Characterization, Production, and Application of Antifungal Metabolites from Probiotic Levilactobacillus and Lactiplantibacillus Strains Isolated from Fermented Olives
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Antifungal Activity of Levilactobacillus and Lactiplantibacillus Cells
2.3. Fermentation and Preparation of the Cell-Free Supernatant (CFS)
2.4. Antimicrobial Activity of CFS from Levilactobacillus and Lactiplantibacillus
2.4.1. Well Diffusion Assay
2.4.2. Mycelium and Biomass Inhibition
in assay/Fungal biomass (g) in assay)/(Fungal colony diameter (mm) in control/
Fungal biomass (g) in control)) × 100
2.5. Characterization of Antifungal Metabolites from Levilactobacillus and Lactiplantibacillus
2.6. Production of Antifungal Metabolites from Levilactobacillus and Lactiplantibacillus
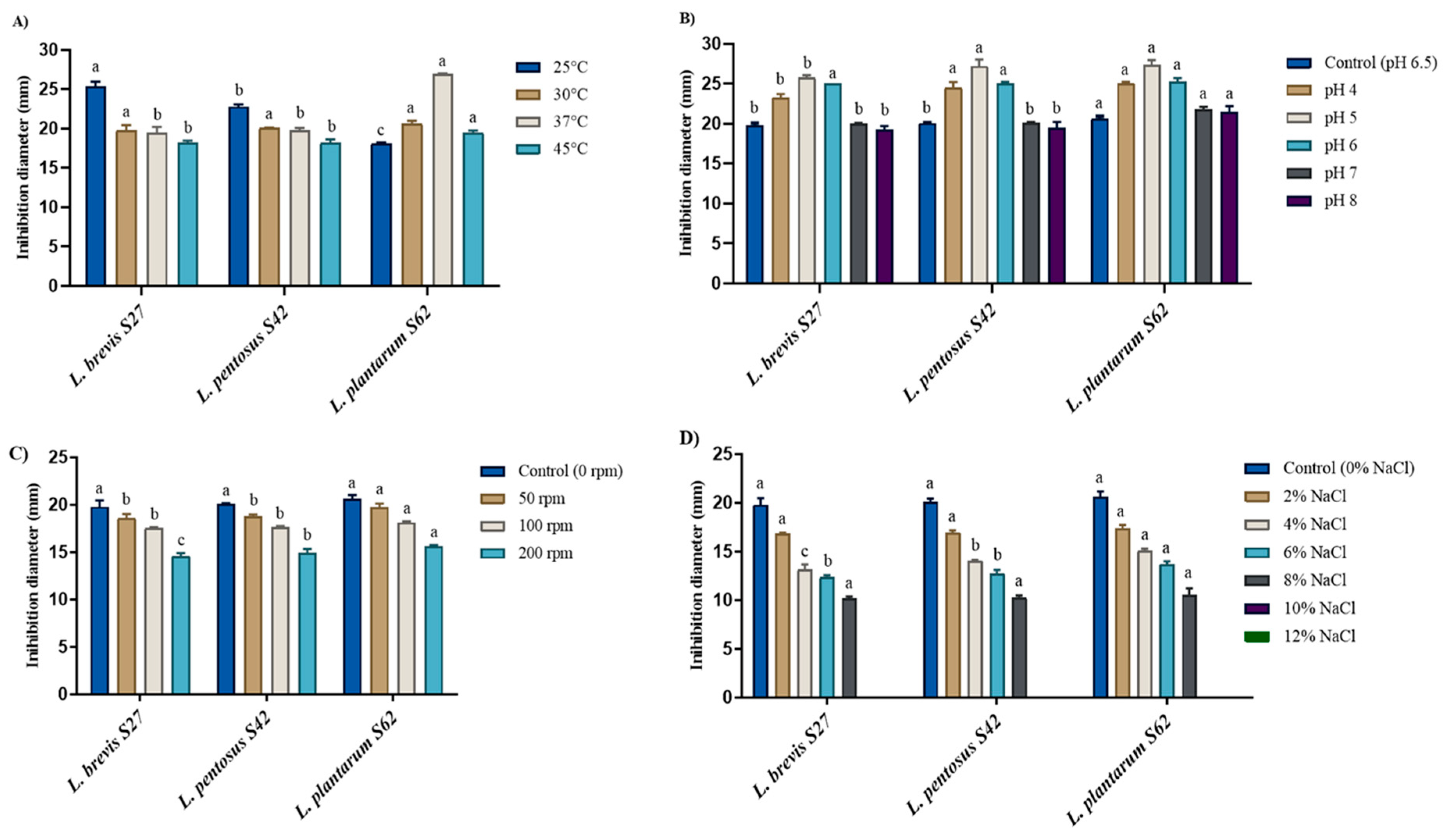
2.6.1. Influence of Temperature
2.6.2. Influence of pH
2.6.3. Influence of Agitation
2.6.4. Influence of NaCl
2.7. Application of Levilactobacillus and Lactiplantibacillus and Their CFS as Bio-Preservative Agents
2.7.1. Application of CFS from Levilactobacillus and Lactiplantibacillus to Yogurt
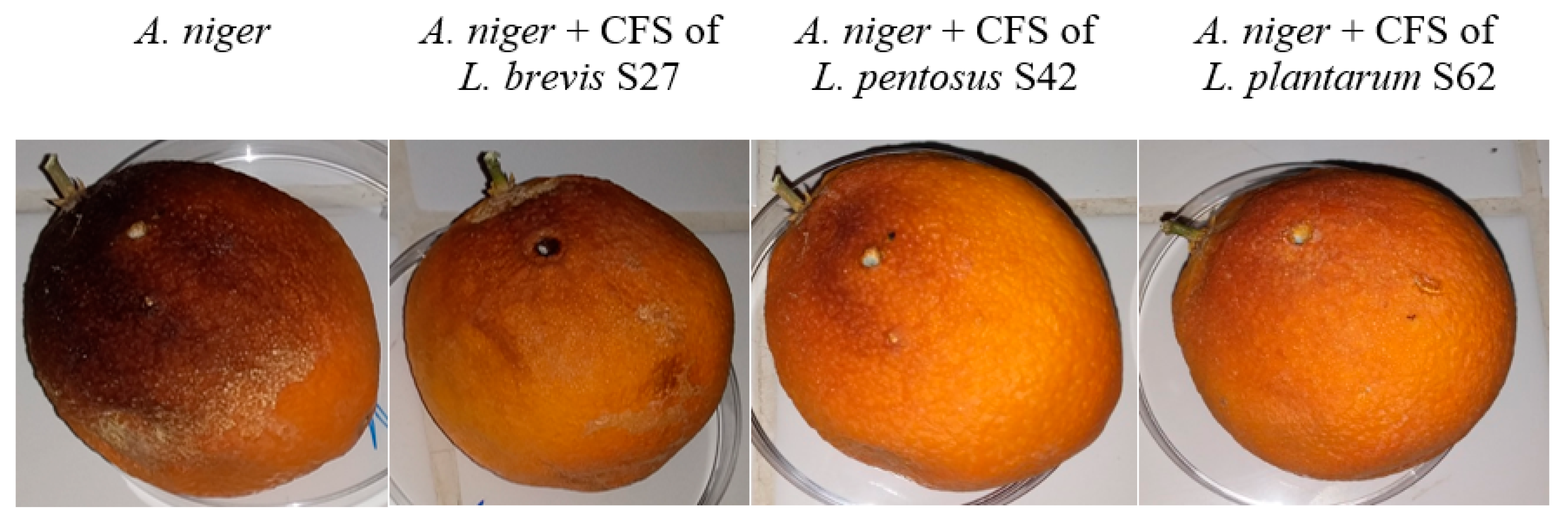
2.7.2. Application of Levilactobacillus and Lactiplantibacillus to Orange Fruit
2.8. Statistical Analysis
3. Results
3.1. Antifungal Activity of Levilactobacillus and Lactiplantibacillus Cells
3.2. Antimicrobial Activity of CFS from Levilactobacillus and Lactiplantibacillus
3.3. Characterization of Antifungal Metabolites from Levilactobacillus and Lactiplantibacillus
3.4. Production of Antifungal Metabolites from Levilactobacillus and Lactiplantibacillus
3.5. Application of Levilactobacillus and Lactiplantibacillus Strains on Yogurt
3.6. Application of CFS from Levilactobacillus and Lactiplantibacillus on Orange Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural occurrence, analysis, and prevention of mycotoxins in fruits and their processed products. Crit. Rev. Food Sci. Nutr. 2014, 54, 64–83. [Google Scholar] [CrossRef]
- Abdel-Nasser, A.; Hathout, A.S.; Badr, A.N.; Barakat, O.S.; Fathy, H.M. Extraction and characterization of bioactive secondary metabolites from lactic acid bacteria and evaluating their antifungal and antiaflatoxigenic activity. Biotechnol. Rep. 2023, 38, e00799. [Google Scholar] [CrossRef]
- Freire, F.D.C.O.; Da Rocha, M.E.B. Impact of mycotoxins on human health. In Fungal metabolites; Springer: Berlin/Heidelberg, Germany, 2017; pp. 239–261. [Google Scholar]
- Luz, C.; Saladino, F.; Luciano, F.B.; Mañes, J.; Meca, G. In vitro antifungal activity of bioactive peptides produced by Lactobacillus plantarum against Aspergillus parasiticus and Penicillium expansum. LWT—Food Sci. Technol. 2017, 81, 128–135. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Warner, J.O. Artificial food additives: Hazardous to long-term health? Arch. Dis. Child. 2024, 109, 882–885. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- EFSA. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 15: Suitability of taxonomic units notified to EFSA until September 2021. EFSA J. 2022, 20, e07045. [Google Scholar] [CrossRef]
- Akpoghelie, P.O.; Edo, G.I.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Owheruo, J.O.; Isoje, E.F.; Igbuku, U.A.; Essaghah, A.E.A.; Makia, R.S.; et al. Lactic acid bacteria: Nature, characterization, mode of action, products and applications. Process Biochem. 2025, 152, 1–28. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Abouloifa, H.; Gaamouche, S.; Ghabbour, N.; El Guerrouj, B.; Karboune, S.; Saalaoui, E.; Asehraou, A. Lactic acid bacteria from Moroccan traditional foods: Techno-functional, health-promoting, nutraceutical value and application as a starter and bio-preservative agent in the food products. Bioresour. Technol. Rep. 2024, 27, 101941. [Google Scholar] [CrossRef]
- Yang, H.; Hao, L.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Functional roles and engineering strategies to improve the industrial functionalities of lactic acid bacteria during food fermentation. Biotechnol. Adv. 2024, 74, 108397. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, V.P.; Rajamani, S. Recent Advancements in Harnessing Lactic Acid Bacterial Metabolites for Fruits and Vegetables Preservation. Probiotics Antimicrob Proteins 2025, 17, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Banicod, R.J.S.; Tabassum, N.; Javaid, A.; Kim, Y.M.; Khan, F. Lactic Acid Bacteria-Derived Secondary Metabolites: Emerging Natural Alternatives for Food Preservation. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Jung, E.S.; Todorov, S.D.; Holzapfel, W.H. Evaluation of antifungal metabolites produced by lactic acid bacteria. Probiotics Antimicrob. Proteins 2023, 15, 1447–1463. [Google Scholar] [CrossRef]
- Batish, K.K.; Utpal, R.; Ram, L.; Grover, S. Antifungal Attributes of Lactic Acid Bacteria—A Review. Crit. Rev. Biotechnol. 1997, 17, 209–225. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Matevosyan, L.A.; Bazukyan, I.L.; Trchounian, A.H. Antifungal activity of lactic acid bacteria isolates and their associations: The effects of Ca and Mg divalent cations. Curr. Microbiol. 2020, 77, 959–966. [Google Scholar] [CrossRef]
- Souza, L.V.; Martins, E.; Moreira, I.; de Carvalho, A.F. Strategies for the Development of Bioprotective Cultures in Food Preservation. Int. J. Microbiol. 2022, 2022, 6264170. [Google Scholar] [CrossRef] [PubMed]
- Abouloifa, H.; Rokni, Y.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; Salah, R.B.; Chihib, N.E.; Saalaoui, E.; Asehraou, A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins 2020, 12, 683–696. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnurer, J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef]
- Guimaraes, A.; Santiago, A.; Teixeira, J.A.; Venancio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef]
- Riolo, M.; Luz, C.; Santilli, E.; Meca, G.; Cacciola, S.O. Antifungal activity of selected lactic acid bacteria from olive drupes. Food Biosci. 2023, 52, 102422. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Liang, N.; Zhao, Z.; Curtis, J.M.; Ganzle, M.G. Antifungal cultures and metabolites of lactic acid bacteria for use in dairy fermentations. Int. J. Food Microbiol. 2022, 383, 109938. [Google Scholar] [CrossRef]
- Volentini, S.I.; Olmedo, G.M.; Grillo-Puertas, M.; Rapisarda, V.A.; Hebert, E.M.; Cerioni, L.; Villegas, J.M. Biological control of green and blue molds on postharvest lemon by lactic acid bacteria. Biol. Control 2023, 185, 105303. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- Gharbi, Y.; Fhoula, I.; Ruas-Madiedo, P.; Afef, N.; Boudabous, A.; Gueimonde, M.; Ouzari, H.-I. In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: Interaction with pathogenic bacteria and the enteric cell line HT29. Ann. Microbiol. 2018, 69, 61–72. [Google Scholar] [CrossRef]
- Chen, H.; Ju, H.; Wang, Y.; Du, G.; Yan, X.; Cui, Y.; Yuan, Y.; Yue, T. Antifungal activity and mode of action of lactic acid bacteria isolated from kefir against Penicillium expansum. Food Control 2021, 130, 108274. [Google Scholar] [CrossRef]
- Chen, O.; Hong, Y.; Ma, J.; Deng, L.; Yi, L.; Zeng, K. Screening lactic acid bacteria from pickle and cured meat as biocontrol agents of Penicillium digitatum on citrus fruit. Biol. Control 2021, 158, 104606. [Google Scholar] [CrossRef]
- Falguni, P.; Shilpa, V.I.J.; Mann, B. Production of proteinaceous antifungal substances from Lactobacillus brevis NCDC 02. Int. J. Dairy Technol. 2010, 63, 70–76. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Guo, Z.; Ji, N.; Sun, Q.; Liu, T.; Li, Y. Antifungal activities of Pediococcus pentosaceus LWQ1 and Lactiplantibacillus plantarum LWQ17 isolated from sourdough and their efficacies on preventing spoilage of Chinese steamed bread. Food Control 2025, 168, 110940. [Google Scholar] [CrossRef]
- Abouloifa, H.; Hasnaoui, I.; Rokni, Y.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; Abousalham, A.; Jaouadi, B.; Saalaoui, E. Antifungal activity of lactic acid bacteria and their application in food biopreservation. In Advances in applied microbiology, Elsevier: Amsterdam, 2022; Volume 120, pp. 33–77.
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z.; Meor Hussin, A.S. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 2020, 109, 106898. [Google Scholar] [CrossRef]
- Barman, S.; Ghosh, R.; Mandal, N.C. Production optimization of broad spectrum bacteriocin of three strains of Lactococcus lactis isolated from homemade buttermilk. Ann. Agrar. Sci. 2018, 16, 286–296. [Google Scholar] [CrossRef]
- Batish, V.K.; Lal, R.; Grover, S. Studies on environmental and nutritional factors on production of antifungal substance by Lactobacillus acidophilus R. Food Microbiol. 1990, 7, 199–206. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Font de Valdez, G.; Gerez, C.L. Optimization of phenyllactic acid production by Pediococcus acidilactici CRL 1753. Application of the formulated bio-preserver culture in bread. Biol. Control 2018, 123, 137–143. [Google Scholar] [CrossRef]
- Sangmanee, P.; Hongpattarakere, T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control 2014, 40, 224–233. [Google Scholar] [CrossRef]
- Muthusamy, K.; Soundharrajan, I.; Srisesharam, S.; Kim, D.; Kuppusamy, P.; Lee, K.D.; Choi, K.C. Probiotic characteristics and antifungal activity of Lactobacillus plantarum and its impact on fermentation of Italian Ryegrass at low moisture. Appl. Sci. 2020, 10, 417. [Google Scholar] [CrossRef]
- Rouse, S.; Harnett, D.; Vaughan, A.; van Sinderen, D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Jurkiewicz, C.; Landgraf, M.; Todorov, S.D.; Franco, B.D.G.M. Effect of proteins, glucose and NaCl on growth, biosynthesis and functionality of bacteriocins of Lactobacillus sakei subsp. sakei 2a in foods during storage at 4 °C: Tests in food models. LWT—Food Sci. Technol. 2018, 95, 167–171. [Google Scholar] [CrossRef]
- Ramos, I.M.; Navajas Porras, B.; Delgado-Osorio, A.; Rufián-Henares, J.Á.; Poveda, J.M. Impact of Lactiplantibacillus plantarum UCLM56 on the bioactive properties of sheep’s milk yogurt before and after in vitro digestion and fermentation. LWT 2025, 228, 118101. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, F.; Zhang, Y.; Zhang, Y.; Wang, H.; Song, J.; Suo, H. A novel strain Lactiplantibacillus plantarum LPP95 isolated from Chinese pickles: Antifungal effect, mechanism, and potential application in yogurt. Food Biosci. 2024, 58, 103640. [Google Scholar] [CrossRef]
- Mélo, E.D.d.; e Silva, P.I.S.; do Oriente, S.F.; Almeida, R.D.; Pessoa, J.M.; França, K.B.; de Gusmão, T.A.S.; de Gusmão, R.P.; Lisboa Oliveira, H.M.; Nascimento, A.P.S. Effect of Commercial Bioprotective Lactic Cultures on Physicochemical, Microbiological, and Textural Properties of Yogurt. Fermentation 2024, 10, 585. [Google Scholar] [CrossRef]
- Ma, J.; Hong, Y.; Deng, L.; Yi, L.; Zeng, K. Screening and characterization of lactic acid bacteria with antifungal activity against Penicillium digitatum on citrus. Biol. Control 2019, 138, 104044. [Google Scholar] [CrossRef]


| LAB Strain | Inhibition Zone | |||
|---|---|---|---|---|
| A. niger | P. digitatum | F. oxysporum | R. oryzae | |
| L. brevisS27 | ++ | ++ | + | − |
| L. pentosusS42 | +++ | +++ | +++ | ++ |
| L. plantarumS62 | +++ | +++ | +++ | ++ |
| LAB Strain | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| Pathogenic Bacteria | Yeast | |||||
| L. monocytogenes ATCC 19117 | S. aureus ATCC 6538 | P. aeruginosa ATCC 49189 | S. enterica ATCC 14028 | R. glutinis UMP22 | C. pelliculosa | |
| L. brevisS27 | 17.05 a ± 0.07 | 17.1 a ± 0.14 | 16.7 ab ± 0.14 | 16.53 b ± 0.39 | 22.78 b ± 0.21 | 19.78 b ± 0.73 |
| L. pentosusS42 | 17.05 a ± 0.07 | 17.15 a ± 0.07 | 16.75 a ± 0.35 | 16.65 ab ± 0.21 | 23.06 ab ± 0.07 | 20.06 b ± 0.34 |
| L. plantarumS62 | 17.1 a ± 0.14 | 17.15 a ± 0.07 | 16.85 a ± 0.07 | 16.9 a ± 0.14 | 23.63 a ± 0.14 | 20.63 a ± 0.40 |
| LAB Strains | Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Mycelium | |||||||
| A. niger | P. digitatum | F. oxysporum | R. oryzae | A. niger | P. digitatum | F. oxysporum | R. oryzae | |
| L. brevisS27 | 78.90 c ± 0.71 | 80.00 c ± 0.54 | 74.69 b ± 0.72 | 57.66 b ± 0.63 | 42.50 c ± 0.14 | 47.55 c ± 0.07 | 43.28 c ± 0.49 | 40.15 b ± 0.14 |
| L.pentosusS42 | 82.77 b ± 0.45 | 82.20 b ± 0.45 | 75.26 b ± 0.56 | 57.19 b ± 0.52 | 48.70 b ± 0.07 | 49.25 b ± 0.28 | 44.55 b ± 0.28 | 40.07 b ± 0.28 |
| L. plantarumS62 | 86.13 a ± 0.13 | 84.50 a ± 0.50 | 78.82 a ± 0.71 | 60.44 a ± 0.61 | 50.08 a ± 0.14 | 55.59 a ± 0.64 | 49.57 a ± 0.07 | 45.64 a ± 0.49 |
| Conditions | LAB Strains | ||
|---|---|---|---|
| L. brevis S27 | L. pentosus S42 | L. plantarum S62 | |
| CFS without treatment (pH 4) | 19.78 b ± 0.73 | 20.06 b ± 0.34 | 20.63 a ± 0.40 |
| Effect of pH | |||
| 4.5 | 19.5 a ± 0.69 | 19.8 a ± 0.28 | 20.2 a ± 0.28 |
| 5 | 18.3 b ± 0.42 | 18.6 b ± 0.28 | 19.05 a ± 0.07 |
| 5.5 | 15.9 b ± 0.28 | 16.05 b ± 0.07 | 16.25 a ± 0.07 |
| 6 | 14.1 b ± 0.14 | 15.25 a ± 0.35 | 15.35 a ± 0.4 |
| 6.5 | 13.85 c ± 0.07 | 14.6 b ± 0.42 | 15.1 a ± 0.14 |
| 7 | 13.8 c ± 0.14 | 14.4 b ± 0.14 | 15.05 a ± 0.07 |
| Effect of Temperature | |||
| 80 °C/10 min | 19.78 b ± 0.73 | 20.06 b ± 0.34 | 20.63 a ± 0.40 |
| 100 °C/10 min | 19.6 b ± 0.42 | 20.0 b ± 0.28 | 20.63 a ± 0.28 |
| Sterilization | 19.5 b ± 0.42 | 19.8 b ± 0.28 | 20.5 a ± 0.42 |
| Effect of storage (4 °C) | |||
| Week 1 | 19.78 b ± 0.73 | 20.06 b ± 0.34 | 20.63 a ± 0.40 |
| Week 2 | 19.55 a ± 0.63 | 19.85 a ± 0.35 | 20.3 a ± 0.14 |
| Week 3 | 18.6 b ± 0.28 | 19.0 a ± 0.4 | 19.2 a ± 0.14 |
| Week 4 | 17.6 b ± 0.28 | 18.0 a ± 0.14 | 18.2 a ± 0.14 |
| Effect ofenzymes | |||
| pH7 + Catalase | 13.75 c ± 0.07 | 14.45 b ± 0.21 | 15.1 a ± 0.14 |
| pH7 + Protease | ND | ND | ND |
| pH7 + Proteinase K | ND | ND | ND |
| pH7 + Lipase | 13.7 c ± 0.42 | 14.48 b ± 0.17 | 15.08 a ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouloifa, H.; Gaamouche, S.; Ghabbour, N.; Hasnaoui, I.; Bentouhami, N.E.; Rokni, Y.; Karboune, S.; Asehraou, A. Characterization, Production, and Application of Antifungal Metabolites from Probiotic Levilactobacillus and Lactiplantibacillus Strains Isolated from Fermented Olives. Fermentation 2025, 11, 661. https://doi.org/10.3390/fermentation11120661
Abouloifa H, Gaamouche S, Ghabbour N, Hasnaoui I, Bentouhami NE, Rokni Y, Karboune S, Asehraou A. Characterization, Production, and Application of Antifungal Metabolites from Probiotic Levilactobacillus and Lactiplantibacillus Strains Isolated from Fermented Olives. Fermentation. 2025; 11(12):661. https://doi.org/10.3390/fermentation11120661
Chicago/Turabian StyleAbouloifa, Houssam, Sara Gaamouche, Nabil Ghabbour, Ismail Hasnaoui, Nour Eddine Bentouhami, Yahya Rokni, Salwa Karboune, and Abdeslam Asehraou. 2025. "Characterization, Production, and Application of Antifungal Metabolites from Probiotic Levilactobacillus and Lactiplantibacillus Strains Isolated from Fermented Olives" Fermentation 11, no. 12: 661. https://doi.org/10.3390/fermentation11120661
APA StyleAbouloifa, H., Gaamouche, S., Ghabbour, N., Hasnaoui, I., Bentouhami, N. E., Rokni, Y., Karboune, S., & Asehraou, A. (2025). Characterization, Production, and Application of Antifungal Metabolites from Probiotic Levilactobacillus and Lactiplantibacillus Strains Isolated from Fermented Olives. Fermentation, 11(12), 661. https://doi.org/10.3390/fermentation11120661






