1. Introduction
Soybean meal is an important protein feed in animal husbandry, with a large yield, wide application, a high crude protein (CP) content, a relatively more balanced amino acid ratio, and easy absorption. In recent years, China’s soybean demand and import dependence have increased. Under the influence of various factors, soybean meal prices have fluctuated by a large proportion in the past 2 years, with a high of approximately 5700 CNY/t. Reducing the amount of soybean meal used or improving its utilization efficiency to reduce dairy farming costs is a challenge many practitioners are eager to solve [
1,
2].
Recently, as a new feed, fermented soybean meal (FSM) has been increasingly used in animal husbandry. It primarily comprises soybean meal—a nitrogen source—and a small proportion of energy feed—a carbon source—that are fermented through inoculation with fungi and bacteria, which may also produce enzymes and nutrients for microbial growth [
3,
4]. FSM contains bacterial protein and microbial metabolites. The digestive enzymes produced by microorganisms, whether naturally occurring or added, pre-digest nutrients, aiding in the rapid absorption of protein and promoting intestinal health post-feeding. FSM is mainly used in pig, poultry, and aquatic animal feeding [
5,
6,
7]. Because the rumen of young ruminants is not fully developed and the digestive system is similar to that of single-stomached animals, recent reports suggest that FSM can improve their production performance and intestinal health. Halidai et al. mixed fermented cottonseed meal and rapeseed into a Hu sheep diet and found that they promoted sugar synthesis and metabolism in the sheep [
8]. FSM shows great potential to improve the protein utilization efficiency and reduce the amount of soybean meal used in animal feeding. Therefore, its application should be further explored and developed for animals with high soybean meal requirements, such as dairy cows.
Although FSM can improve protein utilization, it is easily degraded in the rumen, preventing it from delivering the expected results [
9]. In recent years, tannins and heat treatment have been widely used for rumen protection. The complexes formed when tannins combine with nutrients such as protein and polysaccharides are not easily degraded in neutral environments such as the rumen. However, they can release the bound nutrients in acidic or alkaline environments such as the abomasum and small intestine [
9,
10]. After complex formation, the substrate available for rumen microorganisms is reduced, indirectly reducing ruminal nutrient degradation [
11]. Tannins also inhibit protein-degrading bacteria and reduce digestive enzyme activity [
12]. Studies have shown that the addition of hydrolyzed tannins to legumes is particularly effective, while adding concentrated tannins to alfalfa silage can reduce the protein loss during fermentation [
13,
14]. However, excessive amounts of tannins can lead to adverse effects. For example, Aguerre et al. added 0.45% of a mixture of quebracho and chestnut tannins (2:1 ratio) to the diet of dairy cows. They found that while the milk production was unaffected and the N utilization efficiency was improved, the milk protein yield and nutrient digestibility were adversely affected [
15]. Heat treatment alters the protein structure of soybean meal, exposing more hydrophobic groups and reducing ruminal protein solubility [
16]. Chesini et al. reported that replacing part of a total mixed ration (TMR) with heat-treated soybean meal increased the rumen undegradable protein (RUP) by 0.7%, altering the proportion of digestible amino acids and ultimately increasing the dry matter (DM) intake and milk yield; however, it did not affect the feed efficiency [
17]. Notwithstanding, the aforementioned technologies have rarely been used in FSM. Currently, the processing methods for soybean meal are relatively simple, and few studies have utilized composite technologies to develop an FSM to improve the protein utilization efficiency of soybean meal in dairy production.
This study aimed to explore the degradation characteristics of FSM in the rumen of dairy cows following rumen protection treatment using different methods. Furthermore, we intended to identify the rumen-protected FSM that can provide the most metabolizable protein (MP). This study provides a feasible solution for the efficient use of FSM in dairy cows. It holds significant potential regarding the development and utilization of novel fermented feeds, improving the protein utilization efficiency of soybean meal and reducing the overall soybean meal usage.
2. Materials and Methods
2.1. Animals
This trial was conducted in October 2023 at the Treasure Island Ranch, owned by Beijing Sunlon Livestock Development Co. Ltd., Beijing, China. Three Holstein cows with a mean parity of 2.33 ± 0.58, milk volume of 23.86 ± 5.72 kg/day, and body weight of 646.67 ± 98.33 kg, equipped with permanent rumen fistulas, were selected [
18]. Their diets were formulated according to the nutritional requirements of dairy cows (NRC, 2001) [
19]. The composition and nutritional level of the TMR raw materials are shown in
Table 1. The experimental cattle were fed thrice daily (8:00, 15:00, and 18:30), with free access to drinking water.
2.2. Preparation of Soybean Meal
The diets were designated as follows. The soybean meal treatment was labeled as the SM group (CP: 43.6%); the FSM treatment was labeled as the FS group (CP: 56.58%). The raw materials for the FSM comprised water (30%), soybean (65%), and corn meal (5%). The inoculated microorganisms included
Aspergillus niger,
Aspergillus oryzae,
Bacillus subtilis,
Bacillus amyloliquefaciens,
Lactobacillus plantarum, and
Candida utilis. Enzyme preparations and nutritional additives were added to facilitate aerobic and anaerobic fermentation. Detailed information on the viable counts of the microorganisms, enzyme activities, and their inoculation levels are shown in
Table 2.
The FSM was produced using solid-state fermentation. Aerobic fermentation was conducted at 30 °C for 24 h, followed by 28 °C for 48 h, during which the fermentation bag was turned over every 8 h. Anaerobic fermentation was conducted at 30 °C for 96 h. We obtained invention patents in China for the above formula and production process (CN 118892164 B). The nutritional components of the FSM and soybean meal are shown in
Table 3. The DM content was measured by drying at 105 °C in an oven for 4 h. The organic matter (OM) content was determined by measuring the crude ash content using the combustion method, which is equal to the difference value between DM and crude ash. The CP content was determined using the Kjeldahl nitrogen determination method. An AnkomA2000 fiber analyzer (ANKOM Technology, Macedon, NY, USA) was used to measure the neutral detergent fiber (NDF) and acid detergent fiber (ADF). An AnkomXT15l fat analyzer (ANKOM Technology, Macedon, NY, USA) was used to measure the ether extract (EE). The starch was measured according to AOAC996.11 [
20].
2.3. Rumen Protection Processes
Based on the findings of Jin et al., Berard et al., and Waghorn, we determined that adding tannins at a level of 15 g/kg DM would be effective for the FSM and tolerated by the dairy cows [
21,
22,
23]. The FSM was combined with quebracho tannins (condensed tannins) with a DM content of 1.5% (SILVATEAM, San Michele Mondovì, Italy) and water at 12.5% of the raw weight of the FSM. The FSM was mixed evenly using a blender (FANAI, Shanghai, China). The tannins were thoroughly mixed with the FSM using a large amount of water, forming a light brown granulated mixture designated as the QF group (CP: 54.85%) [
24]. The FSM was also combined with chestnut tannins (hydrolyzed tannins) with a DM content of 1.5% (SILVATEAM) and water at 12.5% of the raw weight of the FSM. The mixture was thoroughly blended, forming a dark brown mixture designated as the CF group (CP: 55.74%).
Lysine is the limiting amino acid for dairy cows. However, it is highly sensitive to heat, with its loss increasing sharply at temperatures exceeding 100 °C [
25,
26]. During the heat treatment, we aimed to reduce the loss of lysine to ensure protein denaturation by placing the FSM on a tray and heating it in an oven at 100 °C for 2 h, forming the HF group (CP: 54.85%). All the samples were dried in an oven at 65 °C for 48 h, crushed using a grinder, and passed through a 1 mm sieve.
2.4. Rumen Degradation Assessment Using the Nylon Bag Method
First, each nylon bag was numbered and weighed. Thereafter, 6 g samples were placed in each bag, and the bags were secured to a hose. Each hose was tied with four nylon bags, with each two nylon bags containing the same sample, and seven hoses were tied to a key tray using nylon thread. When bagging, the hose was placed into the rumen of the cow through the fistula, and the key tray was left outside the body. Next, the fistula plug was covered. Each sample was put into the three fistulated cows, with each cow receiving two nylon bags at each time point and each bag serving as a duplicate. A clear nylon bag was rinsed under running water and used as the sample for 0 h. The time point at which the nylon bags were placed into the rumen of the cow through the fistula was recorded as 0 h. At 2, 6, 12, 16, 24, 36, and 48 h, two nylon bags were removed from the rumen of each cow at each time point, and fermentation was terminated by washing with cold water until the water ran clear.
All the nylon bags were dried in an oven at 65 °C for 48 h and weighed. After calculating the weight of the remaining samples, the remaining samples were taken from six nylon bags of the same sample from the three cows at the same time point. The DM, OM, CP, and NDF were determined to calculate the degradation rate of the sample at this time point. The determination method was the same as that of the FSM in
Section 2.2.
The effective degradation rate (ED) was calculated as follows [
27]:
where
P is the degradation rate of a specific nutrient at time point
t,
a is the rapidly degradable fraction (%),
b is the slowly degradable fraction (%), and
c is the degradation rate of the slowly degradable fraction (%·h
−1). After the values of
a,
b, and
c are obtained, the ED can be calculated;
k represents the rumen outflow rate of the feed, which is 0.0528 (%·h
−1).
The rumen degradable protein (RDP) and RUP were calculated as follows:
where
a is the rapidly degradable fraction (%),
b is the slowly degradable fraction (%),
c is the undegradable fraction (%),
Kd is the degradation rate of the slowly degradable fraction (%·h
−1), and
Kp represents the rumen outflow rate of the feed, where the value here is 0.0528 (%·h
−1) [
28].
The MP was calculated as follows:
where 65.9% is the efficiency of the final transformation of RDP into MP after digestion and absorption in the small intestine, and 80% is the efficiency of the final transformation of RUP into MP after digestion and absorption in the small intestine [
28]. The nutrients in the FSM, mainly carbohydrates such as starch, are lost in the form of heat during fermentation, and the index of DM recovery indicates how much FSM can be obtained from the input raw material (on a DM basis). The MP (DM% × DM recovery) index indicates the proportion of MP that can be obtained from the input raw material of the FSM.
2.5. Rumen Degradation Assessment Using In Vitro Gas Production
The in vitro gas production experiment was conducted using the AGRS-III automated gas recording system with 64 channels and its accompanying software, which allows real-time monitoring and recording of cumulative gas production. The buffer solution was prepared according to the method described by Raab et al. [
29]. Rumen fluid was collected from the same three cannulated cows used for the nylon bag technique. The rumen fluids from the three cows were mixed in equal proportions, maintained at 39 °C in a water bath, and continuously infused with CO
2.
A total of 0.5 g of the sample was weighed into a 125 mL anaerobic fermentation bottle, followed by the addition of 50 mL of buffer solution and 25 mL of rumen fluid. The bottles were continuously purged with N2 to maintain anaerobic conditions. Each sample was prepared in quadruplicate. The fermentation bottles were sequentially connected to the AGRS-III automated gas recording system and incubated at 39 °C in a constant-temperature incubator for 48 h. The gas production was recorded at 2, 6, 12, 16, 24, 36, and 48 h of incubation.
Ørskov’s gas production model was adopted and the gas production was substituted at each time point into the following formula to calculate the gas production parameters:
where
GP is the gas production at time t,
a is the gas production of the rapidly degradable fraction (mL),
b is the gas production of the slowly degradable fraction (mL),
c is the gas production rate of b (×10
3 h/%),
t is the fermentation time (h), and (
a + b) is the theoretical total gas production (mL) [
27].
2.6. Statistical Methods
The experimental data were processed and calculated using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The rumen degradation and in vitro gas production parameters were calculated using SAS 9.4 (method = dud; SAS Institute, Cary, NC, USA). The one-way analysis of variance, followed by Duncan’s multiple range test for multiple comparisons, was conducted using SPSS v19 (SPSS Inc., Chicago, IL, USA). The difference was significant when p < 0.05. A significant trend was considered when 0.05 ≤ p < 0.10.
4. Discussion
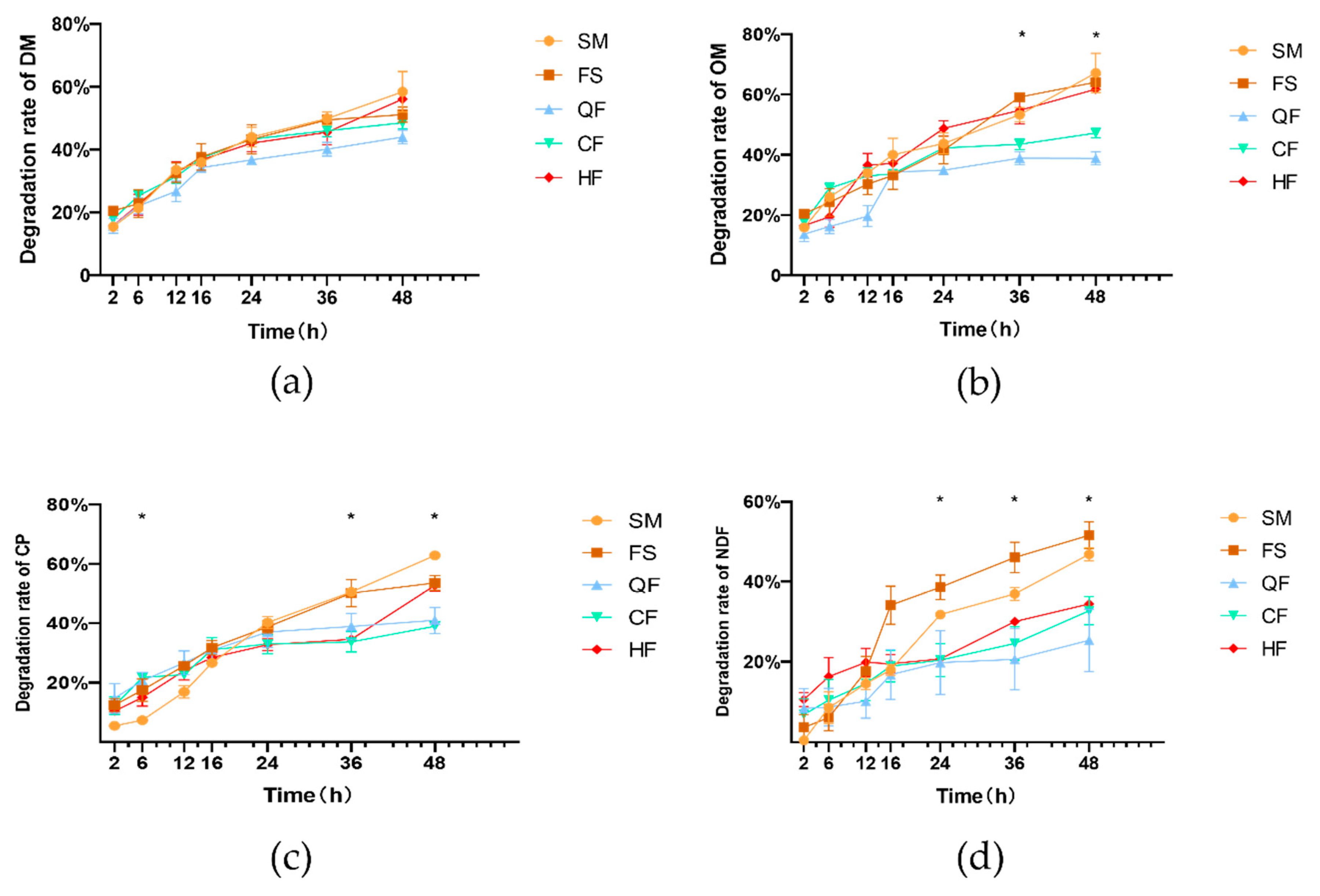
The CP of the FSM is significantly higher than that of soybean meal, and the starch is significantly lower than that of soybean meal. This is because the consumption of non-structural carbohydrates, mainly starch, during the fermentation process not only provides energy for the production of protein for microbial proliferation but also increases the proportion of other nutrients [
30]. Based on the degradation characteristics of the CP, the proportion of the fraction
a in the FSM that was not subjected to rumen protection treatment slightly increased. No significant differences between the SM and FS groups were observed except for the MP (DM% × DM recovery), indicating that the small molecule peptides in the FSM increased after the action of various digestive enzymes. However, since the content of CP in the FSM was much higher than that in soybean meal, the FSM could provide more MP [
31,
32]. The efficiency of CP in providing MP in the rumen and small intestine was 65.9 and 80%, respectively, indicating that the same amount of protein was transferred from the rumen to the small intestine for digestion through the rumen protection treatment, providing 14% more MP. Using the MP (CP%) values of the FS and CF groups as an example (75.07 vs. 77.30%), the difference in the MP (CP%) between the two groups, divided by the improved efficiency, showed that 16% (CP%) of the protein passed through the rumen under the protection of chestnut tannins, compared with 13 and 7% in the QF and HF groups, respectively. The results of the present study are consistent with those of Lavrenčič et al., who concluded that the polyphenol groups of hydrolyzed tannins formed stronger bonds with CP, making their rumen effect superior to that of condensed tannins [
33]. The MP (DM%) of the CF group was the highest. The higher the MP (DM%), the more efficient the feed is in providing MP to dairy cows, which may improve the nitrogen utilization efficiency of dairy cows. However, animal experiments are still needed to determine whether the distribution of nitrogen in the body is affected. Previous studies by Zhang et al. and Aguerre et al. demonstrated that tannins can reduce urinary nitrogen excretion, although only Aguerre et al. reported an improvement in the nitrogen utilization efficiency in dairy cows [
15,
34]. Long-term feeding of feed containing tannins has been shown to positively impact the rumen and whole-gut health due to their antimicrobial properties. Pathogenic bacteria such as
Salmonella,
Pseudomonas, and
Helicobacter pylori are sensitive to tannins [
35,
36]. These effects are linked to the release of hydrogen peroxide and the number of hydroxyl groups of tannins when they are oxidized. However, Cidrini found that while tannins change the rumen microbial population, they do not affect performance [
37].
The amount of tannins added in the current study was 1.5% (DM basis), and whether their protective effects on protein could be further enhanced may be related to the type and amount of tannins added, the soybean meal granularity, the protein structure of the FSM, and the amount of water added. The ratio of RUP and ED of the heat-treated FSM was intermediate between those of the tannin treatment and FS groups, with a
c% lower than that of the other groups. However, no significant effect was observed, likely due to the variations in heating temperature, time, and method, consistent with the results of Lynch and Arce-Cordero [
38,
39]. The CP degradation rate of the HF group was lower than that of the QF and CF groups at 6 h but higher at 48 h. This indicates that heat treatment primarily functions by slowing down the rumen protein degradation rate over a short period [
38,
39]. With the extension of the time, the continuous action of digestive enzymes secreted by rumen microorganisms gradually breaks down the hydrophobic structure, increasing the solubility and increasing the degradation rate. Our results are consistent with those of Deng et al., who used attenuated total reflection Fourier transform infrared spectroscopy to demonstrate that heat treatment altered the ratio of amide I and II, α-helices, and β-sheets. These changes were strongly correlated with the rumen degradation parameters at different time points [
40]. However, heat treatment can somewhat reduce the CP content of the FSM. After 2 h of heating at 100 °C, the CP content of the FSM decreased by 3.08% (FS: 56.58, HF: 54.84%), resulting in higher MP (CP%) and lower MP (DM%) compared with those in the tannin treatment and FS groups. The same heat treatment method is more effective for rumen soybean meal, likely because digestive enzymes partially break down the protein structure of the FSM. Although heating exposes the hydrophobic groups of proteins, the effect of reducing the protein solubility is weakened. However, whether FSM has good palatability as a new type of feed for dairy cows, and whether it will affect the digestion and absorption of other nutrients, still needs to be further verified through animal feeding experiments.
Tannins can bind to both proteins and polysaccharides. Their phenol hydroxyl groups can either chemically react with or physically adsorb to the hydroxyl groups of cellulose [
41,
42]. When combined with fiber, the concentration of monosaccharides in forage reportedly affects the percentage of fiber in the fraction
b%. Some of this fiber is associated with proteins, thus ultimately reducing the ED of NDF and indirectly affecting protein degradation [
43]. Considering the differences in the NDF degradation rate and rumen dynamic parameter interpretation in the QF, CF, and HF groups in this study, the degradation rate did not significantly increase over time under relatively stable rumen environment conditions. This indicates that the binding of tannins and fiber was stronger. The observed negative
a% values of the NDF degradation characteristics in the SM and FS groups may be attributed to the use of a no lag model, which defaulted to a lag of 0 h. While no consensus has been reached on whether the lag time should be included in this model, Broderick suggested that the lag time can be omitted in the model for evaluating the RDP [
44]. However, when evaluating the rumen degradation characteristics of forage NDF, omitting the lag time may result in a higher probability of negative values for a% [
45].
In the current study, compared with the FS group, the addition of quebracho and chestnut tannins and heat treatment reduced the DM degradation rate by 14.4, 2.5, and 2.5%, and the OM degradation rate by 26.8, 6.5, and −1.7%, respectively, consistent with the results of Tabacco et al. [
13]. Lavrenčič collected sheep rumen fluid for in vitro experiments and found that when tannin-rich sweet chestnut and quebracho extracts were mixed with soybean meal, the DM degradation rate decreased with increasing treatment concentrations. In addition, the effect of chestnut tannins was more significant, although the dose of tannins used was 3–12% of the DM. However, the effect was not apparent at an addition level of 3%, possibly due to differences in the tannic acid content in the extracts used or substrate differences [
33]. The protein and fiber in FSM are degraded by various enzymes produced by microorganisms and added enzymes. Tannins may bind more fully to small protein and fiber molecules, reducing the fraction
b% and
c%, and ultimately reducing the ED of the DM. The different binding mechanisms of hydrolyzed and condensed tannins may contribute to the varying degrees of DM and OM degradation. When assessing the degradation characteristics of a particular feed, if the substrate contains tannins and other components that inhibit rumen microorganisms, a highly dynamic method such as the nylon bag method can be used in vivo. This approach enables microorganisms to obtain abundant nutrients and avoid the inhibitory effects of tannins on rumen microorganisms in a limited space [
46]. The results of the in vitro gas production test were also similar to those of the nylon bag method. The addition of tannins reduced the value of
c. Although the CP content in the FSM group was relatively high, the SM group contained more starch. An equal amount of starch can produce more gas than CP [
47]. Moreover, the theoretical total gas production reflects the gas production at 48 h. Combined with the results of the nylon bag method, the rumen-protective effects of the chestnut tannins and heat treatment were somewhat weakened at 48 h. These two points led to non-significant results in terms of the theoretical total gas production. The present study focused solely on the degradation characteristics of rumen-fermented soybean meal using this specific fermentation formulation, which we have obtained a patent for (CN 118892164 B). In this patent, we developed FSM with a nutrient composition similar to that of the FS group through fermentation using the same strain but with a different dosage ratio. After adding the same tannin dose, the rumen in situ incubation revealed that the final MP (DM%) from the FSM with a similar nutrient composition significantly differed. The binding differences between the tannins and the FSM with different fermentation formulations were demonstrated [
48]. The results of this study are affected by the efficiency of tannins in combining with nutrients, as well as the diet, quantity and physiological state of the dairy cows. More rumen in situ incubation or in vitro gas production tests are needed to determine the effect of combining FSM with chestnut tannins. We will further verify the palatability of the FSM with chestnut tannins and its effects on the milk production and nitrogen utilization efficiency of dairy cows through animal experiments.