Abstract
This study aimed to evaluate alkali-treated spent mushroom substrate (Pleurotus eryngii SMS) combined with condensed molasses solubles (CMS) as an alternative forage source for ruminants via in vitro fermentation. Pleurotus eryngii SMS was treated with different chemicals, including NaOH (at 5% in DM of SMS), H2O2 (at 3.5% in DM of SMS), and AHP (at 5% NaOH + 3.5% H2O2 in DM of SMS) to improve its fiber structure and digestibility. The NaOH and AHP treatments significantly increased the total gas production and volatile fatty acid (VFA) concentration and improved digestibility by about 1.5 to 1.8 times. CMS supplementation led to a 21% increase in microbial protein (MCP) synthesis in the NaOH treatment group. A replacement experiment involving a total mixed-ration diet indicated that CMS supplementation increased gas production while reducing dry matter digestibility in the NaOH treatment group. The AHP treatment group performed best at all substitution rates, particularly in digestibility and MCP synthesis. These results suggest that alkali treatment and CMS could improve the nutrient utilization efficiency of Pleurotus eryngii SMS, providing a feasible solution for the resource utilization of agricultural waste.
1. Introduction
Spent mushroom substrate comprises mostly wood shavings mixed with cottonseed hulls, corn cob, or corn stalks supplemented with rice bran or wheat middlings. Calcium carbonate is added to adjust the pH level, and the mixture is sterilized at high temperature and pressure before it is inoculated with pre-cultivated mushroom spores. Spent mushroom substrate (SMS) is the unused substrate and mycelium left after mushroom harvesting. SMSs could be used as animal feed as they contain over 6% crude protein and are rich in minerals such as iron, calcium, magnesium, and phosphorus [1,2]. Studies show that replacing bran in chicken feed with spent mushroom substrate can increase feed intake in chickens without significantly impacting carcass quality, indicating that this substitution is feasible [3]. For ruminants like cows, when processed by fungi, spent mushroom substrates have a reduced fiber content, improving digestibility and feed efficiency, which results in increased milk yield and feed intake in dairy cows [4]. Overall, spent mushroom substrates are a viable feed alternative for different animals and can reduce production costs, but the substitution ratio should be adjusted according to the needs of the animals.
Due to the presence of high levels of indigestible fiber components in SMS, to effectively convert SMS into roughage for ruminants, alkali treatment can be used to break the bonds between hemicellulose, cellulose, and lignin [5]. This disrupts the fiber structure, making it easier for fiber-decomposing microbes to attach to the fibers during the fermentation process in the rumen, thereby improving digestibility. Alkaline treatment is a simple and rapid technique that significantly improves the digestibility of highly lignified forages. Sodium hydroxide (NaOH) treatment breaks the bonds between lignin and cellulose, reducing the levels of NDF, ADF, and ADL and thereby enhancing the digestibility and energy utilization of forage [6]. The treatment of barley straw and sorghum grain with NaOH significantly improved fiber composition, increased gas production, and enhanced digestibility [7]. NaOH treatment of SMS was shown to increase the destruction of plant structures, leading to an increase in the fermentation rate in the rumen. Alkaline hydrogen peroxide (AHP) treatment combines sodium hydroxide with hydrogen peroxide, simultaneously breaking the bonds between fibers and reducing lignin content, making the forage more susceptible to microbial decomposition [8]. Studies have found that AHP treatment significantly improves the digestibility of dry matter and fiber and has a shorter delay in digestion time [9]. These treatment methods greatly enhance the nutritional value and microbial utilization of forages. However, when alkali-treated forage is used as the single feed source, it can lead to a negative nitrogen balance in animals. Cameron et al. [10] also mentioned that when alkali-treated (AHP treatment) wheat straw is used to replace alfalfa hay, it must be supplemented with nitrogen-providing ingredients. This ensures that the animals receive a complete range of nutrients while promoting a positive nitrogen balance in the rumen and supporting microbial growth.
Condensed molasses solubles (CMS) primarily come from fermentation industries. CMS contain high levels of crude protein and soluble carbohydrates, making them a potentially viable feed ingredient, and also help adjust the dietary cation–anion difference (DCAD). CMS are rich in amino acids, vitamins, and microbial protein, making them a cost-effective feed additive [11]. However, proper usage rates are crucial. In a study on dairy cows, Ma et al. [12] demonstrated that including more than 3% CMS in the diet’s total dry matter reduces feed intake, milk yield, and microbial protein synthesis, while 2% is recommended for maintaining rumen health. For beef cattle, replacing 5% of total dry matter intake with CMS has no significant effect, but exceeding 10% reduces weight gain and feed efficiency [13,14]. Combining molasses fermentation liquid with alkali-treated mushroom residues addresses the negative nitrogen balance caused by the treatment. The chloride ions in the molasses fermentation liquid neutralize the sodium ions from the alkaline treatment, allowing for higher usage levels. Additionally, the molasses fermentation liquid provides carbohydrates, non-protein nitrogen, and proteins, offering essential nutrients for microorganisms at different stages of residue digestion, thereby maximizing microbial protein synthesis efficiency.
The aim of this study was to evaluate the feasibility of using spent mushroom substrate supplemented with concentrated molasses solubles as roughage for ruminant animals through a parameter analysis of in vitro fermentation.
2. Materials and Methods
2.1. Spent Mushroom Substrate (SMS) and Chemical Treatments
The spent mushroom (Pleurotus eryngii) substrate (Pleurotus eryngii SMS) used for the experiment was collected from Xinshe District, Taichung City, Taiwan. The samples were stored at 4 °C to determine the moisture content, and the basic components were analyzed immediately. The collected SMS was chemically treated and divided into four groups with four replicates: (1) a control group comprising untreated SMS; (2) a NaOH group treated with a 50% NaOH solution, resulting in a final concentration of 5% of the dry weight of the SMS; (3) a H2O2 group treated with a 35% H2O2 solution, resulting in a final concentration of 3.5% of the dry weight of the SMS; and (4) an AHP treatment group (NaOH + H2O2) mixed with a 50% NaOH solution for 3 min, followed by the addition of a 35% H2O2 solution and another 3 min of mixing. The final concentrations of NaOH and H2O2 matched those of the previous two chemical treatment groups. Each chemical treatment group was then thoroughly mixed in a plastic container and let react at room temperature for three hours. Part of the mixture was then placed in a vacuum bag and stored at −20 °C, and the remaining mixture was dried in a 65 °C oven for one day to stop the reaction. The alkali-treated samples (4 replicates per sampling) were collected at 0, 2, 4, and 7 days after alkaline treatment for a chemical composition assay.
2.2. Chemical Composition Analysis
The SMS’s dry matter (DM, method 7.003), ash (method 942.05), crude protein (CP, method 984.13), acid detergent lignin (ADL, Methods 973.18), and ether extract (EE, Methods 920.39) were determined via the AOAC method [15]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were determined with an ANKOM200 (Ankom Technology, Corporation, Fairport, NY, USA) Fiber Analyzer using the manufacturer-recommended reagents and filter bags (#F57), as described by van Soest et al. [16]. Analysis of NDF was conducted without a heat-stable amylase and expressed exclusive of residual ash; ADF was also expressed exclusive of residual ash. The water-soluble carbohydrate (WSC) content was assayed according to the method described by Morris [17].
2.3. In Vitro Fermentation and Product Analysis
2.3.1. In Vitro Fermentation and In Vitro Dry Matter Digestibility (IVDMD)
In vitro gas production was determined using the rumen fluid collected from two rumen-fistulated Holstein cattle (about 750 kg body weight) fed a diet containing 30% Bermuda grass hay, 30% alfalfa hay, and 40% concentrate (as a DM basis). The rumen fluid was collected separately from each cattle two hours after feeding in the morning. The contents were squeezed through a four-layer cheesecloth and then mixed with prewarmed buffer [18] at a ratio of 1:4 (rumen fluid to buffer) under continuous CO2 flushing. Feed samples were ground to pass a 1 mm screen. After equilibrating the solution with CO2, a 40 mL aliquot of rumen fluid–buffer mixture was added to 50 mL incubation bottles containing 0.4 g samples of the experimental diets. The rumen fluid from each cattle was used as an independent source of inoculation to conduct two separate rounds of in vitro fermentation simultaneously, with four replicates for each diet or chemical treatment sample from each inoculation source. The Rumen fluid–buffer mixture was anaerobically dispensed in each bottle at 39 °C. The bottles were crimped, placed in an incubator at 39 °C, and shaken at regular intervals (every hour). Three bottles containing buffered rumen fluid only were used as blank samples. The gas pressure produced in each bottle was recorded using a pressure transducer (ANKOM RF Gas Production System, USA) for 72 h following the initiation of incubation. The in vitro degradability was determined using an Ankom DaisyII Incubator (Ankom Technology, Macedon, NY, USA) for 48 h. Digestion and sample collection were performed as described by Spanghero et al. [19]. The percentage weight loss was determined and presented as the in vitro DM degradability (IVDMD). The residual NDF was assayed to calculate the in vitro NDF degradability (IVNDFD).
2.3.2. Fermentation Product Analysis
The pH values were determined using a pH meter (F-71, HORIBA Scientific, Kyoto, Japan). The volatile fatty acid (VFA) concentration was analyzed using high-performance liquid chromatography (HPLC) with RexezTM ROA-Organic Acid H+ (8%) column (300 × 4.6 mm, Phenomenex, USA). The sample injection value was 20 μL, and the oven temperature was 39 °C. The flow rate of the mobile phase (0.01N H2SO4) was 0.5mL/min, and the UV detector was set at 210 nm. The ammonia nitrogen was then recorded, as described by Chaney and Marbach [20]. The microbial crude protein (MCP) was conducted according to the purine content method described by Zinn and Owens [21].
2.4. Concentrated Molasses Soluble (CMS) Supplementation and Preparation of Total Mixed Ration
Based on the treatment results, after 4 days of treatment, Pleurotus eryngii SMS samples were selected to sequence the CMS supplementation experiment. After the Pleurotus eryngii SMS underwent alkali treatment and was balanced to room temperature, different alkali-treated samples (after 4 days of treatment) were mixed thoroughly with or without CMS (at 5% DM basis) to evaluate the effect of CMS on in vitro fermentation and digestion. The NaOH- and AHP-treated Pleurotus eryngii SMSs were chosen to replace 0%, 50%, and 100% of Pangola hay in a complete mixed diet based on the fermentation results from SMS supplementation with CMS. A 5% CMS concentration was also paired with these diets (mixed with prepared TMRs) and subjected to further in vitro examination to determine the optimal substitution level (see Table 1). All TMR diets in this study were formulated using the National Research Council [22] recommendation for a multiparous lactating dairy cow (650 kg of BW) producing 25 kg/d of milk containing 3.0% true milk protein and 3.5% milk fat. In the preparation of TMR, water is added to adjust the moisture content, bringing the dry matter of the diet to 50%.

Table 1.
Formulation of total mixed ration and nutrient composition after replacement.
This CMS product was provided by Vedan Company (Taichung, Taiwan). The chemical composition of the CMS was as follows: moisture content, 38.2%; CP content, 30.2%; minor amounts of crude fat, 1.6%; and no crude fiber. In CMS with a high ash content (over 23.6%), chloride is the most common mineral (16.5%), followed by potassium (4.3%) and sodium (2.1%).
2.5. Statistical Analysis
Data were processed using Statistix 8.0 (Analytical Software, Tallahassee, FL, USA) and Tukey’s Honestly Significant Difference Test to compare the treatment groups’ differences. The SAS 9.4 (SAS Institute Inc., Cary, NC, USA)) was employed to investigate the interactions between the chemical treatments and the presence or absence of CMS. The analysis included three substitution rates (0%, 50%, and 100%), three treatment groups (untreated, NaOH-treated, and AHP-treated), and CMS supplementation. Statistical significance was set at p < 0.05.
Gas production kinetics were modeled using a two-phase nonlinear model in GraphPad Prism 7.0 (GraphPad Software, Inc.), with parameter values calculated in MATLAB R2019a (The MathWorks, Inc.). The dynamic degradation model was adjusted to improve the fit by maximizing the R2 value using the following equation:
where () is the initial proportion of the residue, () and () represent rapid and slow degradation portions, () and () are the degradation rates of () and () portions, and () is fermentation time (h). The final gas production value at the end of fermentation (72 h) was considered 100%, and each treatment’s gas production was compared based on its contribution to total production. The dynamic model was modified to ensure the total digestion did not exceed 100%. Tukey’s Honestly Significant Difference Test was used to compare treatment group differences.
3. Results
3.1. Composition Analysis of Alkali Treatment of Pleurotus eryngii SMS
The dry matter content of Pleurotus eryngii SMS is approximately 61%, with a neutral detergent fiber (NDF) content of about 64.6%, an acid detergent fiber (ADF) content of 52.7%, and an acid detergent lignin (ADL) content of 17.5%. The CP of untreated SMS is 8.53%, but the ether extract and WSC contents are very low (0.12% and 0.14%, respectively). Following treatment with NaOH and AHP, NDF, ADF, and ADL contents decreased significantly. As shown in Table 2, the ADF content exhibited a notable reduction only on day 2 post-treatment. Regarding crude protein and ash content, the crude protein levels in the NaOH- and AHP-treated groups were significantly lower than those in the control and H2O2-treated groups, with substantial differences observed across treatment days. Conversely, the ash content in the NaOH and AHP groups significantly increased, though no significant differences were detected between these two groups. After a longer processing period, the nitrogen-free extract (NFE) levels in the H2O2-treated group were the lowest. In contrast, the NaOH and AHP groups exhibited slightly lower values than those of the control group.

Table 2.
The chemical composition (%DM) and total gas production after 72 h fermentation of Pleurotus eryngii SMS after different chemical treatments and time (mean ± SD, n = 4).
3.2. In Vitro Fermentation and Digestion Evaluation of Pleurotus eryngii SMS After Alkali Treatment
Gas Production Performance and Fermentation Products
The gas production of Pleurotus eryngii SMS treated with NaOH and AHP was higher than that of both the control and H2O2 treatment groups (Figure 1 and Table 1). For the H2O2 treatment group, the gas production was higher than that of the control group on days 0 and 2 but declined to levels comparable to the control group by days 4 and 7 (Table 1). As shown in Table 3, the total volatile fatty acid (VFA) concentrations in the NaOH- and AHP-treated groups were higher than those in the control group. Specifically, the NaOH-treated group achieved the highest concentration on day 7, while the AHP-treated group peaked on day 4. The concentrations of acetic acid and propionic acid in the NaOH and AHP groups exceeded those in both the control and H2O2 groups. Notably, butyric acid was undetectable before day 4, possibly due to challenges associated with structural utilization. Regarding ammonia nitrogen concentration, the NaOH and AHP groups exhibited lower levels than the H2O2 group, with concentrations increasing over time. Furthermore, microbial crude protein (MCP) synthesis in the NaOH and AHP groups on days 0 and 2 was higher than in the H2O2 group, with the AHP group reaching the maximum value on day 4. The in vitro dry matter digestibility (IVDMD) and in vitro neutral detergent fiber digestibility (IVNDFD) of Pleurotus eryngii SMS treated with NaOH and AHP were significantly higher than those of the control and H2O2 groups (Table 3). Regarding these factors, the AHP group performed better than the NaOH group. Additionally, the IVDMD and IVNDFD of the H2O2 group on days 0 and 7 of treatment were also significantly higher than those of the control group.
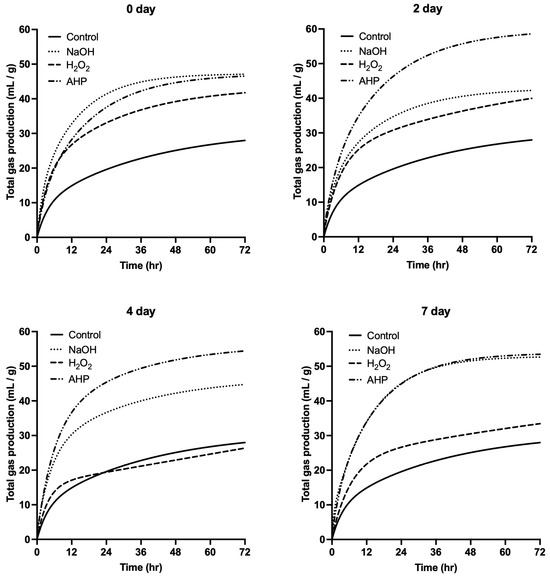
Figure 1.
The total gas production pattern of Pleurotus eryngii SMS after different chemical treatments and time. NaOH: final concentration at 5% DM; H2O2: final concentration at 3.5% DM; AHP: (NaOH + H2O2) treatment.

Table 3.
The fermentation products and in vitro digestibility (72 h incubation) of Pleurotus eryngii SMS after different chemical treatments and time (mean ± SD, n = 4).
3.3. Alkali-Treated SMS with CMS Supplementation
3.3.1. Analysis of Total Gas Production Volume and Kinetic Parameters
The gas production efficiency of the NaOH- and AHP-treated groups was significantly higher than that of the control and H2O2-treated groups, regardless of whether CMS were added (Figure 2). For the H2O2-treated group, the gas production was lower than that of the control group both before and after the addition of CMS. In contrast, the gas production values of other chemically treated cultivation residues increased after CMS addition, with the control group showing the most significant improvement (p < 0.05). As shown in Table 4, the a and b1 values in the NaOH- and AHP-treated groups are lower than those of the control and H2O2-treated groups both before and after CMS addition. This suggests that gas production in the control and H2O2-treated groups primarily originated from rapidly degradable organic matter. Notably, the b1 value in the H2O2-treated group increased significantly after CMS addition, while the b2 value decreased. In contrast, the impact of CMS addition on the NaOH and AHP groups was less pronounced. A significant interaction was observed between the type of chemical treatment and the addition of CMS (p < 0.05), primarily affecting the changes in b1 and b2 values. Before CMS addition, the c1 and c2 values in the control and H2O2-treated groups were lower than those in the NaOH and AHP groups, with the AHP group achieving the highest values. After CMS addition, the c1 values in the control and H2O2-treated groups increased significantly, resulting in steeper gas production curves that shifted closer to the Y-axis. For the slow degradation phase, the c2 values in the NaOH- and AHP-treated groups remained significantly higher than those in the control and H2O2-treated groups after CMS addition. This indicates that Pleurotus eryngii SMSs treated with NaOH and AHP are more readily utilized by microorganisms, demonstrating a higher degradation potential.
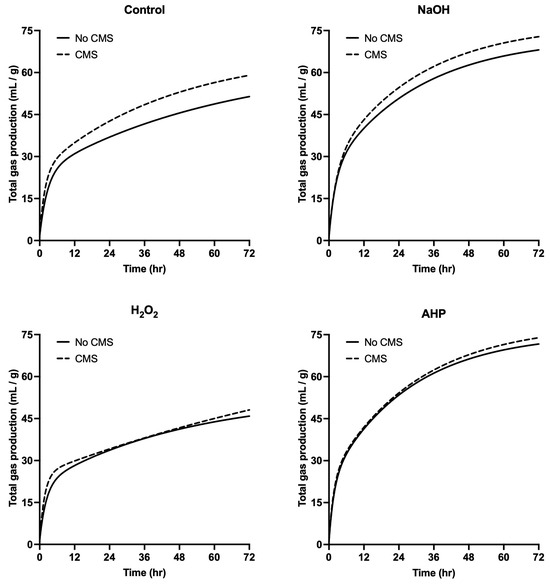
Figure 2.
Effect of CMS supplementation on total gas production pattern of Pleurotus eryngii SMS after different chemical treatments for 4 days. No CMS: without CMS supplementation; CMS: supplementation of CMS at 5% DM; NaOH: final concentration at 5% DM; H2O2: final concentration at 3.5% DM; AHP: (NaOH + H2O2) treatment.

Table 4.
The effect of CMS supplementation on the in vitro gas production kinetics parameters of Pleurotus eryngii SMS after different chemical treatments (mean ± SD, n = 4).
3.3.2. Fermentation Product Analysis
In terms of volatile fatty acid (VFA) concentrations, as shown in Table 5, the acetic acid and propionic acid levels in the NaOH- and AHP-treated groups before CMS addition were significantly higher than those in the control and H2O2-treated groups. The butyric acid concentration in the NaOH- and AHP-treated groups was slightly higher than in other groups, though the difference was not statistically significant. After CMS addition, the concentrations of acetic acid, propionic acid, and butyric acid in the NaOH- and AHP-treated groups showed an increasing trend, but the differences remained non-significant.

Table 5.
The effect of CMS supplementation on the fermentation products (72 h) of Pleurotus eryngii SMS after different chemical treatments for 4 days (mean ± SD, n = 4).
The ammonia nitrogen concentrations were similar across all treatment groups before CMS addition, with slightly higher levels observed in the control and H2O2-treated groups (Table 5). After CMS addition, ammonia nitrogen concentrations increased significantly in the NaOH- and AHP-treated groups. Regarding microbial protein synthesis efficiency, the control and H2O2-treated groups, characterized by lower dry matter digestibility, had significantly higher microbial protein synthesis efficiency compared to the NaOH- and AHP-treated groups (Table 5). After CMS addition, all groups demonstrated increased dry matter digestion, with the NaOH-treated group exhibiting the most pronounced improvement. Furthermore, microbial protein synthesis efficiency increased significantly in the control, NaOH-treated, and H2O2-treated groups following CMS addition, with the NaOH-treated group showing the most significant improvement.
Regarding pH changes, there were no significant differences among the treatment groups before CMS addition (Table 6). However, after CMS addition, the NaOH-treated group exhibited the highest pH value, while the pH values of the control, H2O2-treated, and AHP-treated groups decreased slightly. The control group showed the most significant pH decline.

Table 6.
The effect of CMS supplementation on in vitro dry matter digestibility (IVDMD) and pH value (at 72 h) of Pleurotus eryngii SMS after different chemical treatments for 4 days (mean ± SD, n = 4).
3.4. The Effect of CMS Supplementation and Substitution of Alkali-Treated SMS to Pangola Hay in the Total Mixed Ration (TMR)
NaOH and AHP treatments effectively reduce the fiber content of Pleurotus eryngii SMSs and significantly enhance their in vitro digestibility. After four days of treatment, the NaOH and AHP groups exhibited higher concentrations of volatile fatty acids and improved microbial protein synthesis efficiency, making them suitable as raw materials to replace Pangola hay. Additionally, a substitution rate test for the control group was conducted to evaluate the feasibility of directly using Pleurotus eryngii SMS.
Regarding gas production, as shown in Table 7, the control group exhibited a downward trend as the TMR substitution rate increased. However, the differences between substitution rates were not significant. After adding the CMS, the gas production value increased in all treatment groups, with the best performance observed at a 50% substitution rate. In the NaOH treatment group, the gas production value at a 100% substitution rate significantly increased after CMS addition. While the gas production value at a 100% substitution rate in the AHP group was the lowest, it still exhibited an increasing trend after CMS addition. However, the change was not statistically significant. For pH changes, the pH value at the end of fermentation (72 h) increased with the substitution rate, reaching its highest value at 100% substitution. Both the control and NaOH treatment groups exhibited significant increases in pH after CMS addition, particularly at the 50% substitution rate. Conversely, in the AHP group, CMS addition resulted in a decrease in pH, with the most pronounced decrease at the 50% substitution rate.

Table 7.
The effect of alkaline treatment and CMS supplementation on total gas production, in vitro dry matter digestibility (IVDMD), and pH of the TMR with different levels of Pleurotus eryngii SMS replacement after different chemical treatments (mean ± SD, n = 4).
Volatile fatty acid concentrations are shown in Table 8. In the control group, the acetic acid and butyric acid concentrations decreased as the substitution rate increased, reaching their lowest values at 100% substitution. The concentration of propionic acid was the lowest at a 50% substitution rate. In contrast, the NaOH treatment group exhibited decreased volatile fatty acid concentrations after CMS addition, while the AHP group saw a significant increase in acetic acid, propionic acid, and butyric acid concentrations, especially at 50% and 100% substitution rates. The ammonia nitrogen concentration in the control group increased slightly with higher substitution rates. While CMS addition improved ammonia nitrogen concentration, the change was not significant. In the NaOH treatment group, CMS addition significantly increased the ammonia nitrogen concentration, which peaked at a 100% substitution rate. In the AHP treatment group, CMS increased the ammonia nitrogen concentration, which resulted in the highest microbial protein synthesis efficiency at the 50% substitution rate, demonstrating excellent performance. Both the NaOH and AHP treatments enhanced the utilization of Pleurotus eryngii SMS. In the control group, a 50% substitution rate combined with CMS significantly increased gas production and total volatile fatty acid concentrations, although the microbial protein synthesis efficiency slightly decreased. In the NaOH treatment group, though it reduced dry matter digestibility and microbial protein synthesis efficiency, CMS addition at a 50% substitution rate significantly increased gas production. The AHP treatment group performed best at all substitution rates, highlighting its strong potential as a TMR raw material.

Table 8.
The effect of alkaline treatment method and CMS supplementation on fermentation products of the TMR with different levels Pleurotus eryngii SMS replacement (mean ± SD, n = 4).
4. Discussion
4.1. Effect of Alkali Treatment on Pleurotus eryngii SMS
4.1.1. Changes in Fiber Structure
Research indicates that only alkali treatment (NaOH) and oxidizing alkali treatment (AHP) significantly reduce the residual fiber content in Pleurotus eryngii SMS. Chaudhry and Miller [23] reported that treating wheat straw with NaOH led to a substantial decrease in NDF content, with the best results achieved by adding 50 g of NaOH per kilogram of dry matter. However, the effects on ADF and ADL were relatively limited. Myung and Kennelly (1989) [9] observed that treating straw with 50% NaOH and 1% H2O2 significantly reduced the ADL content and had a minimal impact on the NDF and ADF contents. In this study, the NaOH and AHP treatments effectively reduced the NDF, ADF, and ADL contents of Pleurotus eryngii SMS, indicating significant alterations in the fiber structure. Additionally, H2O2 treatment showed a relatively strong effect on reducing hemicellulose content, though its impact on the cellulose content was limited. This may be attributed to the high moisture content and loose fiber structure of the residues. However, alkali treatments may result in some nutrient loss, with Wanapat et al. [6] noting that the crude protein content of wheat straw declined after NaOH treatment. Despite this, NaOH and AHP treatments enhance the microbial utilization of the substrate by modifying the fiber structure. In contrast, the effects of H2O2 treatment are limited, with some nutrients remaining inaccessible due to their encapsulation within the fiber structure.
4.1.2. Fermentation and Total Gas Production Characteristics
The NaOH and AHP treatments significantly increased gas production and volatile fatty acid concentrations in Pleurotus eryngii SMS, demonstrating improved fermentation efficiency (Table 3). The AHP treatment group exhibited superior uniformity and stability, supporting long-term fermentation, and changes in ammonia nitrogen concentrations further validated these results. The NaOH and AHP treatments initially reduced ammonia nitrogen concentrations, but these increased over time, suggesting that nutrient release following treatment is closely linked to microbial activity. The NaOH and AHP treatments markedly improved the in vitro digestibility of the Pleurotus eryngii SMS. Wilson and Pigden [24] found that the in vitro digestibility of wheat straw and poplar wood was significantly enhanced after NaOH treatment, with higher NaOH concentrations yielding better results. Wang et al. [25] also observed that NaOH-treated wheat straw exhibited increased volume, swelling capacity, and reduced porosity, all of which were strongly correlated with improved digestibility. Myung and Kennelly [9] further demonstrated that AHP-treated rice straw showed significantly higher dry matter and NDF digestibility in the in situ tests than NaOH-treated samples.
4.2. Effects of Alkali Treatment Combined with CMS on Pleurotus eryngii SMS Interaction Between Total Gas Production and Fermentation Characteristics
The interaction between alkalization treatments (NaOH, AHP, H2O2) and CMS addition significantly influenced gas production parameters, particularly the c1 (fast fermentation phase) and c2 (slow fermentation phase) values. Coupled with the analysis of fermentation products (Table 5), these findings underscore the alterations in nutrient use efficiency resulting from the various chemical treatments in combination with CMS. After CMS addition, the NaOH treatment group showed a marked improvement in microbial protein synthesis efficiency, consistent with a significant reduction in ammonia nitrogen concentration. This suggests that microbial uptake of nitrogen sources increased, promoting protein synthesis. Although the substrate digestion rates were lower in the control group and the H2O2 treatment group, they unexpectedly exhibited higher microbial protein synthesis efficiency. This phenomenon may not result from increased protein synthesis but could instead be an artifact of matrix degradation and uneven nutrient distribution. According to studies by Zali et al. [26] and Fernández et al. [27], while adding sugar beet CMS tends to increase ammonia nitrogen concentrations, the differences are generally not statistically significant. Bas et al. [28] noted that AHP-treated wheat straw exhibited lower organic matter digestibility, leading to a decline in microbial protein synthesis efficiency. Similarly, this study found that the microbial protein synthesis efficiency of the AHP-treated group was lower than that of the NaOH-treated group, possibly due to reduced substrate availability following treatment. NaOH treatment combined with CMS significantly enhanced substrate digestibility and fermentation product yields, particularly gas production performance and volatile fatty acid concentrations (Table 4 and Table 5).
In contrast, the AHP and H2O2 treatment groups exhibited more limited effects. Notably, the AHP treatment group may have experienced reduced substrate availability during the treatment process, which constrained its potential benefits. The control group, lacking improvements in fiber structure, demonstrated significantly poorer performance, emphasizing the critical role of alkalization in enhancing substrate utilization.
4.3. Effects of Pleurotus eryngii SMS Combined with CMS on the Total Mixed Ration
An increasing trend in pH was observed with higher CMS substitution levels in TMR, potentially due to the carbohydrate source provided by CMS or their non-protein nitrogen [29]. A previous study found that adding 4% CMS to the TMR diet of Merino sheep caused a slight but insignificant decrease in rumen pH, likely due to the increased supply of available carbohydrates and feed intake [30]. Similarly, Ma et al. [12] reported that adding 1% to 4% CMS to the diet of lactating cows significantly reduced rumen pH, illustrating the correlation between substrate utilization efficiency and pH fluctuations during fermentation. However, a higher pH may indicate improved substrate utilization efficiency, warranting further investigation into its impact on long-term fermentation stability. Although CMS supplementation provided additional carbohydrate sources, VFA concentrations decreased in certain treatment groups, which suggests that microbial carbon utilization efficiency plays a key role. In the AHP treatment group, CMS addition significantly increased butyric acid concentrations. Similar findings were reported by Potter et al. [14] and O’Reilly [30] in beef cattle and Merino sheep diets, respectively, demonstrating that the sugars in CMS stimulate butyric acid production. However, the increase in gas production may be linked to microbial lysis rather than higher VFA concentrations [31].
Additionally, microorganisms may utilize VFAs to synthesize proteins or lipids, resulting in decreased VFA concentrations [32]. The influence of CMS on microbial protein synthesis efficiency depends on the balance between carbohydrate and nitrogen sources within the substrate. While CMS provide a rapidly available nitrogen source, insufficient carbohydrates may limit its ability to enhance microbial protein synthesis. In this study, CMS addition did not significantly reduce ammonia nitrogen concentrations, suggesting lower nitrogen utilization efficiency [33], particularly in untreated or poorly treated substrates.
5. Conclusions
This study confirms that combining chemical treatments and CMS can significantly enhance the nutrient utilization efficiency of Pleurotus eryngii SMS, offering a practical solution for agricultural waste resource utilization. Among the treatments tested, the combination of AHP treatment and CMS yielded the most favorable results, though the NaOH treatment group demonstrated consistent improvements. The in vitro results indicated no effect on digestion and fermentation products when alkali-treated Pleurotus eryngii SMS replaced grass hay by up to 50% in the present diet. Future research should focus on optimizing treatment methods and CMS supplementation levels and exploring the interactions between microbial utilization and matrix degradation. Additionally, animal studies should be conducted to validate these results, thereby providing a stronger scientific foundation for the efficient resource use of agricultural by-products.
Author Contributions
Conceptualization, B.-Y.C. and H.-T.W.; Methodology, H.-T.W.; Software, B.-Y.C.; Formal analysis, P.-S.W.; Data curation, B.-Y.C. and P.-S.W.; Writing—original draft, B.-Y.C.; Writing—review & editing, H.-T.W.; Project administration, H.-T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Science and Technology, Taiwan (MOST 108-2622-B-002-009-CC3).
Institutional Review Board Statement
All animal care procedures used in this study were approved by the Institutional Animal Care and Use Committee of the National Taiwan University (approval no: NTU-110-EL-00068).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bae, J.S.; Kim, Y.I.; Jung, S.H.; Oh, Y.G.; Kwak, W.S. Evaluation on feed-nutritional value of spent mushroom (Pleurotus osteratus, Pleurotus eryngii, Flammulina velutupes) substrates as a roughage source for ruminants. J. Anim. Sci. Technol. 2006, 48, 237–246. [Google Scholar] [CrossRef]
- Oh, Y.K.; Lee, W.M.; Choi, C.W.; Kim, K.H.; Hong, S.K.; Lee, S.C.; Seol, Y.J.; Kwak, W.S.; Choi, N.J. Effects of spent mushroom substrates supplementation on rumen fermentation and blood metabolites in Hanwoo steers. Asian-Australas. J. Anim. Sci. 2010, 23, 1608–1613. [Google Scholar] [CrossRef]
- Baptista, F.; Almeida, M.; Paié-Ribeiro, J.; Barros, A.N.; Rodrigues, M. Unlocking the potential of spent mushroom substrate (sms) for enhanced agricultural sustainability: From environmental benefits to poultry nutrition. Life 2023, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, H.; Shafyee-Varzeneh, H.; Farahpoor, A.; Moayyer, A. Recycling of mushroom compost wheat straw in the diet of feedlot calves with two physical forms. Int. J. Recycl. Org. Waste Agric. 2014, 3, 3. [Google Scholar] [CrossRef]
- Gould, J.M. Studies on the mechanism of alkaline peroxide delignification of agricultural residues. Biotechnol. Bioeng. 1985, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M.; Sundstøl, F.; Garmo, T.H. A comparison of alkali treatment methods to improve the nutritive value of straw. I. Digestibility and metabolizability. Anim. Feed Sci. Technol. 1985, 12, 295–309. [Google Scholar] [CrossRef]
- Parnian, F.; Taghizadeh, A.; Paya, H.; Nobari, B. In vitro fermentation response to alkaline treated sorghum grain. J. Biosci. Biotech. 2014, 3, 23–28. [Google Scholar]
- Gould, J.M. Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification. Biotechnol. Bioeng. 1984, 26, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Myung, K.; Kennelly, J. Effect of alkaline hydrogen peroxide treatment of rice straw on in sacco ruminal digestibility. Asian-Australas. J. Anim. Sci. 1989, 2, 311–314. [Google Scholar] [CrossRef]
- Cameron, M.G.; Fahey, G.C.; Clark, J.H.; Merchen, N.R.; Berger, L.L. Effects of feeding alkaline hydrogen peroxide-treated wheat straw-based diets on digestion and production by dairy cows. J. Dairy Sci. 1990, 73, 3544–3554. [Google Scholar] [CrossRef] [PubMed]
- Moeini, M. Effect of molasses distillers condensed soluble on nutrients digestibility, performance and some blood biological parameters in lambs. Annu. Res. Rev. Biol. 2014, 4, 443–450. [Google Scholar] [CrossRef]
- Ma, J.; Ma, C.; Fan, X.; Shah, A.M.; Mao, J. Use of condensed molasses fermentation solubles as an alternative source of concentrates in dairy cows. Anim. Biosci. 2021, 34, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.G.; Ammerman, C.B.; Henry, P.R.; Becker, H.N.; Palmer, A.Z. Effect of sugarcane condensed molasses solubles, sugarcane molasses and monensin on performance and volatile fatty acid production in finishing steers. Anim. Feed Sci. Technol. 1985, 12, 275–283. [Google Scholar] [CrossRef]
- Potter, S.G.; Moya, A.; Henry, P.R.; Palmer, A.Z.; Becker, H.N.; Ammerman, C.B. Sugarcane condensed molasses solubles as a feed ingredient for finishing cattle. J. Anim. Sci. 1985, 60, 839–846. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrate with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Menke, H.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Spanghero, M.; Boccalon, S.; Gracco, L.; Gruber, L. NDF degradability of hays measured in situ and in vitro. Anim. Feed Sci. Technol. 2003, 104, 201–208. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Zinn, R.A.; Owens, F.N. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Can. J. Anim. Sci. 1986, 66, 157–166. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th rev. ed.; National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Chaudhry, A.S.; Miller, E.L. The effect of sodium hydroxide and alkaline hydrogen peroxide on chemical composition of wheat straw and voluntary intake, growth and digesta kinetics in store lambs. Anim. Feed Sci. Technol. 1996, 60, 69–86. [Google Scholar] [CrossRef]
- Wilson, R.K.; Pigden, W.J. Effect of a sodium hydroxide treatment on the utilization of wheat straw and poplar wood by rumen microorganisms. Can. J. Anim. Sci. 1964, 44, 122–123. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Wu, Y.M.; Ye, J. Improvement of organic matter digestibility along with changes of physical properties of rice straw by chemical treatments. J. Anim. Feed Sci. 2006, 15, 147–157. [Google Scholar] [CrossRef]
- Zali, A.; Eftekhari, M.; Fatehi, F.; Ganjkhanlou, M. Effect of vinasse (condensed molasses solubles) on performance and meat chemical composition of Holstein male calves. Ital. J. Anim. Sci. 2017, 16, 515–520. [Google Scholar] [CrossRef]
- Fernández, B.; Bodas, R.; López-Campos, Ó.; Andrés, S.; Mantecón, A.R.; Giráldez, F.J. Vinasse added to dried sugar beet pulp: Preference rate, voluntary intake, and digestive utilization in sheep. J. Anim. Sci. 2009, 87, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Bas, F.J.; Stern, M.D.; Fahey, G.C., Jr. Alkaline hydrogen peroxide-treated wheat straw as a source of energy for ruminal bacteria in continuous culture. J. Anim. Sci. 1989, 67, 2081–2088. [Google Scholar] [CrossRef]
- Stemme, K.; Gerdes, B.; Harms, A.; Kamphues, J. Beet-vinasse (condensed molasses solubles) as an ingredient in diets for cattle and pigs-nutritive value and limitations. J. Anim. Physiol. Anim. Nutr. 2005, 89, 179–183. [Google Scholar] [CrossRef]
- O’Reilly, K. Effect of Condensed Molasses Solubles on Intake Growth Performance Digestibility and Certain Rumen Parameters of Sheep. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2017. [Google Scholar]
- Blümmel, M.; Ørskov, E.R. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Technol. 1993, 40, 109–119. [Google Scholar] [CrossRef]
- Emmanuel, B.; Milligan, L.P.; Turner, B.V. The metabolism of acetate by rumen microorganisms. Can. J. Microbiol. 1974, 20, 183–185. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).