Synergistic Effect Enhances Aromatic Profile in Beer Brewing Through Mixed-Culture Fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Fermentation Conditions
2.3. Determination of Sugar Residue Content
2.4. Volatile Compound Analysis
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
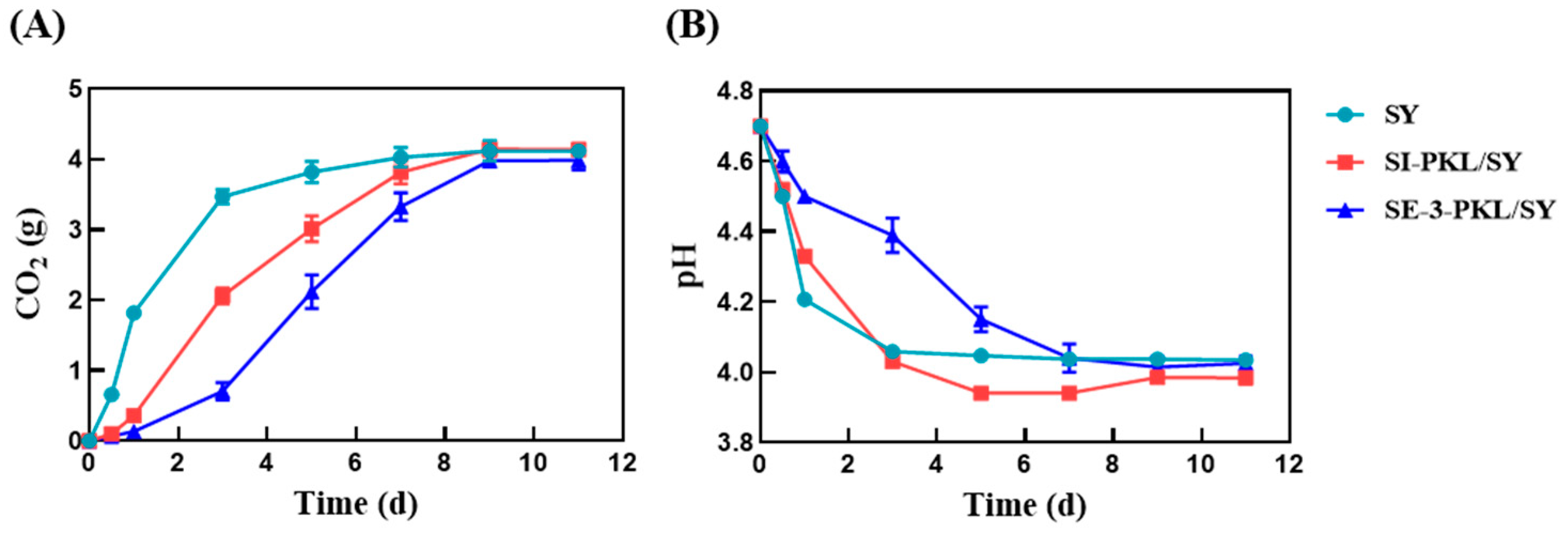
3.1. Fermentation Process Monitoring
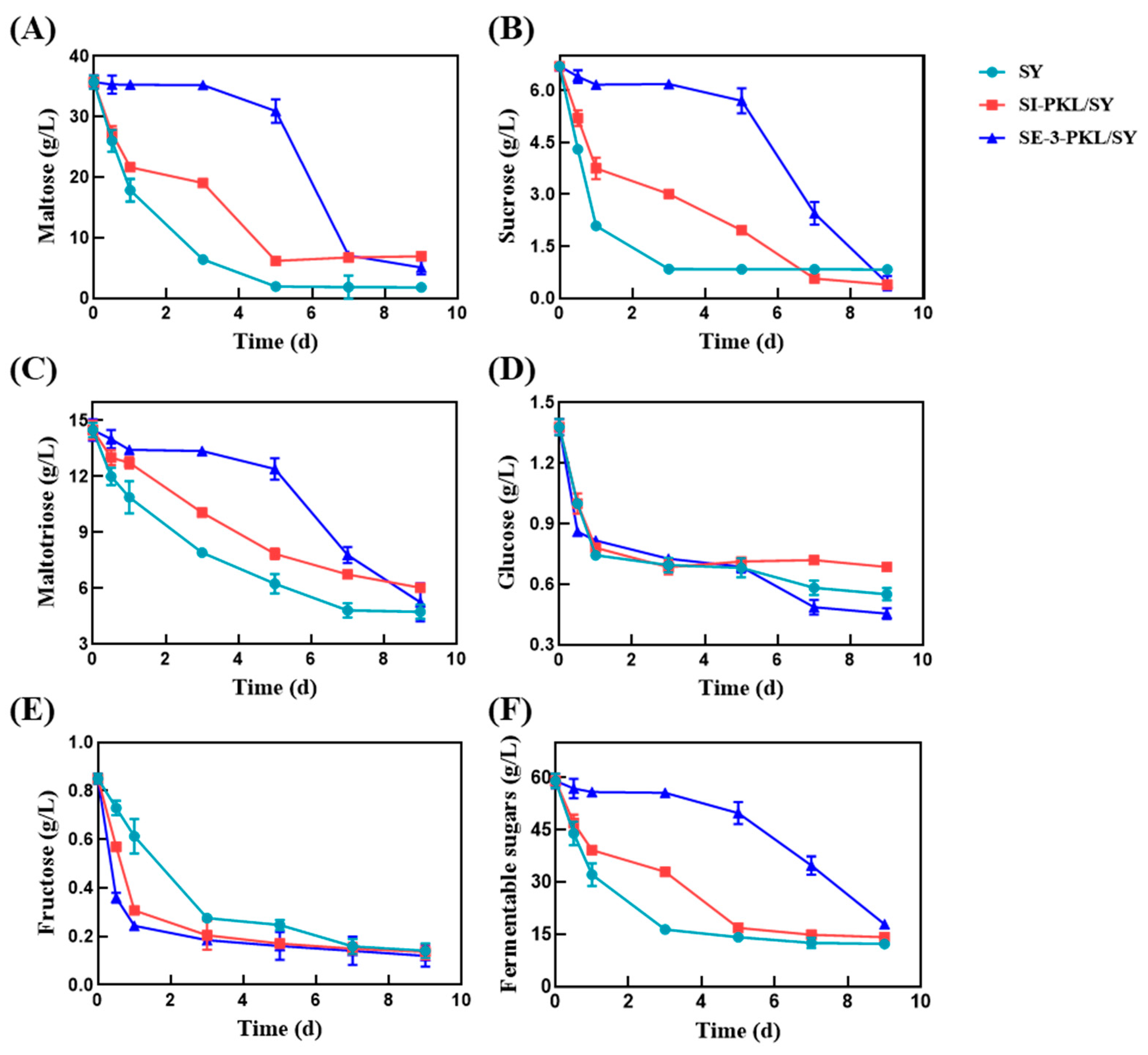
3.2. Analysis of Fermentable Sugars
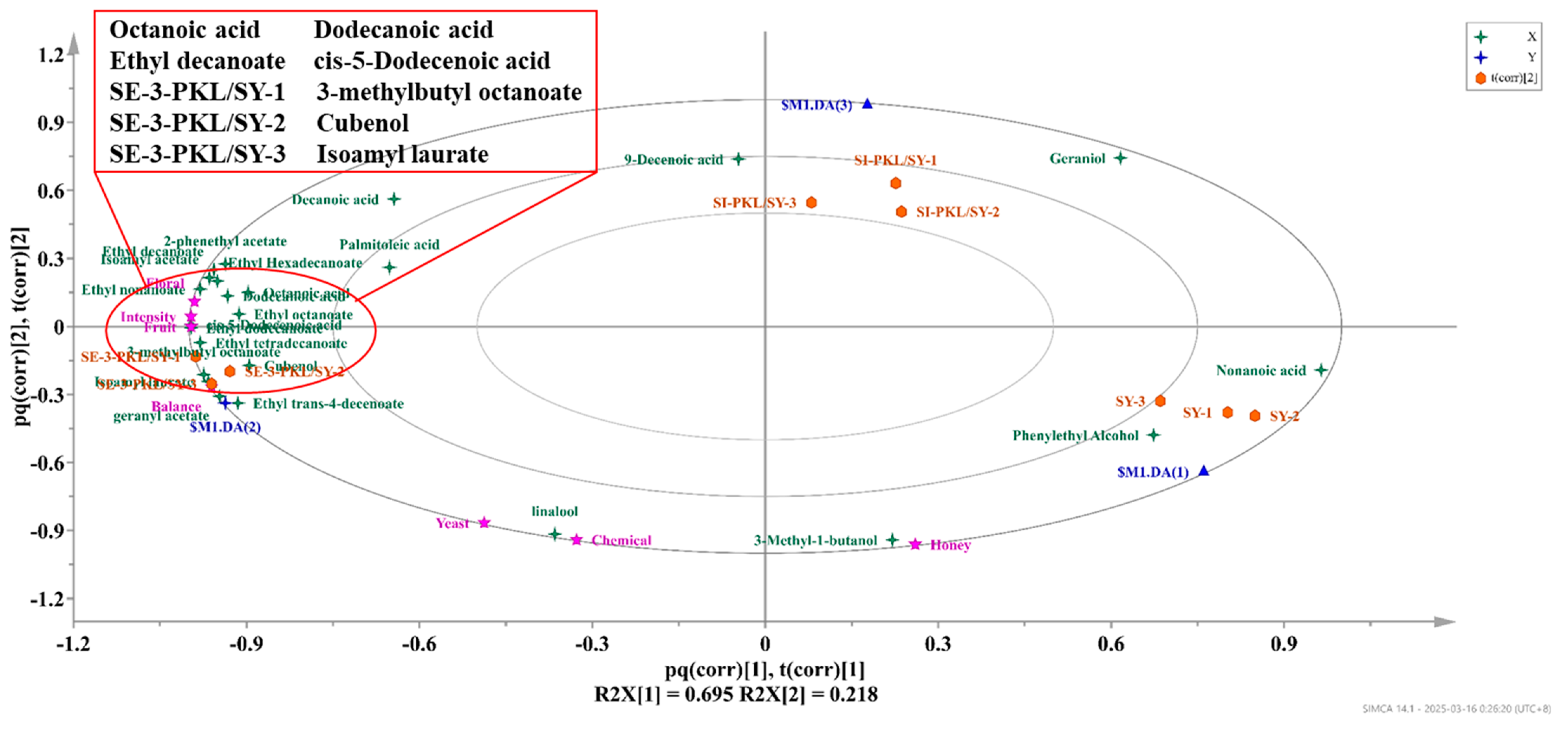
3.3. Key Aroma Compounds of Co-Fermentation of P. kluyveri and S. cerevisiae
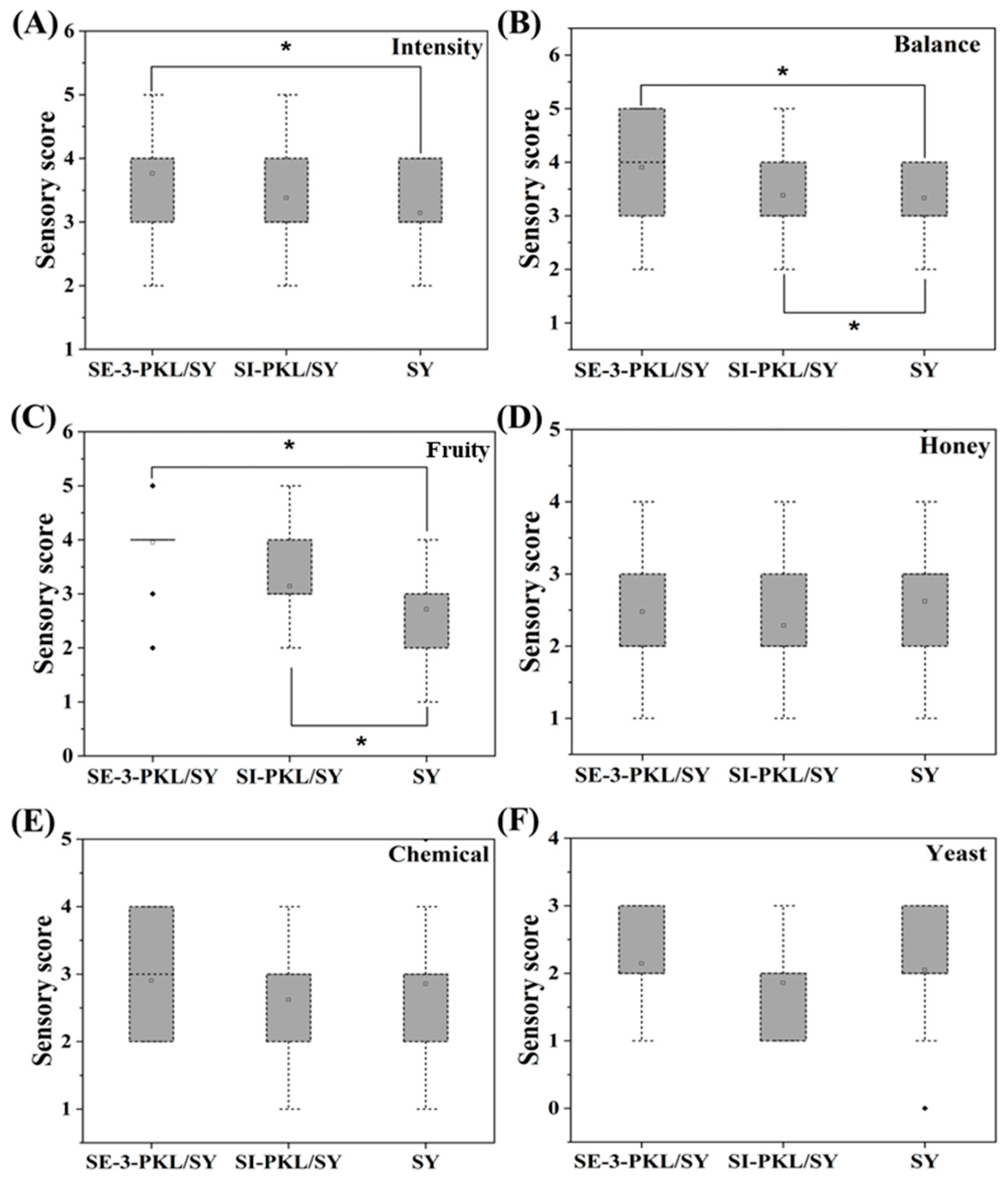
3.4. The Influence of Mixed-Fermentation on the Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larroque, M.N.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, Y.; Tang, K. Aroma of Icewine: A Review on How Environmental, Viticultural, and Oenological Factors Affect the Aroma of Icewine. J. Agric. Food Chem. 2021, 69, 6943–6957. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef]
- Liu, S.X.; Laaksonen, O.; Yang, B.R. Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol. 2019, 80, 25–39. [Google Scholar] [CrossRef]
- Shayevitz, A.; Abbott, E.; Van Zandycke, S.; Fischborn, T. The Impact of Lactic and Acetic Acid on Primary Beer Fermentation Performance and Secondary Re-Fermentation during Bottle-Conditioning with Active Dry Yeast. J. Am. Soc. Brew. Chem. 2021, 80, 258–269. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Yin, J.; Ma, N.; Tao, Y. Adjustment of impact odorants in Hutai-8 rose wine by co-fermentation of Pichia fermentans and Saccharomyces cerevisiae. Food Res. Int. 2022, 153, 110959. [Google Scholar] [CrossRef]
- Schober, D.; Wacker, M.; Schmarr, H.-G.; Fischer, U. Understanding the Contribution of Co-Fermenting Non-Saccharomyces and Saccharomyces Yeasts to Aroma Precursor Degradation and Formation of Sensory Profiles in Wine Using a Model System. Fermentation 2023, 9, 931. [Google Scholar] [CrossRef]
- Lu, Y.; Voon, M.K.W.; Chua, J.-Y.; Huang, D.; Lee, P.-R.; Liu, S.-Q. The effects of co- and sequential inoculation of Torulaspora delbrueckii and Pichia kluyveri on chemical compositions of durian wine. Appl. Microbiol. Blot. 2017, 101, 7853–7863. [Google Scholar] [CrossRef]
- Gao, M.; Hu, J.; Wang, X.; Zhang, H.; Du, Z.; Ma, L.; Du, L.; Zhang, H.; Tian, X.; Yang, W. Effects of Pichia kluyveri on the favor characteristics of wine by co-fermentation with Saccharomyces cerevisiae. Eur. Food Res. Technol. 2023, 249, 1449–1460. [Google Scholar] [CrossRef]
- Hong, K.; Li, C.; Ai, J.; Han, X.; Han, B.; Qin, Q.; Deng, H.; Wu, T.; Zhao, X.; Huang, W.; et al. Biogenic amines degradation ability of Saccharomyces cerevisiae I45 and Pichia sp. NW5 & LB60 and their application in beer fermentation. Food Res. Int. 2025, 202, 115726. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Lin, Y.C.; Lin, Y.W.; Zhang, Y.W.; Huang, D.W. The Potential of Co-Fermentation with Pichia kluyveri and Saccharomyces cerevisiae for the Production of Low-Alcohol Craft Beer. Foods 2024, 13, 3794. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Magalhães, F.; Kuivanen, J.; Gibson, B. A deletion in the STA1 promoter determines maltotriose and starch utilization in STA1+ Saccharomyces cerevisiae strains. Appl. Microbiol. Biotechnol. 2019, 103, 7597–7615. [Google Scholar] [CrossRef] [PubMed]
- Meier-Dornberg, T.; Kory, O.I.; Jacob, F.; Michel, M.; Hutzler, M. Saccharomyces cerevisiae variety diastaticus friend or foe?—Spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Res. 2018, 18, foy023. [Google Scholar] [CrossRef]
- Brickwedde, A. Maltose and Maltotriose Metabolism in Brewing-Related Saccharomyces Yeasts. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Qian, M.; Ruan, F.; Zhao, W.; Dong, H.; Bai, W.; Li, X.; Huang, X.; Li, Y. The dynamics of physicochemical properties, microbial community, and flavor metabolites during the fermentation of semi-dry Hakka rice wine and traditional sweet rice wine. Food Chem. 2023, 416, 135844. [Google Scholar] [CrossRef]
- Xue, D.-D.; He, T.-P.; You, M.-C.; Song, H.-L.; Gong, L.; Pan, W. Effects of different treatments on fishy odor of fish soups. J. Aquat. Food Prod. Technol. 2018, 27, 722–732. [Google Scholar] [CrossRef]
- Cayhan, G.G.; Selli, S. Characterization of the key aroma compounds in cooked grey mullet (Mugil cephalus) by application of aroma extract dilution analysis. J. Agric. Food Chem. 2011, 59, 654–659. [Google Scholar] [CrossRef]
- Hong, K.; Xu, Z.; Wang, L.; Johnpaul, A.; Cheng, Y.; Lv, C.; Ma, C. Varietal differences in the phytochemical components’ accumulation and aroma profile of three Humulus lupulus cultivars. Food Control 2022, 132, 108499. [Google Scholar] [CrossRef]
- Wang, J.; Yan, J.; Zhang, W.; Zhang, Y.; Dong, Z.; Luo, H.; Liu, M.; Su, J. Comparison of potential Wickerhamomyces anomalus to improve the quality of Cabernet Sauvignon wines by mixed fermentation with Saccharomyces cerevisiae. LWT 2022, 173, 114285. [Google Scholar] [CrossRef]
- Alberto, B.G.J.; Diego, C.T.J.; Coronel Domnguez, A.J.; Fernndez Zabala, C.; Carlos, S.O. Pichia kluyveri Strain and Uses There of 2012, ES2382843A1. Available online: https://patents.google.com/patent/WO2012066176A1/en (accessed on 24 May 2012).
- Miguel, G.A.; Carlsen, S.; Almeida-Faria, R.; Saerens, S.; Arneborg, N. Amino acid preference and fermentation performance of Pichia kluyveri strains in a synthetic wort. Food Sci. Technol. 2024, 199, 116059. [Google Scholar] [CrossRef]
- Yabaci Karaoglan, S.; Jung, R.; Gauthier, M.; Kin, L.T.; Dostálek, P. Maltose-Negative Yeast in Non-Alcoholic and Low-Alcoholic Beer Production. Fermentation 2022, 8, 273. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Liu, S.Q. Impact of simultaneous fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii on volatile and non-volatile constituents in beer. LWT 2018, 91, 26–33. [Google Scholar] [CrossRef]
- Gonçalves, C.; Wisecaver, J.H.; Kominek, J.; Oom, M.S.; Leandro, M.J.; Shen, X.; Opulente, D.A.; Zhou, X.; Peris, D.; Kurtzman, C.P.; et al. Evidence for loss and reacquisition of alcoholic fermentation in a fructophilic yeast lineage. eLife 2018, 7, e33034. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Rosa, A.L. Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris. Fermentation 2021, 7, 87. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profle and volatile aroma composition of reduced alcohol merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef]
- Hori, H.; Fujii, W.; Hatanaka, Y.; Suwa, Y. Effects of fusel oil on animal hangover models. Alcohol. Clin. Exp. Res. 2003, 27, 37S–41S. [Google Scholar] [CrossRef]
- Zhu, H.; Xing, Y.; Akan, O.D.; Yang, T. Alcohol-Induced Headache with Neuroinflammation: Recent Progress. Fermentation 2023, 9, 184. [Google Scholar] [CrossRef]
- Yerolla, R.; Mehshan, K.M.M.; Roy, N.; Harsha, N.S.; Pavan Ganesh, M.P.; Besta, C.S. Beer fermentation modeling for optimum flavor and performance. IFAC-PapersOnLine 2022, 55, 381–386. [Google Scholar] [CrossRef]
- Williams, C.; Stander, M.A.; Medvedovici, A.; Buica, A. Volatile terpenoid profiling in gin and beer—A targeted approach. J. Food Compos. Anal. 2023, 118, 105178. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, C.; Wang, L.; Hong, K.; Ma, C.; Lv, C. Potential enzymes involved in beer monoterpenoids transformation: Structures, functions and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 2082–2092. [Google Scholar] [CrossRef]
- Liu, P.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Effect of unsaturated fatty acids on intra-metabolites and aroma compounds of Saccharomyces cerevisiae in wine fermentation. Foods 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, C.; Yang, D.; Liu, H.; Xue, J.; Duan, C.; Yan, G. Effects of three indigenous non-Saccharomyces yeasts and their pairwise combinations in co-fermentation with Saccharomyces cerevisiae on volatile compounds of Petit Manseng wines. Food Chem. 2021, 368, 130807. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef] [PubMed]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Mendez-Zamora, A.; Gutierrez-Avendano, D.O.; Arellano-Plaza, M.; De la Torre Gonzalez, F.J.; Barrera-Martinez, I.; Gschaedler Mathis, A.; Casas-Godoy, L. The non-Saccharomyces yeast Pichia kluyveri for the production of aromatic volatile compounds in alcoholic fermentation. FEMS Yeast Res. 2021, 20, foaa067. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Res. 2017, 41 (Suppl. S1), S95–S128. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef]
- King, A.; Richard, D.J. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T-delbrueckii/S-cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
| Aroma Compounds | Content (mg/L) | OAVs | Odor Descriptors | ||||
|---|---|---|---|---|---|---|---|
| SY | SE-3-PKL/SY | SI-PKL/SY | SY | SE-3-PKL/SY | SI-PKL/SY | ||
| ethanol | 2.10% ± 0.35a | 2.07% ± 0.39a | 2.08% ± 0.28a | ||||
| 3-Methyl-1-butanol | 95.94 ± 3.35a | 49.99 ± 3.17c | 77.19 ± 3.37b | >1 | >1 | >1 | Fruity, apple, banana |
| Isoamyl acetate | 1.16 ± 0.28 c | 14.35 ± 0.19a | 8.15 ± 0. 23b | >1 | >1 | >1 | Banana, fruity, sweet |
| 2-Phenylethanol | 37.48 ± 0.92a | 31.24 ± 2.13b | 30.33 ± 0.58b | >1 | >1 | >1 | Rose, pollen, perfume |
| Ethyl octanoate | 1.96 ± 0.23b | 2.52 ± 0.20b | 3.41 ± 0.25a | >1 | >1 | >1 | Floral, apple, beer, burnt |
| 2-Phenethyl acetate | 0.18 ± 0.06c | 2.16 ± 0.13a | 1.42 ± 0.04b | >1 | >1 | >1 | Fruity, floral, pleasant |
| Linalool | 0.064 ± 0.003b | 0.075 ± 0.002a | 0.066 ± 0.002b | >1 | >1 | >1 | Citrus, floral, fruity |
| Geraniol | 0.045 ± 0.001b | 0.022 ± 0.004c | 0.070 ± 0.007a | >1 | >1 | >1 | Citrus, floral, rose |
| Ethyl laurate | 0.019 ± 0.002c | 0.552 ± 0.012a | 0.201 ± 0.024b | >1 | >1 | >1 | Fruity, strawberry, pleasant |
| Sensory Descriptors | 3-Methyl-1-butanol | Isoamyl Acetate | 2-Phenylethanol | Ethyl Octanoate | 2-Phenethyl Acetate | Ethyl Laurate | Linalool | Geraniol | |
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Pearson cor. | −0.103 | 0.284 * | −0.332 | 0.185 | 0.271 * | 0.294 * | 0.181 | 0.199 |
| Sig. | 0.420 | 0.024 | 0.008 | 0.146 | 0.032 | 0.019 | 0.155 | 0.119 | |
| Balance | Pearson cor. | −0.002 | 0.261 * | −0.221 | 0.272 * | 0.251 * | 0.297 * | 0.266 * | −0.260 * |
| Sig. | 0.989 | 0.039 | 0.081 | 0.031 | 0.047 | 0.018 | 0.035 | 0.040 | |
| Yeast | Pearson cor. | 0.137 | 0.033 | 0.005 | 0.169 | 0.033 | 0.085 | 0.187 | −0.134 |
| Sig. | 0.283 | 0.797 | 0.966 | 0.187 | 0.795 | 0.508 | 0.142 | 0.294 | |
| Fruity | Pearson cor. | −0.127 | 0.513 ** | −0.370 | 0.422 ** | 0.503 ** | 0.521** | 0.299 * | −0.350 ** |
| Sig. | 0.321 | 0.000 | 0.003 | 0.001 | 0.000 | 0.000 | 0.017 | 0.005 | |
| Chemical | Pearson cor. | 0.113 | 0.022 | −0.048 | −0.042 | 0.012 | 0.047 | 0.131 | −0.106 |
| Sig. | 0.377 | 0.866 | 0.708 | 0.743 | 0.929 | 0.717 | 0.305 | 0.410 | |
| Honey | Pearson cor. | 0.135 | −0.057 | 0.076 | −0.099 | −0.068 | −0.039 | 0.087 | −0.035 |
| Sig. | 0.293 | 0.657 | 0.555 | 0.438 | 0.596 | 0.764 | 0.499 | 0.786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Y.; Yu, X.; Hong, K. Synergistic Effect Enhances Aromatic Profile in Beer Brewing Through Mixed-Culture Fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus. Fermentation 2025, 11, 148. https://doi.org/10.3390/fermentation11030148
Rong Y, Yu X, Hong K. Synergistic Effect Enhances Aromatic Profile in Beer Brewing Through Mixed-Culture Fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus. Fermentation. 2025; 11(3):148. https://doi.org/10.3390/fermentation11030148
Chicago/Turabian StyleRong, Youyan, Xiaoxue Yu, and Kai Hong. 2025. "Synergistic Effect Enhances Aromatic Profile in Beer Brewing Through Mixed-Culture Fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus" Fermentation 11, no. 3: 148. https://doi.org/10.3390/fermentation11030148
APA StyleRong, Y., Yu, X., & Hong, K. (2025). Synergistic Effect Enhances Aromatic Profile in Beer Brewing Through Mixed-Culture Fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus. Fermentation, 11(3), 148. https://doi.org/10.3390/fermentation11030148





