.-L.; Herman, B.; Lemasters, J.J. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am. J. Physiol.-Cell Physiol. 1989, 257, C347–C354. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 145–153. [Google Scholar]
- Lloret, A.; Fuchsberger, T.; Giraldo, E.; Vina, J. Reductive stress: A new concept in Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 206–211. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Responses to reductive stress in the cardiovascular system. Free Radic. Biol. Med. 2017, 109, 114–124. [Google Scholar] [CrossRef]
- Yu, Q.; Lee, C.F.; Wang, W.; Karamanlidis, G.; Kuroda, J.; Matsushima, S.; Sadoshima, J.; Tian, R. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J. Am. Heart Assoc. 2014, 3, e000555. [Google Scholar] [CrossRef]
- Dialynas, G.; Shrestha, O.K.; Ponce, J.M.; Zwerger, M.; Thiemann, D.A.; Young, G.H.; Moore, S.A.; Yu, L.; Lammerding, J.; Wallrath, L.L. Myopathic lamin mutations cause reductive stress and activate the nrf2/keap-1 pathway. PLoS Genet. 2015, 11, e1005231. [Google Scholar] [CrossRef]
- Rydström, J. Mitochondrial NADPH, transhydrogenase and disease. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 721–726. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Cantó, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, P.A.; Laviolette, L.A.; Kelleher, J.K.; Iliopoulos, O.; Stephanopoulos, G. Cofactor Balance by Nicotinamide Nucleotide Transhydrogenase (NNT) Coordinates Reductive Carboxylation and Glucose Catabolism in the Tricarboxylic Acid (TCA) Cycle. J. Biol. Chem. 2013, 288, 12967–12977. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Schwörer, S.; Berisa, M.; Kyung, Y.J.; Ryu, K.W.; Yi, J.; Jiang, X.; Cross, J.R.; Thompson, C.B. Mitochondrial NADP (H) generation is essential for proline biosynthesis. Science 2021, 372, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kou, J.; Qin, J.; Li, L.; Zhang, Z.; Pan, Y.; Xue, Y.; Du, W. NADPH levels affect cellular epigenetic state by inhibiting HDAC3–Ncor complex. Nat. Metab. 2021, 3, 75–89. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef]
- Bera, A.K.; Ho, N.W.; Khan, A.; Sedlak, M. A genetic overhaul of Saccharomyces cerevisiae 424A (LNH-ST) to improve xylose fermentation. J. Ind. Microbiol. Biotechnol. 2011, 38, 617–626. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Yang, H.-K.; Zhang, W.-G. NADPH metabolism: A survey of its theoretical characteristics and manipulation strategies in amino acid biosynthesis. Crit. Rev. Biotechnol. 2018, 38, 1061–1076. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Shi, T.; Sun, G.; Gao, N.; Zhao, X.; Guo, X.; Ni, X.; Yuan, Q.; Feng, J. CRISPR-assisted rational flux-tuning and arrayed CRISPRi screening of an L-proline exporter for L-proline hyperproduction. Nat. Commun. 2022, 13, 891. [Google Scholar] [CrossRef]
- Li, S.; Ye, Z.; Moreb, E.A.; Hennigan, J.N.; Castellanos, D.B.; Yang, T.; Lynch, M.D. Dynamic control over feedback regulatory mechanisms improves NADPH flux and xylitol biosynthesis in engineered E. coli. Metab. Eng. 2021, 64, 26–40. [Google Scholar] [CrossRef]
- Ohnishi, J.; Katahira, R.; Mitsuhashi, S.; Kakita, S.; Ikeda, M. A novel gnd mutation leading to increased L-lysine production in Corynebacterium glutamicum. FEMS Microbiol. Lett. 2005, 242, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Z.; Ruan, H.-Z.; Chen, X.-L.; Zhang, F.; Zhang, W. Equilibrium of the intracellular redox state for improving cell growth and L-lysine yield of Corynebacterium glutamicum by optimal cofactor swapping. Microb. Cell Factories 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Y.; Liu, D.; Chen, Z. Efficient mining of natural NADH-utilizing dehydrogenases enables systematic cofactor engineering of lysine synthesis pathway of Corynebacterium glutamicum. Metab. Eng. 2019, 52, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care—Associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef]
- Diep, B.A.; Chan, L.; Tattevin, P.; Kajikawa, O.; Martin, T.R.; Basuino, L.; Mai, T.T.; Marbach, H.; Braughton, K.R.; Whitney, A.R. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 2010, 107, 5587–5592. [Google Scholar] [CrossRef]
- Novick, R.P.; Ross, H.; Projan, S.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993, 12, 3967. [Google Scholar] [CrossRef]
- Cheung, G.Y.; Wang, R.; Khan, B.A.; Sturdevant, D.E.; Otto, M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011, 79, 1927–1935. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Xu, L.; Zhu, K.; Feng, Y.; Pan, J.; Si, M.; Zhang, L.; Shen, X. Transcriptional control of the phenol hydroxylase gene phe of Corynebacterium glutamicum by the AraC-type regulator PheR. Microbiol. Res. 2018, 209, 14–20. [Google Scholar] [CrossRef]
- Küberl, A.; Mengus-Kaya, A.; Polen, T.; Bott, M. The iron deficiency response of Corynebacterium glutamicum and a link to thiamine biosynthesis. Appl. Environ. Microbiol. 2020, 86, e00065-20. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Xu, J.; Ruan, H.; Zhang, W. Deciphering the crucial roles of AraC-type transcriptional regulator Cgl2680 on NADPH metabolism and L-lysine production in Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2020, 36, 82. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ouyang, S.-p.; Kim, J.; Chen, G.-Q. The impact of PHB accumulation on l-glutamate production by recombinant Corynebacterium glutamicum. J. Biotechnol. 2007, 132, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Che, Y.; Milse, J.; Yin, Y.-J.; Liu, L.; Ruckert, C.; Shen, X.-H.; Qi, S.-W.; Kalinowski, J.; Liu, S.-J. The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J. Biol. Chem. 2006, 281, 10778–10785. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Reinhardt, C.A.; Zbinden, G. A rapid cell membrane permeability test using flourescent dyes and flow cytometry. Cell Biol. Toxicol. 1986, 2, 247–255. [Google Scholar] [CrossRef]
- Jeucken, A.; Molenaar, M.R.; van de Lest, C.H.; Jansen, J.W.; Helms, J.B.; Brouwers, J.F. A comprehensive functional characterization of Escherichia coli lipid genes. Cell Rep. 2019, 27, 1597–1606.e1592. [Google Scholar] [CrossRef]
- Brands, M.; Gutbrod, P.; Dörmann, P. Lipid analysis by gas chromatography and gas chromatography–mass spectrometry. In Plant Lipids: Methods and Protocols; Springer: New York, NY, USA, 2021; pp. 43–57. [Google Scholar]
- Xiao, W.; Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Vogelsang, L.; Dietz, K.-J. Regulatory thiol oxidation in chloroplast metabolism, oxidative stress response and environmental signaling in plants. Biochem. J. 2020, 477, 1865–1878. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Ginez, L.D.; Osorio, A.; Vázquez-Ramírez, R.; Arenas, T.; Mendoza, L.; Camarena, L.; Poggio, S. Changes in fluidity of the E. coli outer membrane in response to temperature, divalent cations and polymyxin-B show two different mechanisms of membrane fluidity adaptation. FEBS J. 2022, 289, 3550–3567. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Kilic, G.; Wojtaszek, P.A.; Qadri, I.; Fitz, J.G. Volume-sensitive Tyrosine Kinases Regulate Liver Cell Volume through Effects on Vesicular Trafficking and Membrane Na+ Permeability*. J. Biol. Chem. 2003, 278, 44632–44638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Han, R.; Yu, F.; Liu, K.; Zhao, M.; Liu, Y.; Xue, Z.; Zhao, S. Phosphatase A1 accessory protein PlaS from Serratia marcescens controls cell membrane permeability, fluidity, hydrophobicity, and fatty acid composition in Escherichia coli BL21. Int. J. Biol. Macromol. 2023, 253, 126776. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Ueno, T.; Nagumo, H.; Sato, Y.; Sakai-Kato, K. Enhancing the Endocytosis of Phosphatidylserine-Containing Liposomes through Tim4 by Modulation of Membrane Fluidity. Mol. Pharm. 2022, 19, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G.; Bertoli, E.; Curatola, G.; Mazzanti, L.; Bigi, A. Lipid protein interactions in mitochondria: Spin and fluorescence probe studies on the effect of n-alkanols on phospholipid vesicles and mitochondrial membranes. Arch. Biochem. Biophys. 1976, 172, 278–288. [Google Scholar] [CrossRef]


| Strains or Plasmids | Relevant Description | Sources |
|---|---|---|
| Strains | ||
| ATCC 13032 | Wild-type C. glutamicum | China Center for Type Culture Collection (CCTCC) |
| XQ-5 | An L-tryptophan producing strain, derived from C. glutamicum ATCC 13032 | [30] |
| TQ-1 | Strain XQ-5ΔputA::pntAB | This study |
| TQ-2 | Mutant with deletion of Cgl2680 in the genome of TQ-1 | This study |
| TQ-3 | Strain TQ-2 harboring the pTRCmob-Cgl2680 | This study |
| Plasmids | ||
| pK18mobsacB | Gene deletion/insertion vector, Kan R, “R” stands for “resistance”. Sucrose S, “S” stands for “solvent”. sucide vector | [31] |
| pK18mobsacB-ΔCgl2680 | pK18mobsacB with a Cgl2680 deletion construct | This study |
| pK18mobsacB-ΔputA::pntAB | pK18mobsacB with a construct replacing the putA with the pntAB. | This study |
| pTRCmob | E. coli-C. glutamicum shuttle expression vector with a derepressed Ptrc promoter by deletion of the lacI gene, Kan R, “R” stands for “resistance”. | [32] |
| pTRCmob-Cgl2680 | pTRCmob with a Cgl2680 insertion construct | This study |
| Primer Name | Primer Sequence (5′→3′) |
|---|---|
| Cgl2680-up-F | ATCCTATTGACGCTGCCTGAGGATATTTCC |
| pK18-Cgl2680-R | TCAGGCAGCGTCAATAGGATCCCCGGGTACCGAGCTCGAATTCGTAATC |
| Cgl2680-down-F | GGTACGCACCGGTTTTTGTTCTCAGGCGGACATC |
| Cgl2680-up-R | AACAAAAACCGGTGCGTACCACAATAGAGTCTTAGATC |
| pK18-Cgl2680-F | TGATGGCAGCAAGTCTAGAGTCGACCTGCAGGCATGCAAGCTTG |
| Cgl2680-down-R | CTCTAGACTTGCTGCCATCATCAGGTACTTC |
| putA-up-F | ATCCCTCTGCCCGCTTGAATTCAACTGGG |
| pK18-putA-R | ATTCAAGCGGGCAGAGGGATCCCCGGGTACCGAGCT |
| putA-down-F | GCTCTGTAATCGTTTTGCGCATGGGTCGC |
| PntAB-F | AACCACCATGCGAATTGGCATACCAAGAGAACGG |
| PntAB-R | GCGCAAAACGATTACAGAGCTTTCAGGATTGCATCCACGC |
| putA-up-R | TGCCAATTCGCATGGTGGTTCTCCTTCAAGATCAGCGGTG |
| pK18- putA-F | CTGGAGTGCCTACAGACTTCTCAAAGAACTC |
| putA-down-R | AGGCACTCCAGTCTAGAGTCGACCTGCAGGCATGC |
| Cgl2680-F | GACCATGGAATTCATGACTGCTCAACCAGCCCACGAG |
| pTRCmob-Cgl2680-R | CAGTCATGAATTCCATGGTCTGTTTCCTGTGTGAAATTGTTATCCG |
| pTRCmob-Cgl2680-F | GAGTGATTCTAGAGTCGACCTGCAGGCATGCAAG |
| Cgl2680-R | GGTCGACTCTAGAATCACTCTTGAAAGGATTGACGCTGCGATTCC |
| Strains | Branched Mercaptan Contentation (μmol/Dry Weight) |
|---|---|
| TQ-1 | 2.86 ± 0.42 |
| TQ-2 | 3.52 ± 0.58 |
| TQ-3 | 2.48 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Tian, S.; Gong, Z.; Xu, J. Physiological Function of AtrN in Regulating Intracellular NADPH Levels and the Anti-Reductive Stress Response in Corynebacterium glutamicum. Fermentation 2025, 11, 149. https://doi.org/10.3390/fermentation11030149
Xu G, Tian S, Gong Z, Xu J. Physiological Function of AtrN in Regulating Intracellular NADPH Levels and the Anti-Reductive Stress Response in Corynebacterium glutamicum. Fermentation. 2025; 11(3):149. https://doi.org/10.3390/fermentation11030149
Chicago/Turabian StyleXu, Guotao, Shuping Tian, Zhihan Gong, and Jianzhong Xu. 2025. "Physiological Function of AtrN in Regulating Intracellular NADPH Levels and the Anti-Reductive Stress Response in Corynebacterium glutamicum" Fermentation 11, no. 3: 149. https://doi.org/10.3390/fermentation11030149
APA StyleXu, G., Tian, S., Gong, Z., & Xu, J. (2025). Physiological Function of AtrN in Regulating Intracellular NADPH Levels and the Anti-Reductive Stress Response in Corynebacterium glutamicum. Fermentation, 11(3), 149. https://doi.org/10.3390/fermentation11030149






