Abstract
The improvement in poultry production performance varies with different microbial strains used in fermented feed. This study investigates the efficacy of Lactiplantibacillus-plantarum-ZG7-fermented feed (ZG7-FF) on the productivity of laying hens. Results indicated that ZG7-FF significantly reduced the daily feed intake while increasing egg weight and decreasing the feed-to-egg ratio during peak production (p < 0.05), in addition to enhancing the late-phase laying rate (p < 0.05). Further intestinal morphological results showed that ZG7-FF significantly increased the length of villi in each intestinal segment, most significantly in the duodenum and jejunum (p < 0.01). ZG7-FF also significantly increased the abundance of the phylum Desulfobacterota, while showing a notable increase in the abundance of Cyanobacteria. Conversely, there was a significant reduction in the abundance of intestinal Firmicutes (p < 0.05), specifically Limosilactobacillus and Ligilactobacillus. The LEfSe (LDA Effect Size) analysis indicated that the differential species in the duodenum associated with ZG7-FF are primarily Bifidobacteriales and Aeriscardovia. In contrast, the jejunum is predominantly composed of Cyanobacteria, while the colon is mainly characterized by Enterococcus. Non-targeted metabolomics revealed that ZG7-FF drives the suppression of key metabolites, including 3-hydroxybutyric acid, ethylnitronate, 6-chlorocoumarin-3-carboxylic acid, lotaustralin, and oleoylcarnitine, while enriching pathways related to amino acid metabolism. The downregulated metabolites were functionally linked to ABC transporters and neuroactive ligand–receptor interactions. Correlation analyses demonstrated positive associations between Limosilactobacillus/Ligilactobacillus and suppressed metabolites, whereas Enterococcus and chloroplast-related taxa exhibited negative correlations. In summary, the administration of ZG7-FF significantly enhances intestinal morphology, reduces feed intake, increases egg weight, decreases ingredient usage, elevates the abundance of intestinal Enterococcus, and diminishes the overall microbial load.
1. Introduction
The layer farming industry is increasingly demanding green, efficient, and environmentally sustainable feed additives [1]. Fermented feed, as an innovative feed resource, has gained significant attention due to its potential to enhance production performance, egg quality, gut health, and immunity in layers, while reducing disease incidence [2]. Fermentation, mediated by beneficial microorganisms such as lactic acid bacteria, Bacillus, Yeast, and Lactiplantibacillus plantarum (LP), improves the nutritional value of feed by degrading anti-nutritional factors, producing bioactive metabolites, and modulating gut microbiota [3]. Among these, LP has emerged as a promising probiotic due to its ability to enhance production performance, gut health, and immune function in poultry [4].
LP fermented feed (LP-FF) has been shown to significantly improve egg production rate, feed intake, and egg quality in layers [5]. For instance, the LP strain FRT4 alleviates fatty liver hemorrhagic syndrome (FLHS) by modulating gut microbiota and metabolic pathways, thereby enhancing egg production [6]. LP-FF also improves eggshell thickness, albumen height, and the Haugh unit, indicating its role in optimizing nutrient absorption and metabolism [7]. Furthermore, LP-FF positively influences gut microbiota composition by increasing the abundance of Firmicutes and reducing Spirochaetes and Desulfobacterota, which are associated with improved nutrient digestion and reduced inflammation. The production of short-chain fatty acids (SCFAs) by LP-FF further enhances gut health by inhibiting pathogenic bacteria and promoting beneficial microbial populations [8].
In addition to its effects on gut health, LP-FF enhances systemic immunity by increasing the activity of antioxidant enzymes (e.g., superoxide dismutase and glutathione peroxidase) and reducing oxidative stress markers such as malondialdehyde (MDA) [9]. LP-FF also modulates immune-related gene expression, providing layers with enhanced resistance to infections and inflammatory conditions. The antimicrobial and anti-inflammatory properties of LP-FF, mediated by organic acids and bacteriocins, further contribute to its efficacy in preventing diseases such as salpingitis and colibacillosis [10].
Despite these advancements, the functional efficacy of LP strains varies significantly due to differences in genetic background and metabolic capabilities. For example, acid and bile salt tolerance, which are critical for survival and colonization in the gastrointestinal tract, vary among strains [11]. Similarly, strain-specific enzyme systems, such as phytase and β-mannanase, determine the ability to degrade anti-nutritional factors in feed [12]. Recent studies have also highlighted the role of strain-specific metabolic profiles, including SCFAs and B vitamins, in modulating gut microbiota and host immune responses [13].
In this context, our team has isolated and characterized a novel LP strain, ZG7 (deposited under GDMCC No. 65517), which exhibits superior acid and bile salt tolerance due to high-copy genes such as gadB and bglA. Whole-genome sequencing revealed that ZG7 possesses enhanced metabolic activity under low pH and high bile salt conditions, along with the ability to efficiently degrade fibrous substrates. Notably, ZG7-fermented feed (ZG7-FF) contains elevated levels of phenyllactic acid and γ-aminobutyric acid (GABA), which synergistically mitigate oxidative stress and enhance calcium transport in the eggshell gland.
This study aims to evaluate the effects of ZG7-FF on the production performance, egg quality, gut morphology, microbiota, and metabolic profiles of layers. By elucidating the mechanisms underlying the benefits of ZG7-FF, this research provides a scientific foundation for its application in layer farming, contributing to the development of sustainable and efficient poultry production systems.
2. Materials and Methods
2.1. Strain and Animals
The Lactiplantibacillus plantarum ZG7 strain used in this study was isolated from traditional Tibetan pickles, sourced from local herders, and has been deposited in the China Center for Type Culture Collection (CCTCC) under the accession number or GDMCC No. 65517. Whole-genome sequencing of ZG7 revealed the presence of high-copy acid resistance genes (gadB) and β-glucosidase-encoding genes (bglA), which confer exceptional tolerance to low pH and high bile salt conditions (according to a journal review). These genetic traits enable ZG7 to maintain metabolic activity in harsh gastrointestinal environments while efficiently degrading fibrous substrates, making it an ideal candidate for feed fermentation applications.
For the animal trial, 480 healthy HY-LINE VARIETY BROWN (28 weeks old) hens were randomly allocated into two groups: a control group fed a basal diet and an experimental group supplemented with ZG7-fermented feed (ZG7-FF). The hens were housed in a controlled environment with ad libitum access to feed and water, and all procedures were conducted in accordance with institutional animal care guidelines.
2.2. Experimental Design
This study employed a Completely Randomized Block Design (CRBD) to evaluate the effects of fermented feed on laying-hen performance. A total of 480 healthy HY-LINE VARIETY BROWN hens, aged 28 weeks and with a similar production performance, were randomly divided into two groups, each comprising six replicates (40 hens per replicate). The experimental groups were as follows: the control group (CON), fed a basal diet (corn–soybean meal type, containing 16.5% crude protein and 11.2 MJ/kg metabolizable energy) (Table 1) and the fermented-feed group (LP-FF), fed a solid-state fermented feed prepared using ZG7, where the basal diet was inoculated with the strain at a concentration of 1 × 106 CFU/g and fermented at 37 °C for 48 h, resulting in a final product with a pH of 4.2 ± 0.1. The fermented feed preparation was carried out as follows: The Lactiplantibacillus plantarum ZG7 strain was first activated in MRS liquid medium (37 °C, 24 h) and then inoculated into the mixed feed (adjusted to 40% moisture content) at a 10% inoculation rate. Fermentation was carried out in a temperature- and humidity-controlled fermenter (37 °C, 85% humidity) for 48 h, with manual turning and aeration for 10 min every 12 h.

Table 1.
Basal dietary composition and nutritional level (air-dry basis).
Housing and management: The hens were housed in a three-tier cage system under controlled environmental conditions. They were provided with ad libitum access to feed and water, with a 16L:8D light cycle and at a temperature range of 20–25 °C. The trial lasted for 12 W, during which weekly measurements of egg production rate, egg weight, and feed intake were recorded to calculate the feed conversion ratio (FCR).
Sample collection and analysis: At weeks 0, 6, and 12 of the trial, 10 eggs per replicate were randomly collected for quality assessment. The parameters measured included eggshell strength (N), eggshell thickness (mm), and the Haugh unit.
2.3. Intestinal Morphological Determination
After the experiment, 10 hens were randomly selected from each group and euthanized, and tissues from the duodenum, colon, mid-jejunum, and terminal ileum (approximately 2 cm in length) were rapidly collected and immediately fixed in pre-cooled 4% paraformaldehyde (PFA, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany, Cat. No. 158127) for 24 h at 4 °C. Following fixation, the tissues underwent dehydration in a gradient of ethanol (70–100%), clearing in xylene, and embedding in paraffin using a Leica EG1160 (leica, Wetzlar, Germany) embedding machine. Continuous longitudinal sections (4 μm thick) were cut using a Leica RM2255 microtome and mounted onto adhesive slides (Citotest, Nangjing, China Cat. No. 188105) for subsequent use [14]. The sections were dewaxed, rehydrated, and stained with hematoxylin (Sigma-Aldrich, Cat. No. H9627) for 5 min for HE staining, followed by bluing in running water for 10 min. They were then stained with eosin (Sigma-Aldrich, Cat. No. HT110316) for 2 min, dehydrated in a gradient of ethanol, and mounted with neutral balsam. The stained sections were observed under a Nikon Eclipse (Tokyo, Japan) Ci-L optical microscope. Five intact villus-crypt structures were randomly selected from each sample, and the following parameters were measured using image analysis software (ImageJ 1.53t, NIH): villus height (VH), defined as the vertical distance from the tip of the villus to the crypt opening; crypt depth (CD), defined as the depth from the base of the crypt to the crypt opening; and the villus height-to-crypt-depth ratio (VH/CD), which comprehensively assesses intestinal absorption and regenerative capacity (method based on that in [14]). Negative controls (unstained sections) and positive controls (known healthy intestinal tissue sections) were included in each staining batch.
2.4. The Method for 16S rRNA Sequencing of Intestinal Contents
At the end of the experiment, 10 hens were randomly selected from each group, fasted for 12 h, and then euthanized. Approximately 0.5 g of cecal contents was aseptically collected and immediately placed in a sterile cryovial. The samples were rapidly frozen in liquid nitrogen and then transferred to −80°C for storage. Total microbial DNA was extracted using the QIAamp PowerFecal Pro DNA Kit (Qiagen, Dusseldorf, Germany, Cat. No. 51804). The specific steps included mixing the samples with lysis buffer (containing protease K), vortexing, incubating at 65 °C for 30 min, and purifying using magnetic beads to remove residual host DNA. The concentration and purity of the DNA were checked using a NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA) with an A260/280 ratio of 1.8–2.0, and the integrity was verified by 1% agarose gel electrophoresis.
Primer design targeted the V3–V4 region of the bacterial 16S rRNA gene, with primer sequences 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), containing Illumina bridge PCR-compatible adapter [15]. The PCR reaction mixture (25 μL) contained 2× KAPA HiFi HotStart ReadyMix (Roche, Basel, Switzerland), and the cycling parameters were as follows: initial denaturation at 95 °C for 3 min; 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s; final extension at 72 °C for 5 min. PCR products were purified using AMPure XP magnetic beads (Beckman Coulter, San Diego, CA, USA, Cat. No. A63881), and the library quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, San Diego, CA, USA) with a fragment size of ~550 bp.
Paired-end sequencing (2 × 250 bp) was performed on the Illumina NovaSeq 6000 (San Diego, CA, USA) platform, with an average of 50,000 reads per sample [16]. Raw data were processed using QIIME2 (version 2023.2) as follows: low-quality sequences (q < 20, containing N bases, or primer mismatches) were removed using the DADA2 plugin. ASV generation was based on the DADA2 algorithm for denoising and generating amplicon sequence variants (ASVs). Taxonomic annotation was performed using the Silva database (v138) with a confidence threshold of 0.7. Diversity analysis included calculation of α-diversity (Shannon index, Chao1 index) and β-diversity (Bray–Curtis distance, PCoA visualization). Functional prediction was based on PICRUSt2 for microbial metabolic pathways (KEGG database, level 3).
For quality control and statistical validation, negative controls were included in each batch of extractions, including no-template controls (NTC) and environmental DNA controls, to ensure no exogenous contamination. Reproducibility was verified by randomly selecting 10% of the samples for technical replicate sequencing, with a Bray–Curtis similarity of > 95% between groups. Statistical analysis was performed using R language (v4.2.1) for LEfSe analysis (LDA score > 3.0, p < 0.05), and differential metabolic pathways were analyzed using ANOVA with STAMPv2.1.3 software (corrected FDR, q < 0.05) [17].
2.5. Untargeted Metabolomics Analysis
At the end of the experiment, 10 laying hens from each group were randomly selected, fasted for 12 h, and then euthanized. Duodenal, jejunal, and cecal contents (approximately 0.2 g each) were aseptically collected, immediately transferred to pre-cooled cryotubes, flash-frozen in liquid nitrogen, and stored at −80 °C. Metabolite extraction was performed using an 80% methanol–water solution (containing 0.1% formic acid). The detailed extraction procedure was as follows: samples were mixed with pre-cooled extraction solvent (sample–solvent = 1:10, w/v), vortexed for 1 min, and subjected to ice-bath ultrasonication for 30 min (40 kHz, 4 °C). The mixture was then centrifuged (12,000× g, 15 min, 4 °C), and the supernatant was filtered through a 0.22 μm nylon membrane (Millipore, MA, USA, Cat. No. SLGV033RB). The filtrate was vacuum-concentrated to dryness and reconstituted in 100 μL of a 50% acetonitrile–water solution for LC–MS analysis.
The chromatographic conditions were as follows: the column was an ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 μm, Waters); the mobile phases were A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile); the gradient elution was 0–15 min, 5–95% B; and the flow rate was 0.3 mL/min. The column temperature was 40 °C. The mass spectrometry conditions were as follows: the instrument was a Q Exactive HF-X mass spectrometer (Thermo Fisher); the ion source was electrospray ionization (ESI); the scanning mode was positive/negative ion switching (m/z 50–1500); the resolution was 120,000; and the collision energy was 20–40 eV. Raw data were processed using Progenesis QI 3.0 (Waters) for peak extraction, alignment, and noise reduction. Metabolite features with RSD < 30% (based on QC samples) were retained. Metabolite identification was performed by matching against the HMDB (v5.0), KEGG (2023 version), and an in-house ZG7 strain metabolite database, with a mass error tolerance of <5 ppm and MS/MS fragment similarity >70%.
2.6. Statistical Analysis
Statistical analysis was performed using SIMCA-P 14.1 for Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA). Metabolites with a Variable Importance in Projection (VIP) value of >1.0 and a-value of <0.05 were considered statistically significant. Pathway enrichment analysis was conducted using MetaboAnalyst 5.0, with KEGG pathways analyzed via the hypergeometric test and adjusted for the false discovery rate (FDR) at q < 0.05. To explore the interactions between gut microbiota and metabolites, differential metabolites were integrated with 16S rRNA sequencing data using Cytoscape 3.9.1, constructing a “microbiota–metabolite–host” interaction network. This integrative approach provided insights into the complex relationships among gut microbiota, metabolic profiles, and host physiology.
3. Results
3.1. Effect of ZG7-FF on the Production Performance of Laying Hens
A 12-week feeding trial (n = 480, HY-LINE VARIETY BROWN, 28 weeks old) was conducted to evaluate the effects of ZG7-FF-fermented feed on production performance. The results showed that the egg production rate in the ZG7-FF group was 89.5 ± 1.82% (Table 2), which was significantly higher than that of the control group (82.15 ± 2.31%) (p < 0.01). The peak egg production period (>80% egg production rate) was extended to 31 ± 3.21 weeks in the ZG7-FF group, representing a 29.2% and 14.8% increase compared to the control group (24.12 ± 2.31 weeks) (p < 0.05). The feed conversion ratio (FCR) in the ZG7-FF group was 2.18 ± 0.12 kg/kg, which was significantly lower than that of the control group (2.45 ± 0.15 kg/kg) (p < 0.05), indicating a 13.3% improvement in feed utilization. Eggshell thickness was significantly increased in the ZG7-FF group (3.85 ± 0.27 mm) compared to the control group (3.53 ± 0.33 mm) (p < 0.05) (Table 3). Mortality in the ZG7-FF group decreased to 1.8 ± 0.35%, which was significantly lower than that of the control group (4.25 ± 0.52%) (p < 0.01). These findings suggest that ZG7-FF-fermented feed significantly enhances egg production efficiency and egg quality by improving nutrient utilization, regulating gut health, and modulating immune status, providing a reliable technical solution for antibiotic-free farming.

Table 2.
Effects of ZG7-FF on the production performance of laying hens.

Table 3.
Effects of ZG7-FF on the egg quality of laying hens.
3.2. Effect of ZG7-FF on the Intestinal Morphology of Laying Hens
H&E staining combined with digital morphometric analysis was used to systematically evaluate the effects of ZG7-FF on the mucosal structure of different intestinal segments (duodenum, jejunum, ileum, and colon) in laying hens. The results showed that the duodenal villus height (VH) in the ZG7-FF group increased by 28.7% compared to the control group (CON: 423.5 ± 18.2 μm vs. ZG7-FF: 545.1 ± 22.4 μm, p < 0.001). The VH/crypt depth (V/C) ratio in the ZG7-FF group increased by 0.24. In the jejunum (KC) and ileum (HC), the V/C ratios in the ZG7-FF group increased to 1.68 ± 0.21 (KC) and 1.99 ± 0.12 (HC), representing 14.9% and 7.0% improvements compared to the control group (KC: 1.43 ± 0.14, HC: 1.85 ± 0.06) (p < 0.001), indicating enhanced intestinal absorption function and reduced crypt cell proliferation pressure (Table 4). Notably, ZG7-FF exhibited specific regulatory effects on colonic mucosal morphology: the crypt depth (CD) in the ZG7-FF group was significantly reduced by 18.9% compared to the control group (CON: 105.16 ± 3.42 μm vs. ZG7-FF: 85.25 ± 6.31 μm), suggesting potential improvements in the colonic microenvironment through the regulation of mucus secretion and barrier repair.

Table 4.
Effect of ZG7-FF on intestinal morphological structure of laying hens.
3.3. Effect of ZG7-FF on the Gut Microbiota of Laying Hens
The 16S rRNA sequencing analysis of different intestinal segments revealed that ZG7-FF feed significantly altered the gut microbiota structure, with notable reductions in the abundance of Ligilactobacillus and Limosilactobacillus across all four intestinal segments (Figure 1A–E). Additionally, ZG7-FF significantly increased the relative abundance of Firmicutes in the duodenum, jejunum, and ileum, while increasing Desulfobacterota abundance. Interestingly, the relative abundance of Firmicutes and Desulfobacterota in the colon showed opposite trends compared to the other segments. ZG7-FF also exhibited a trend of upregulating the abundance of other microbial groups, such as Lactobacillus, Enterococcus, Cyanobacteria, and Actinobacteriota, although these changes were not statistically significant (Figure 1A–M). LEfSe (LDA Effect Size) analysis identified several microbial taxa with significant differences (LDA score > 2.0, p < 0.05). At the phylum level, ZG7-FF increased the proportions of Cyanobacteria, Enterococcus, and Bacteroidota, suggesting that fermented feed may influence host function by modulating energy metabolism and immune-related pathways. At the genus level, probiotic groups such as Allidiomarina and Veillonella were significantly enriched in the ZG7-FF group (LDA score = 4.8) (Figure 2). These microbial changes were consistent with improvements in intestinal barrier function, reduced inflammatory cytokine levels, and enhanced nutrient absorption efficiency, indicating that ZG7-FF may regulate laying-hen health through the “microbiota–gut–physiology axis”.
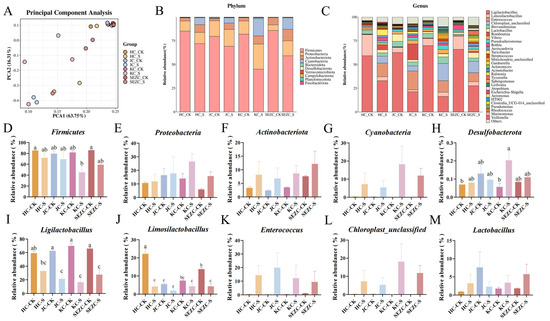
Figure 1.
Impact of ZG7-FF on the gut microbiota of laying hens. (A) Principal component analysis (PCA) of microbial communities across intestinal segments. (B) Top 10 phylum-level microbial abundances in different intestinal segments. (C) Top 30 genus-level microbial abundances in different intestinal segments. (D–M) Microbial taxa at phylum and genus levels showing distinct or trending differences across intestinal segments.
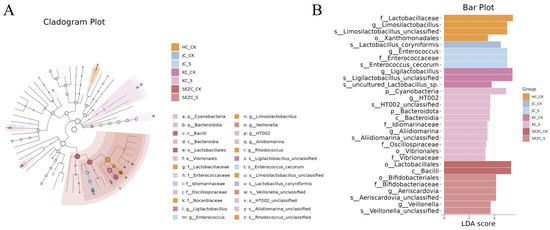
Figure 2.
LEfSe (LDA Effect Size) analysis of structural changes in gut microbiota of laying hens fed ZG7-FF. (A) Phylogenetic cladogram from LEfSe results displays microbial taxa with intergroup differences across taxonomic levels (phylum to genus). Red and green nodes represent taxa significantly enriched in respective groups, while yellow nodes indicate non-significant taxa. (B) Bar plot of LDA scores (>2.0) highlighting taxa with significant differential abundances between groups; bar length reflects the effect size (LDA score).
3.4. Effect of ZG7-FF on the Intestinal Metabolites of Laying Hens
Non-targeted metabolomics analysis (UHPLC-QTOF-MS) revealed that ZG7-FF significantly altered the metabolite composition of intestinal contents (PLS-DA model, R2Y = 0.85, Q2 = 0.72, p < 0.001) (Figure 3). The metabolic responses exhibited spatial heterogeneity across different intestinal segments (SE: duodenum, KC: jejunum, HC: ileum, JC: colon), with the most significant changes observed in the duodenum (VIP > 1.5) (Figure 4). Key metabolites in all four segments, including 3-Hydroxybutyric acid, Ethylnitronate, 6-Chlorocoumarin-3-carboxylic acid, 2-Furyl(oxo)acetic acid, and others, were significantly downregulated, with the most pronounced decreases in the duodenum (Figure 5).
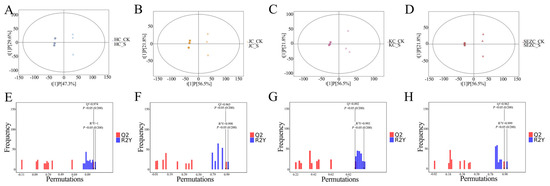
Figure 3.
OPLS–DA score plots. (A–D) and permutation test results (E–H) of gut-content metabolites in laying hens (positive ion mode) between ZG7-fermented feed and control groups.
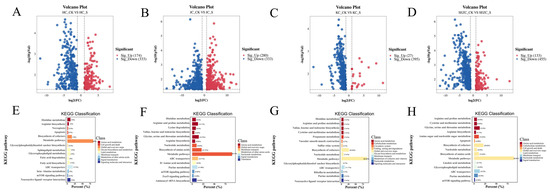
Figure 4.
Differential metabolites in gut contents of laying hens between ZG7-FF and control groups. (A–D) Volcano plots of differentially expressed metabolites, with red and blue dots indicating upregulated and downregulated metabolites, respectively. (E–H) KEGG pathway classification of differential metabolites. The x-axis shows the percentage of annotated metabolites per pathway, and the y-axis lists enriched KEGG pathways.
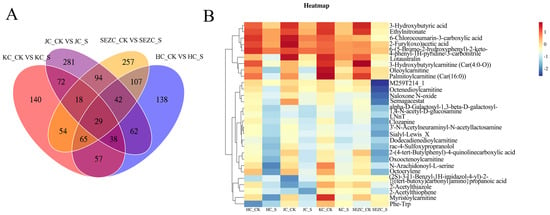
Figure 5.
Heatmap of differential metabolites. (A) Shared and unique metabolite categories across samples. (B) Heatmap illustrating relative abundance of differential metabolites. Red indicates higher abundance, blue indicates lower abundance. Rows represent metabolites, columns represent samples.
3.5. Microbiota–Metabolite Interaction Network
Spearman correlation analysis (R > 0.6, FDR < 0.05) revealed positive correlations between Ligilactobacillus and metabolites such as 2-Acetylthiophene, 3-Hydroxybutyric acid, and Ethylnitronate. Limosilactobacillus was positively correlated with LNnT alpha-D-Galactosyl-1,3-beta-D-galactosyl-1,4-N-acetyl-D-glucosamine. Conversely, Chloroplast—unclassified showed negative correlations with metabolites such as clozapine, Sialyl-Lewis_X, and Oxooctenoylcarnitine (Figure 6).
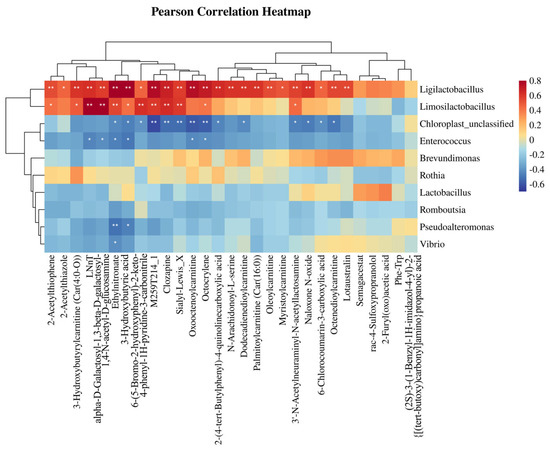
Figure 6.
Correlation analysis between differential gut microbiota and metabolites. Heatmap colors denote correlation strength (red: positive; blue: negative). Asterisks (*) indicate significance levels: * p < 0.05, ** p < 0.01.
4. Discussion
The present study demonstrates that ZG7-FF significantly enhances the production performance of laying hens, as evidenced by improvements in egg production rate, feed efficiency, eggshell quality, and mortality reduction. The 12-week feeding trial revealed that hens fed ZG7-FF achieved a markedly higher egg production rate and an extended peak laying period compared to the control group. These results align with previous reports suggesting that fermented feeds optimize nutrient bioavailability through the microbial pre-digestion of complex substrates (e.g., fibers and proteins), thereby improving energy allocation for reproductive processes [18]. The reduced feed conversion ratio further supports this hypothesis, indicating enhanced metabolic efficiency and nutrient utilization in ZG7-FF-fed hens.
The observed increase in eggshell thickness may be attributed to improved mineral absorption, particularly calcium, which is critical for shell formation. Fermentation-derived organic acids (e.g., lactic acid) and probiotic metabolites likely enhanced intestinal absorption efficiency by modulating gut pH and promoting enterocyte proliferation [19]. Notably, the significant reduction in mortality underscores the dual role of ZG7-FF in both nutritional and health management. The enrichment of beneficial taxa (e.g., Lactobacillus and Bifidobacterium) and suppression of opportunistic pathogens, as revealed by our earlier microbiome analysis, may have strengthened the intestinal barrier integrity and systemic immunity, thereby reducing susceptibility to infections and stress-related mortality [20].
Importantly, the prolonged peak laying period and elevated egg production rate suggest that ZG7-FF mitigates the physiological decline typically observed in aging hens. This could be linked to the anti-inflammatory effects of SCFAs and the sustained energy supply from enhanced fiber degradation by cellulolytic bacteria, which collectively delay metabolic exhaustion. These findings position ZG7-FF as a promising alternative to antibiotic growth promoters, aligning with global trends in sustainable poultry production.
ZG7-FF profoundly modulates the intestinal morphology in laying hens, with segment-specific effects on the mucosal architecture. The observed increase in duodenal villus height and elevated villus height-to-crypt-depth (V/C) ratios across the small intestine (duodenum, jejunum, and ileum) strongly suggest an enhanced nutrient absorption capacity. Enlarged villi, as documented in ZG7-FF-fed hens, are typically associated with an expanded surface area for nutrient transport, which aligns with the improved feed efficiency and egg production rates reported in our earlier findings. Notably, the elevated V/C ratios further indicate reduced crypt cell proliferation pressure, implying a shift toward energy conservation for epithelial maintenance rather than compensatory regeneration—a phenomenon often linked to diminished intestinal inflammation or oxidative stress [21].
The colon-specific reduction in crypt depth highlights ZG7-FF’s unique regulatory role in hindgut ecology. Shallower crypts in the colon may reflect improved mucosal barrier integrity, as excessive crypt hyperplasia is frequently correlated with microbial dysbiosis or luminal toxin exposure [22]. This morphological adaptation could be driven by ZG7-FF-derived metabolites (e.g., short-chain fatty acids, SCFAs), which are known to enhance mucus secretion, tighten junctional complexes, and suppress pathogenic colonization. Such mechanisms would synergize with our prior microbiome data showing ZG7-FF-induced enrichment of Bifidobacterium, a genus recognized for its mucogenic and anti-inflammatory properties [23].
The segment-dependent responses underscore the importance of regional microbiota–host crosstalk. In the small intestine, villus elongation likely stems from direct nutrient- or microbial-metabolite-stimulated epithelial proliferation (e.g., via mTOR or Wnt/β-catenin pathways). In contrast, colonic crypt remodeling may involve the SCFA-mediated modulation of stem cell dynamics, as butyrate has been shown to balance crypt proliferation and differentiation through histone deacetylase inhibition [24]. These adaptations collectively suggest that ZG7-FF optimizes gut homeostasis by spatially fine-tuning the mucosal structure to match luminal nutrient availability and microbial loads.
While this study delineates the morphological effects of ZG7-FF, future work should integrate transcriptomic or proteomic analyses to elucidate molecular drivers (e.g., tight-junction proteins, immune markers) underlying these changes. Nevertheless, the consistent improvements in intestinal architecture and production performance position ZG7-FF as a multifunctional feed additive capable of enhancing both economic and physiological outcomes in commercial poultry systems.
This study revealed that ZG7-FF-fermented feed exerts segment-specific modulatory effects on the gut microbiota of laying hens, with notable implications for intestinal homeostasis and host physiology. The observed reduction in Ligilactobacillus and Limosilactobacillus abundance across all intestinal segments suggests that ZG7-FF may selectively inhibit certain lactic acid bacteria (LAB) strains, potentially through competitive exclusion or metabolite-mediated suppression (e.g., bacteriocins or organic acids). Intriguingly, ZG7-FF induced divergent shifts in the relative abundance of Firmicutes and Desulfobacterota between the small intestine (duodenum, jejunum, ileum) and colon. The decreased Firmicutes (a phylum enriched in carbohydrate-metabolizing taxa) in the proximal gut may reflect altered energy harvest dynamics [25], while its concurrent increase in the colon could enhance fiber fermentation capacity—a hypothesis supported by the elevated Desulfobacterota (sulfate-reducing bacteria) in the small intestine, which may participate in mucosal sulfur metabolism and redox balance regulation. Desulfobacterota includes sulfate-reducing bacteria in this cohort primarily mediated by sulfite detoxification by converting dietary sulfites (common in poultry feed preservatives) into less toxic sulfide derivatives, reducing epithelial oxidative stress [26,27].
The LEfSe-identified enrichment of Cyanobacteria, Enterococcus, and Bacteroidota at the phylum level highlights ZG7-FF’s role in reshaping microbial functional guilds. Cyanobacteria are rarely reported in poultry microbiota studies; their increased abundance might be linked to fermented feed-derived bioactive compounds (e.g., phycobiliproteins) that modulate microbial interactions [28]. The genus-level proliferation of Allidiomarina (a halophilic bacterium with protease activity) and Veillonella (a propionate-producing taxon) suggests enhanced protein utilization and anti-inflammatory short-chain fatty acid (SCFA) production, respectively. These changes align with our physiological data showing improved gut barrier function and reduced systemic inflammation, potentially mediated by SCFA-driven upregulation of tight-junction proteins (e.g., occludin) and suppression of NF-κB signaling.
The paradoxical responses between intestinal segments (e.g., opposing trends in Firmicutes abundance) underscore the spatial heterogeneity of microbial niches. In the small intestine, reduced Firmicutes may decrease competition for simple carbohydrates, favoring Desulfobacterota-mediated sulfate reduction to support mucosal detoxification. Conversely, colonic Firmicutes enrichment could enhance complex polysaccharide fermentation, synergizing with Bacteroidota to boost SCFA output—a critical driver of colonocyte health and barrier maintenance. Such compartmentalized microbiota remodeling likely reflects adaptive strategies to optimize nutrient extraction and pathogen resistance along the gut axis [29]. While the upregulation of Lactobacillus and Enterococcus did not reach statistical significance, their trending increase hints at ZG7-FF’s potential to foster a balanced microbial community resilient to dysbiosis. This is further supported by the significant LEfSe biomarkers (e.g., Veillonella), which are associated with improved feed efficiency and immune tolerance in poultry. Nevertheless, the functional consequences of Ligilactobacillus depletion warrant further investigation, as certain strains within this genus are considered to be beneficial for gut health. Our findings demonstrate that ZG7-FF re-programs the gut microbiota in a segment-dependent manner, primarily through enriching functionally synergistic taxa (e.g., SCFA producers) while suppressing less competitive LAB species [30]. These microbial shifts correlate with enhanced intestinal barrier integrity and nutrient absorption, providing a mechanistic basis for ZG7-FF’s efficacy in promoting laying-hen performance. Future studies should employ metatranscriptomics to validate inferred metabolic pathways and isolate strain-specific contributions to host phenotypes.
The present study provides compelling evidence that dietary supplementation with ZG7-FF induces significant alterations in the intestinal metabolome of laying hens, as revealed by non-targeted metabolomic profiling (UHPLC-QTOF-MS). The robust PLS-DA model (R2Y = 0.85, Q2 = 0.72, p < 0.001) demonstrated a clear separation between ZG7-FF-treated and control groups, underscoring the profound regulatory effects of ZG7-FF on intestinal metabolism. Notably, the spatial heterogeneity of metabolic responses across intestinal segments highlights the segment-specific functional adaptations of the gastrointestinal tract, with the duodenum exhibiting the most pronounced metabolic reprogramming (VIP > 1.5). This observation aligns with the duodenum’s role as the primary site for nutrient absorption and enzymatic digestion, suggesting its heightened sensitivity to dietary interventions [31].
The marked downregulation of key metabolites in SE, including 3-Hydroxybutyric acid Ethylnitronate, 6-Chlorocoumarin-3-carboxylic acid2-Furyl(oxo)acetic acid, and acylcarnitines (e.g., Oleoylcarnitine, Palmitoylcarnitine), implies a systemic modulation of energy metabolism and mitochondrial β-oxidation. Specifically, the suppression of 3-Hydroxybutyric acid derivatives, critical ketone bodies involved in energy homeostasis, may reflect ZG7-FF’s role in redirecting lipid catabolism pathways. Concurrently, the reduced levels of medium- and long-chain acylcarnitines (Car(16:0), Car(4:0-O)) suggest attenuated fatty acid transport into mitochondria, potentially limiting hepatic ketogenesis and altering energy partitioning [32]. These findings are particularly relevant to poultry production, as dysregulated lipid metabolism in laying hens is closely associated with hepatic steatosis and impaired egg quality.
The spatial attenuation of metabolic effects from SE to JC further underscores the duodenum’s metabolic dominance. The identification of Lotaustralin—a cyanogenic glycoside with antioxidative properties—as a downregulated metabolite in SE raises intriguing questions about ZG7-FF’s interaction with xenobiotic detoxification pathways [33]. This observation, coupled with the reduction in 6-Chlorocoumarin derivatives (phase II metabolism intermediates), may indicate enhanced hepatic clearance or altered gut–liver axis signaling, warranting further investigation into ZG7-FF’s hepatoprotective potential.
While this study elucidates ZG7-FF’s segment-specific metabolic impacts, limitations persist. The functional implications of altered metabolites on egg production parameters remain to be validated through targeted assays. Future work should integrate transcriptomic and proteomic approaches to unravel the molecular mechanisms underlying these metabolic shifts, particularly focusing on PPARα-mediated lipid metabolism and Nrf2-regulated detoxification pathways. Furthermore, dose–response studies are warranted to optimize ZG7-FF supplementation strategies for maximal productive performance and intestinal health in commercial poultry systems.
These findings position ZG7-FF as a novel dietary modulator of intestinal metabolism, offering a scientific foundation for developing precise nutrition strategies to enhance nutrient utilization and metabolic resilience in high-yield laying hens. The spatial resolution of metabolic responses provided herein advances our understanding of intestinal-segment-specific nutrient–drug interactions in avian species.
5. Conclusions
In summary, ZG7-FF significantly enhances the intestinal absorption efficiency and barrier integrity in laying hens by differentially regulating the morphological structure of various intestinal segments (villus elongation, crypt shallowing, and V/C ratio optimization). These effects were significantly superior to those observed in the non-fermented-feed group, providing critical evidence for the targeted functional analysis of probiotic-fermented feeds.
Author Contributions
Z.L. and W.L.: Funding acquisition, Investigation, writing—original draft preparation, writing—review and editing, data curation, methodology, formal analysis. S.P.: Conceptualization, writing—review and editing, methodology. H.W.: data curation, methodology, formal analysis. M.Z.: Validation, Data curation, methodology. F.L.: Validation, conceptualization, supervision. L.Z.: Data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the Fujian Provincial Competitive Public Welfare Project (2023R1076), Key Scientific and Technological Project of the Fujian Academy of Agricultural Sciences (KJZD202404), 2022 “Tianshan Talents” Training Program of the Xinjiang Uygur Autonomous Region—Young Top Science and Technology Talents Project (2022TSYCJC0022).
Institutional Review Board Statement
The animal care and use protocol was approved by the Institutional Animal Care and Use Committee at the Institute of Animal Husbandry and Veterinary Medicine of the Fujian Academy of Agricultural Sciences (202307FJ015).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank all of the members of the laboratory for their support and constructive comments, and all authors included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singha Roy, A.; Kesavan Pillai, S.; Ray, S.S. Layered Double Hydroxides for Sustainable Agriculture and Environment: An Overview. ACS Omega 2022, 7, 20428–20440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, B.; Li, J.; Zhu, L. Effects of Fermented Feed on Growth Performance, Immune Response, and Antioxidant Capacity in Laying Hen Chicks and the Underlying Molecular Mechanism Involving Nuclear Factor-ΚB. Poult. Sci. 2020, 99, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, G.; Liu, L.; Luo, J.; Peng, X. Anti-Inflammatory Activity of β-Glucans from Different Sources before and after Fermentation by Fecal Bacteria In Vitro. J. Sci. Food Agric. 2024, 104, 1116–1131. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Biasato, I.; Dabbou, S.; Colombino, E.; Rantsiou, K.; Squara, S.; Gariglio, M.; Capucchio, M.T.; Gasco, L.; Cordero, C.E.; et al. Lactiplantibacillus plantarum, Lactiplantibacillus pentosus and Inulin Meal Inclusion Boost the Metagenomic Function of Broiler Chickens. Anim. Microbiome 2023, 5, 36. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Li, S.; Zhang, H.; Liu, Z. Effects of Tea Residues-Fermented Feed on Production Performance, Egg Quality, Antioxidant Capacity, Caecal Microbiota, and Ammonia Emissions of Laying Hens. Front. Vet. Sci. 2023, 10, 1195074. [Google Scholar] [CrossRef]
- Li, D.; Cai, H.; Liu, G.; Han, Y.; Qiu, K.; Liu, W.; Meng, K.; Yang, P. Lactiplantibacillus Plantarum FRT4 Attenuates High-Energy Low-Protein Diet-Induced Fatty Liver Hemorrhage Syndrome in Laying Hens through Regulating Gut-Liver Axis. J. Anim. Sci. Biotechnol. 2024, 15, 31. [Google Scholar] [CrossRef]
- Niu, K.-M.; Wang, Y.F.; Liang, X.; Zhai, Z.; Liu, J.; Wang, R.; Chen, G.; Wu, X. Impact of Fermented Broussonetia Papyrifera on Laying Performance, Egg Quality, Lipid Metabolism, and Follicular Development of Laying Hens. Poult. Sci. 2023, 102, 102569. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Lin, F.; Yan, L.; Wu, H.; Zhou, H.; Guo, Q.; Lin, B.; Xie, B.; Xu, Y.; et al. Duck Compound Probiotics Fermented Diet Alters the Growth Performance by Shaping the Gut Morphology, Microbiota and Metabolism. Poult. Sci. 2024, 103, 103647. [Google Scholar] [CrossRef]
- Chen, S.; Mei, H.; Xu, L.; Zhan, L.; Yang, Y.; Zhao, D.; Bao, G.; Li, X.; Cao, Z. Impact of Fermented Feed of Soybean Hulls and Rapeseed Cake on Immunity, Antioxidant Capacity, and Gut Microbiota in Chahua Chicken. Poult. Sci. 2024, 103, 103451. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and Improvement of Acid Resistance in Lactic Acid Bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ljungh, A.; Wadström, T. Lactic Acid Bacteria as Probiotics. Curr. Issues Intest. Microbiol. 2006, 7, 73–89. [Google Scholar]
- Wang, D.; Pham, V.T.; Steinert, R.E.; Zhernakova, A.; Fu, J. Microbial Vitamin Production Mediates Dietary Effects on Diabetic Risk. Gut Microbes 2022, 14, 2154550. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Y.; Mackowiak, B.; Gao, B. MicroRNAs as Regulators, Biomarkers and Therapeutic Targets in Liver Diseases. Gut 2021, 70, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Rajendram, R.; Preedy, V.R. Effect of Alcohol Consumption on the Gut. Dig. Dis. 2005, 23, 214–221. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Sufyan, A.; Ahmad, N.; Shahzad, F.; Embaby, M.G.; AbuGhazaleh, A.; Khan, N.A. Improving the Nutritional Value and Digestibility of Wheat Straw, Rice Straw, and Corn Cob through Solid State Fermentation Using Different Pleurotus Species. J. Sci. Food Agric. 2022, 102, 2445–2453. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented Corn-Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef]
- Kogut, M.H.; Genovese, K.J.; Swaggerty, C.L.; He, H.; Broom, L. Inflammatory Phenotypes in the Intestine of Poultry: Not All Inflammation Is Created Equal. Poult. Sci. 2018, 97, 2339–2346. [Google Scholar] [CrossRef]
- Akiyama, T.; Oishi, K.; Wullaert, A. Bifidobacteria Prevent Tunicamycin-Induced Endoplasmic Reticulum Stress and Subsequent Barrier Disruption in Human Intestinal Epithelial Caco-2 Monolayers. PLoS ONE 2016, 11, e0162448. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. Inflammation: Friend or Foe for Animal Production? Poult. Sci. 2018, 97, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate Specifically Modulates MUC Gene Expression in Intestinal Epithelial Goblet Cells Deprived of Glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ding, R.; Chen, X.; Lu, Y.; Shi, J.; Lü, Y.; Tang, B.; Zhang, W.; Ye, C.; Yuan, M.; et al. Firmicutes and Blautia in Gut Microbiota Lessened in Chronic Liver Diseases and Hepatocellular Carcinoma Patients: A Pilot Study. Bioengineered 2021, 12, 8233–8246. [Google Scholar] [CrossRef]
- Dyksma, S.; Pester, M. Growth of Sulfate-Reducing Desulfobacterota and Bacillota at Periodic Oxygen Stress of 50% Air-O2 Saturation. Microbiome 2024, 12, 191. [Google Scholar] [CrossRef]
- Huang, C.-B.; Xiao, L.; Xing, S.-C.; Chen, J.-Y.; Yang, Y.-W.; Zhou, Y.; Chen, W.; Liang, J.-B.; Mi, J.-D.; Wang, Y.; et al. The Microbiota Structure in the Cecum of Laying Hens Contributes to Dissimilar H2S Production. BMC Genom. 2019, 20, 770. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Oliphant, K.; Ali, M.; D’Souza, M.; Hughes, P.D.; Sulakhe, D.; Wang, A.Z.; Xie, B.; Yeasin, R.; Msall, M.E.; Andrews, B.; et al. Bacteroidota and Lachnospiraceae Integration into the Gut Microbiome at Key Time Points in Early Life Are Linked to Infant Neurodevelopment. Gut Microbes 2021, 13, 1997560. [Google Scholar] [CrossRef]
- Sevgili, A.; Can, C.; Ceyhan, D.I.; Erkmen, O. Molecular Identification of LAB and Yeasts from Traditional Sourdoughs and Their Impacts on the Sourdough Bread Quality Characteristics. Curr. Res. Food Sci. 2023, 6, 100479. [Google Scholar] [CrossRef]
- Palmada, N.; Cater, J.E.; Cheng, L.K.; Suresh, V. Modelling Flow and Mixing in the Proximal Small Intestine. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 2496–2499. [Google Scholar] [CrossRef]
- Panov, A.V.; Mayorov, V.I.; Dikalova, A.E.; Dikalov, S.I. Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria. Int. J. Mol. Sci. 2022, 24, 379. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The Higher the Better? Differences in Phenolics and Cyanogenic Glycosides in Sambucus Nigra Leaves, Flowers and Berries from Different Altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).