Influence of Essential Oils on Inhibiting Biogenic Amine-Producing Bacteria in Xinjiang Smoked Horsemeat Sausage
Abstract
1. Introduction
2. Materials and Methods
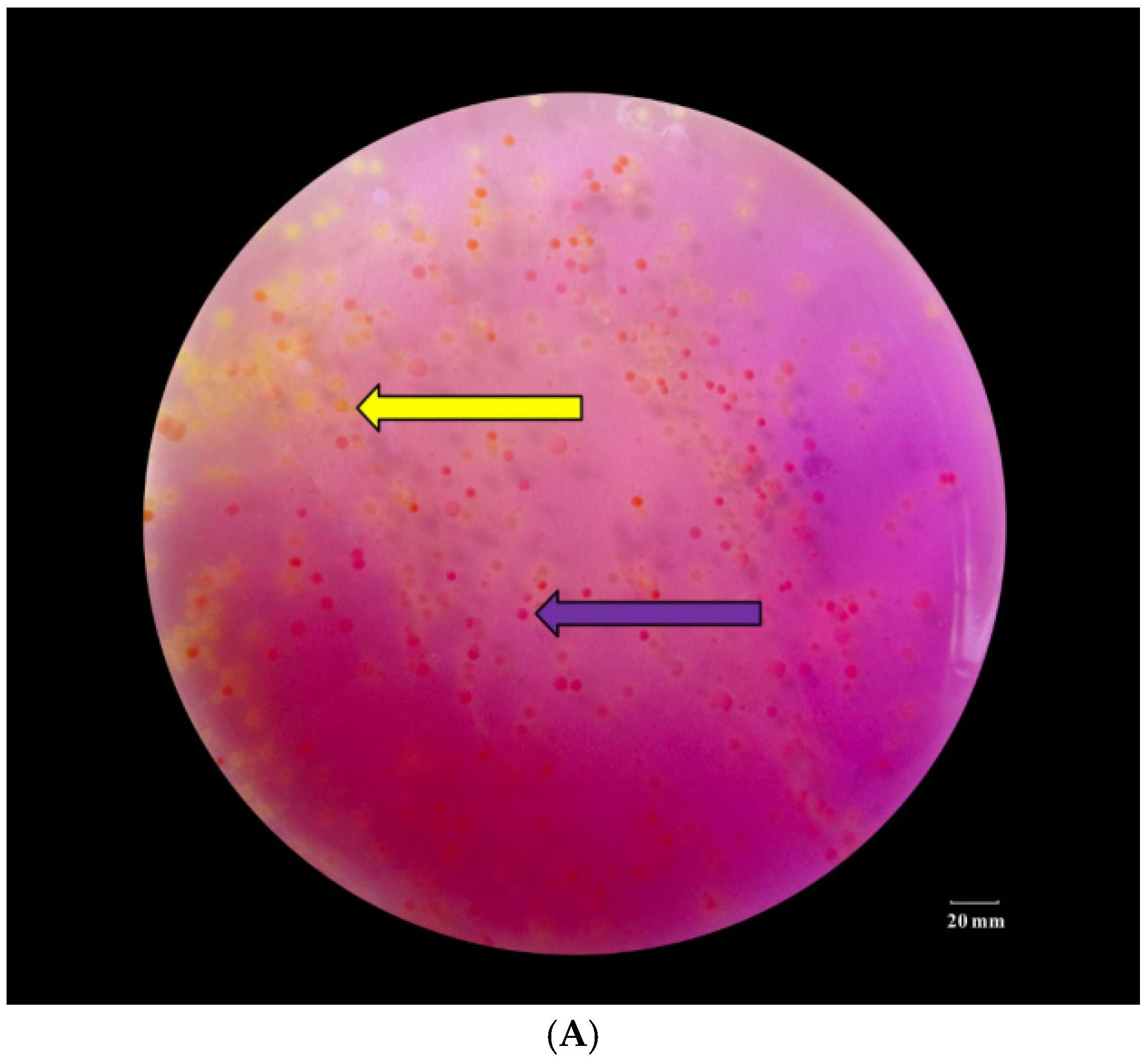
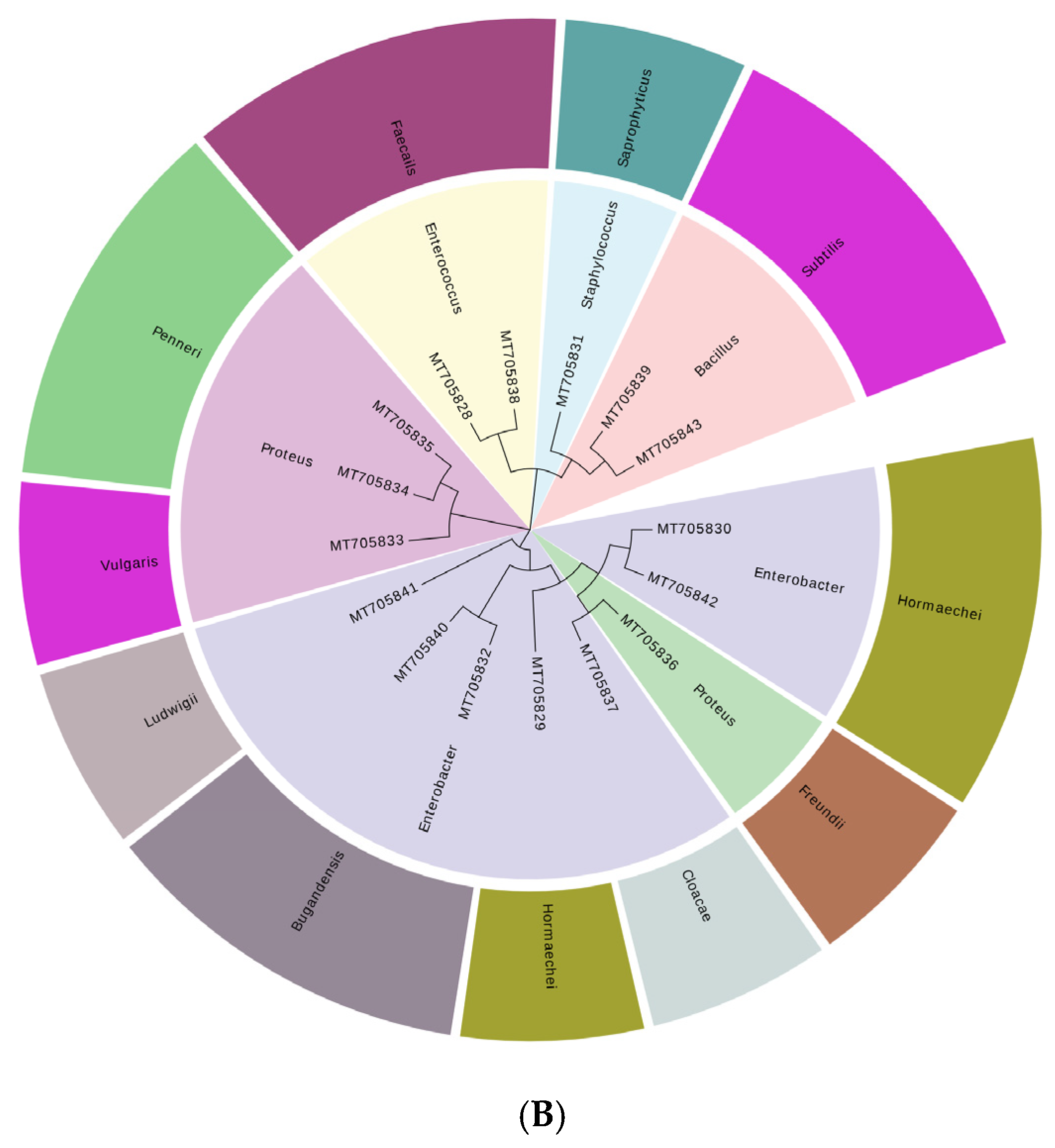
2.1. Isolation and Identification of the Dominant Bacteria Producing BAs in Xinjiang Smoked Horsemeat Sausage
2.2. Detection of Genes for Amino Acid Decarboxylase in Bacteria
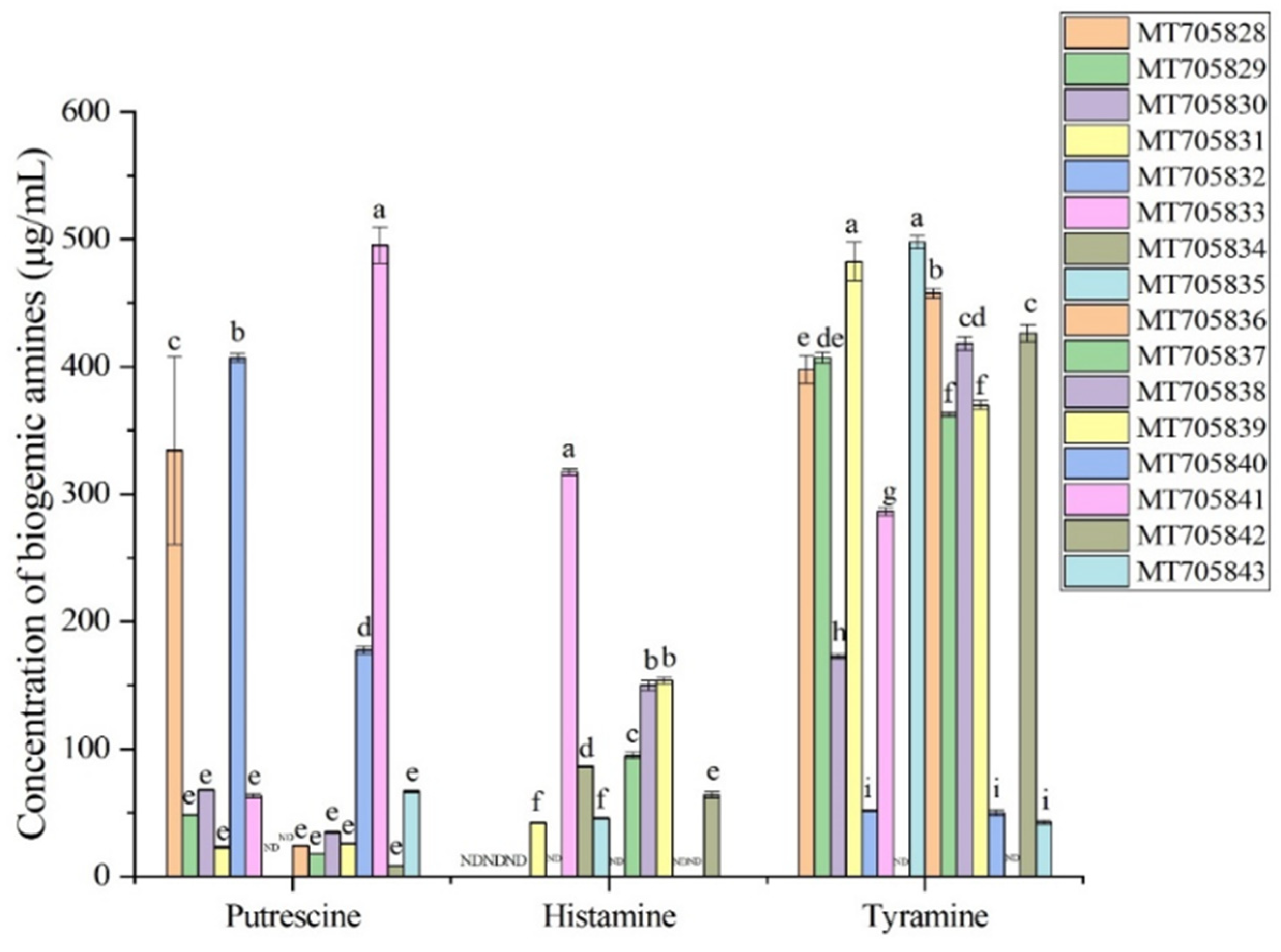
2.3. Analysis BA Production in Isolated Bacterial Strains from Xinjiang Smoked Horsemeat Sausage
2.4. Determining the Minimal Inhibitory Concentration of Essential Oils on Specific Bacterial BA Production
2.5. Determining the Effect of Essential Oil on Specific Bacterial Growth
2.6. Determining the Effect of Essential Oil on Gene Expression in Specific Bacteria
2.7. Statistical Analysis
3. Results
3.1. Dominant Bacteria Producing BA in Xinjiang Smoked Horsemeat Sausage
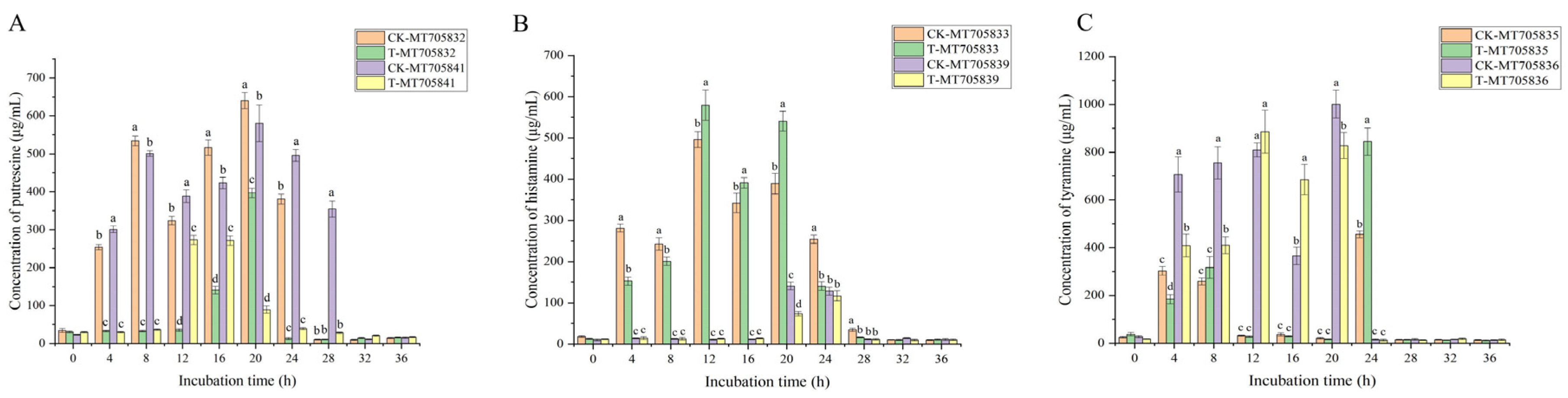
3.2. Effect of Essential Oils on BA Production by Strains Isolated from Xinjiang Smoked Horsemeat Sausage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.H.; Li, Y.Z.; Lu, S.L.; Yue, J.P. Impact of Cinnamaldehyde on the Formation of Biogenic Amines, Microbiological, Physicochemical, and Sensory Quality of Smoked Horsemeat Sausage. LWT-Food Sci. Technol. 2024, 195, 115832. [Google Scholar] [CrossRef]
- Komprda, T.; Sladkova, P.; Dohnal, V. Biogenic Amine Content in Dry Fermented Sausages as Influenced by a Producer, Spice Mix, Starter Culture, Sausage Diameter and Time of Ripening. Meat Sci. 2009, 83, 534–542. [Google Scholar] [CrossRef]
- Papavergou, E.J.; Savvaidis, I.N.; Ambrosiadis, I.A. Levels of Biogenic Amines in Retail Market Fermented Meat Products. Food Chem. 2012, 135, 2750–2755. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Holzapfel, W.H. Improved Screening Procedure for Biogenic Amine Production by Lactic Acid Bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Roigsagues, A.X.; HernandezHerrero, M.; LopezSabater, E.I.; RodriguezJerez, J.J.; MoraVentura, M.T. Histidine Decarboxylase Activity of Bacteria Isolated from Raw and Ripened Salchichon, a Spanish Cured Sausage. J. Food Prot. 1996, 59, 516–520. [Google Scholar] [CrossRef]
- Suzzi, G.; Gardini, F. Biogenic Amines in Dry Fermented Sausages: A Review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Ansorena, D.; Montel, M.C.; Rokka, M.; Talon, R.; Eerola, S.; Rizzo, A. Analysis of Biogenic Amines in Northern and Southern European Sausages and Role of Flora in Amine Production. Meat Sci. 2002, 61, 141–147. [Google Scholar] [CrossRef]
- Houicher, A.; Bensid, A.; Regenstein, J.M.; Ozogul, F. Control of Biogenic Amine Production and Bacterial Growth in Fish and Seafood Products Using Phytochemicals as Biopreservatives: A Review. Food Biosci. 2021, 39, 100807. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ulubelen, A. Cardioactive and Antibacterial Terpenoids from Some Salvia Species. Phytochemistry 2003, 64, 395–399. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Landete, J.M.; Arena, M.E.; Pardo, I.; de Nadra, M.C.M.; Ferrer, S. The Role of Two Families of Bacterial Enzymes in Putrescine Synthesis from Agmatine via Agmatine Deiminase. Int. Microbiol. 2010, 13, 169–177. [Google Scholar] [CrossRef]
- Wongsariya, K.; Bunyapraphatsara, N.; Yasawong, M.; Chomnawang, M.T. Development of Molecular Approach Based on PCR Assay for Detection of Histamine Producing Bacteria. J. Food Sci. Technol. 2016, 53, 640–648. [Google Scholar] [CrossRef]
- Liu, F.; Du, L.H.; Wu, H.H.; Wang, D.Y.; Zhu, Y.Z.; Geng, Z.M.; Zhang, M.H.; Xu, W.M. Effects of Storage Temperature on Tyramine Production by Enterococcus faecalis R612Z1 in Water-Boiled Salted Ducks. J. Food Prot. 2014, 77, 1804–1808. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.Y.; Zhang, J.H.; An, J.S.; Zhang, C.; Chen, T. Research Progress of Biogenic Amines in Fermented Sausages: A Review. Int. Food Res. J. 2022, 29, 223–235. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The Dietary Biogenic Amines Tyramine and Histamine Show Synergistic Toxicity towards Intestinal Cells in Culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative Analysis of the in Vitro Cytotoxicity of the Dietary Biogenic Amines Tyramine and Histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef]
- Nychas, G.J.; Arkoudelos, J.S. Staphylococci: Their Role in Fermented Sausages. J. Appl. Bacteriol. 1990, 69, 167S–188S. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Lee, B.; Her, J.Y.; Lee, K.G.; Lee, J.H. Safety and Technological Characterization of Coagulase-Negative Staphylococci Isolates from Traditional Korean Fermented Soybean Foods for Starter Development. Int. J. Food Microbiol. 2016, 236, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.H.; Wu, X.; Liu, W.Q.; Takahashi, H.; Xie, S.Y.; Ohshima, C.; He, Q.L. Prevalence of Histamine-Forming Bacteria in Two Kinds of Salted Fish at Town Markets of Guangdong Province of South China. J. Food Prot. 2022, 85, 956–960. [Google Scholar] [CrossRef]
- Lu, S.L.; Xu, X.L.; Zhou, G.H.; Zhu, Z.Y.; Meng, Y.; Sun, Y.M. Effect of Starter Cultures on Microbial Ecosystem and Biogenic Amines in Fermented Sausage. Food Control 2010, 21, 444–449. [Google Scholar] [CrossRef]
- Onal, A. A Review: Current Analytical Methods for the Determination of Biogenic Amines in Foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Wojcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic Amines: Formation, Action and Toxicity—A Review. J. Sci. Food Agric. 2020, 101, 2634–2640. [Google Scholar] [CrossRef]
- Satomi, M.; Furushita, M.; Oikawa, H.; Yoshikawa-Takahashi, M.; Yano, Y. Analysis of a 30 Kbp Plasmid Encoding Histidine Decarboxylase Gene in Tetragenococcus halophilus Isolated from Fish Sauce. Int. J. Food Microbiol. 2008, 126, 202–209. [Google Scholar] [CrossRef]
- Yu, H.H.; Huang, Y.L.; Lu, L.L.; Liu, Y.H.; Tang, Z.G.; Lu, S.L. Impact of Thyme Microcapsules on Histamine Production by Proteus Bacillus in Xinjiang Smoked Horsemeat Sausage. Foods 2021, 10, 2491. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Chuang, T.C.; Lin, H.C.; Huang, Y.R.; Lin, C.M.; Kung, H.F.; Tsai, Y.H. Histamine Content and Histamine-Forming Bacteria in Dried Milkfish (Chanos) Products. Food Chem. 2009, 114, 933–938. [Google Scholar] [CrossRef]
- Houicher, A.; Kuley, E.; Ozogul, F.; Bendeddouche, B. Effect of Natural Extracts (Mentha spicata L. and Artemisia campestris) on Biogenic Amine Formation of Sardine Vacuum-Packed and Refrigerated (Sardina pilchardus) Fillets. J. Food Process. Preserv. 2015, 39, 2393–2403. [Google Scholar] [CrossRef]
- del Rio, B.; Linares, D.; Ladero, V.; Redruello, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Putrescine Biosynthesis in Lactococcus lactis Is Transcriptionally Activated at Acidic pH and Counteracts Acidification of the Cytosol. Int. J. Food Microbiol. 2016, 236, 83–89. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Y.; Xu, R.X.; Qian, B.; Zhou, J.W.; Xia, X.L. Primary and Secondary Succession Mediate the Accumulation of Biogenic Amines during Industrial Semidry Chinese Rice Wine Fermentation. Appl. Environ. Microbiol. 2020, 86, e01177-20. [Google Scholar] [CrossRef]
- del Rio, B.; Linares, D.M.; Ladero, V.; Redruello, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Putrescine Production via the Agmatine Deiminase Pathway Increases the Growth of Lactococcus Lactis and Causes the Alkalinization of the Culture Medium. Appl. Microbiol. Biotechnol. 2015, 99, 897–905. [Google Scholar] [CrossRef]
- Marcobal, A.; De las Rivas, B.; Landete, J.M.; Tabera, L.; Munoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- Palusiak, A. Immunochemical Properties of Proteus penneri Lipopolysaccharides-One of the Major Proteus sp Virulence Factors. Carbohydr. Res. 2013, 380, 16–22. [Google Scholar] [CrossRef]
- Uddin, M.J.; Haque, F.; Jabeen, I.; Shuvo, S.R. Characterization and Whole-Genome Sequencing of an Extreme Arsenic-Tolerant Citrobacter freundii SRS1 Strain Isolated from Savar Area in Bangladesh. Can. J. Microbiol. 2023, 69, 44–52. [Google Scholar] [CrossRef]
- Durlu-Ozkaya, F.; Ayhan, K.; Vural, N. Biogenic Amines Produced by Enterobacteriaceae Isolated from Meat Products. Meat Sci. 2001, 58, 163–166. [Google Scholar] [CrossRef]
- Maifreni, M.; Frigo, F.; Bartolomeoli, I.; Innocente, N.; Biasutti, M.; Marino, M. Identification of the Enterobacteriaceae in Montasio Cheese and Assessment of Their Amino Acid Decarboxylase Activity. J. Dairy Res. 2013, 80, 122–127. [Google Scholar] [CrossRef]
- Delbes-Paus, C.; Pochet, S.; Helinck, S.; Veisseire, P.; Bord, C.; Lebecque, A.; Coton, M.; Desmasures, N.; Coton, E.; Irlinger, F.; et al. Impact of Gram-Negative Bacteria in Interaction with a Complex Microbial Consortium on Biogenic Amine Content and Sensory Characteristics of an Uncooked Pressed Cheese. Food Microbiol. 2012, 30, 74–82. [Google Scholar] [CrossRef]
- Yu, H.H.; Li, P.C.; Yin, P.C.; Cai, J.X.; Jin, B.Y.; Zhang, H.P.; Lu, S.L. Bacterial Community Succession and Volatile Compound Changes in Xinjiang Smoked Horsemeat Sausage during Fermentation. Food Res. Int. 2023, 174, 113656. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.X.; Du, L.H.; Wang, D.Y.; Zhu, Y.Z.; Geng, Z.M.; Xu, X.X.; Xu, W.M. Effect of NaCl Treatments on Tyramine Biosynthesis of Enterococcus Faecalis. J. Food Prot. 2015, 78, 940–945. [Google Scholar] [CrossRef]
- Perez, M.; Galles-Enriquez, M.; Nes, I.; Martin, M.C.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Tyramine Biosynthesis Is Transcriptionally Induced at Low pH and Improves the Fitness of Enterococcus Faecalis in Acidic Environments. Appl. Microbiol. Biotechnol. 2015, 99, 3547–3558. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Ladero, V.; Redruello, B.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. The Putrescine Biosynthesis Pathway in Lactococcus Lactis Is Transcriptionally Regulated by Carbon Catabolic Repression, Mediated by CcpA. Int. J. Food Microbiol. 2013, 165, 43–50. [Google Scholar] [CrossRef]
- del Rio, B.; Ladero, V.; Redruello, B.; Linares, D.M.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Lactose-Mediated Carbon Catabolite Repression of Putrescine Production in Dairy Lactococcus Lactis Is Strain Dependent. Food Microbiol. 2015, 48, 163–170. [Google Scholar] [CrossRef]
- Kimura, B.; Takahashi, H.; Hokimoto, S.; Tanaka, Y.; Fujii, T. Induction of the Histidine Decarboxylase Genes of Photobacterium damselae Subsp. damselae (Formally P. histaminum) at Low pH. J. Appl. Microbiol. 2009, 107, 485–497. [Google Scholar] [CrossRef]
- Satomi, M.; Mori-Koyanagi, M.; Shozen, K.; Furushita, M.; Oikawa, H.; Yano, Y. Analysis of Plasmids Encoding the Histidine Decarboxylase Gene in Tetragenococcus Muriaticus Isolated from Japanese Fermented Seafoods. Fish. Sci. 2012, 78, 935–945. [Google Scholar] [CrossRef]
- Satomi, M.; Furushita, M.; Oikawa, H.; Yano, Y. Diversity of Plasmids Encoding Histidine Decarboxylase Gene in Tetragenococcus spp. Isolated from Japanese Fish Sauce. Int. J. Food Microbiol. 2011, 148, 60–65. [Google Scholar] [CrossRef]
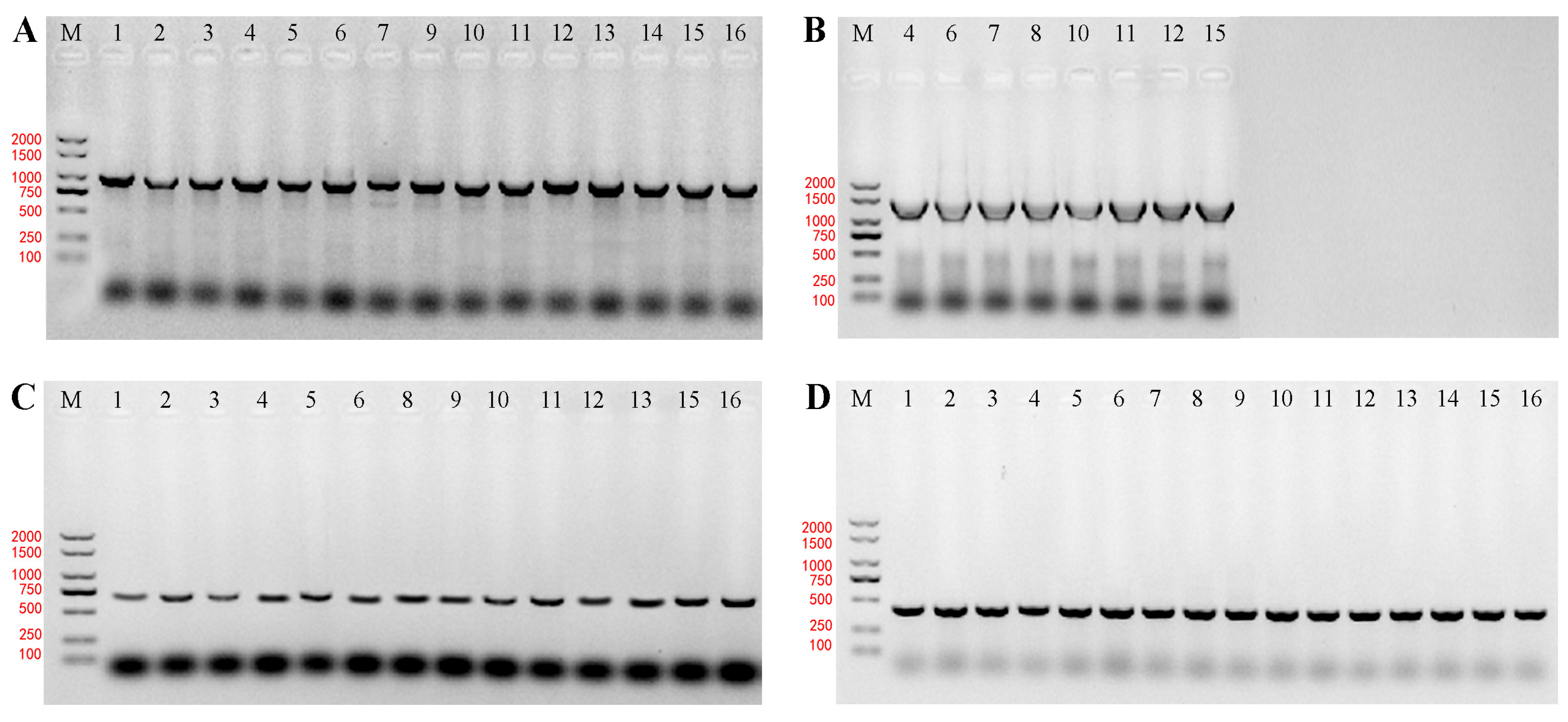

| Enzymes | Primer | Sequence (5′-3′) | Procedures of PCR |
|---|---|---|---|
| agmatine deiminase [12] | AguA-F/R | GACTGGACDTTYAAGGS/YTGGGG TGYTGRGTRATRCARTGR | A |
| histidine decarboxylase [13] | Hdc_2F/R | TGGGGTTATGTSACCATTGG GTRTGGCCGTTACGYGARCC | B |
| tyrosine decarboxylase [14] | Tdc-F/R | AACTATCGTATGGATATCAACG TAGTCAACCATATTGAAATCTGG | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhang, F.; Lu, S. Influence of Essential Oils on Inhibiting Biogenic Amine-Producing Bacteria in Xinjiang Smoked Horsemeat Sausage. Fermentation 2025, 11, 129. https://doi.org/10.3390/fermentation11030129
Li R, Zhang F, Lu S. Influence of Essential Oils on Inhibiting Biogenic Amine-Producing Bacteria in Xinjiang Smoked Horsemeat Sausage. Fermentation. 2025; 11(3):129. https://doi.org/10.3390/fermentation11030129
Chicago/Turabian StyleLi, Ruiting, Fanfan Zhang, and Shiling Lu. 2025. "Influence of Essential Oils on Inhibiting Biogenic Amine-Producing Bacteria in Xinjiang Smoked Horsemeat Sausage" Fermentation 11, no. 3: 129. https://doi.org/10.3390/fermentation11030129
APA StyleLi, R., Zhang, F., & Lu, S. (2025). Influence of Essential Oils on Inhibiting Biogenic Amine-Producing Bacteria in Xinjiang Smoked Horsemeat Sausage. Fermentation, 11(3), 129. https://doi.org/10.3390/fermentation11030129







