From Raw Milk Microbiome to Cheese: The Challenge of Indigenous Natural Starter Culture Exploitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plan
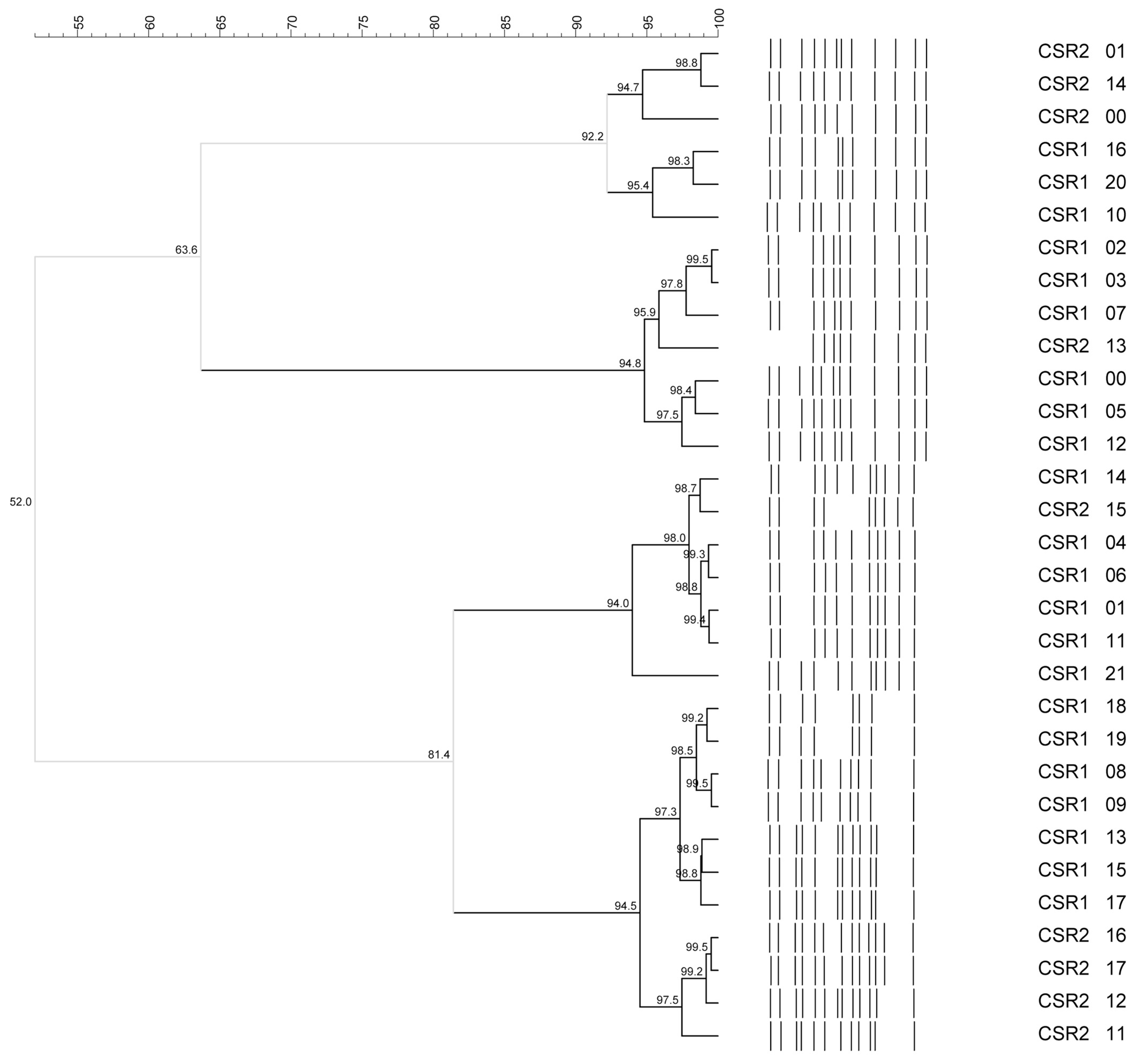
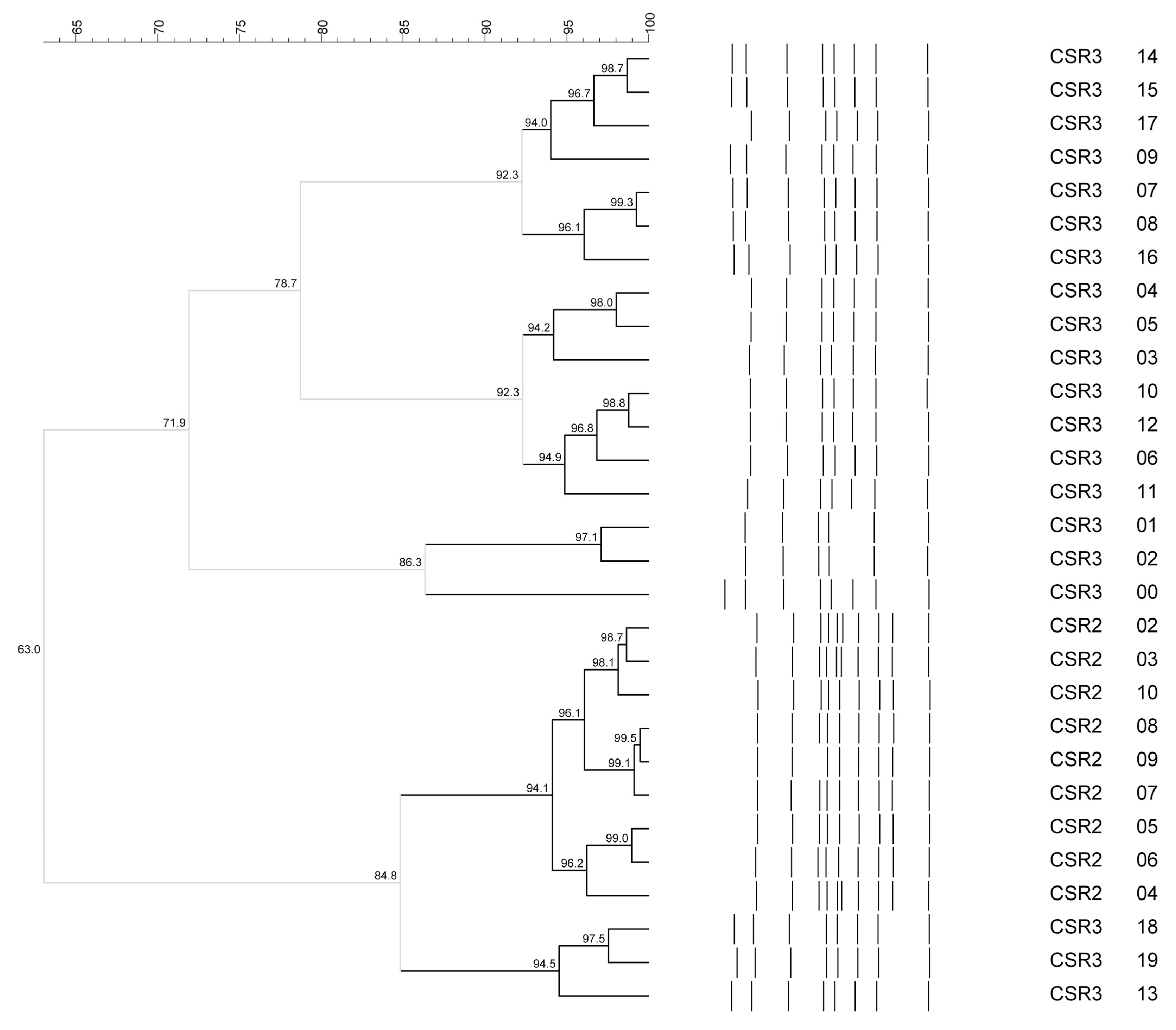
2.2. Identification and Biodiversity Evaluation of Bacterial Isolates for Str-Mix and Lb-Mix Preparation
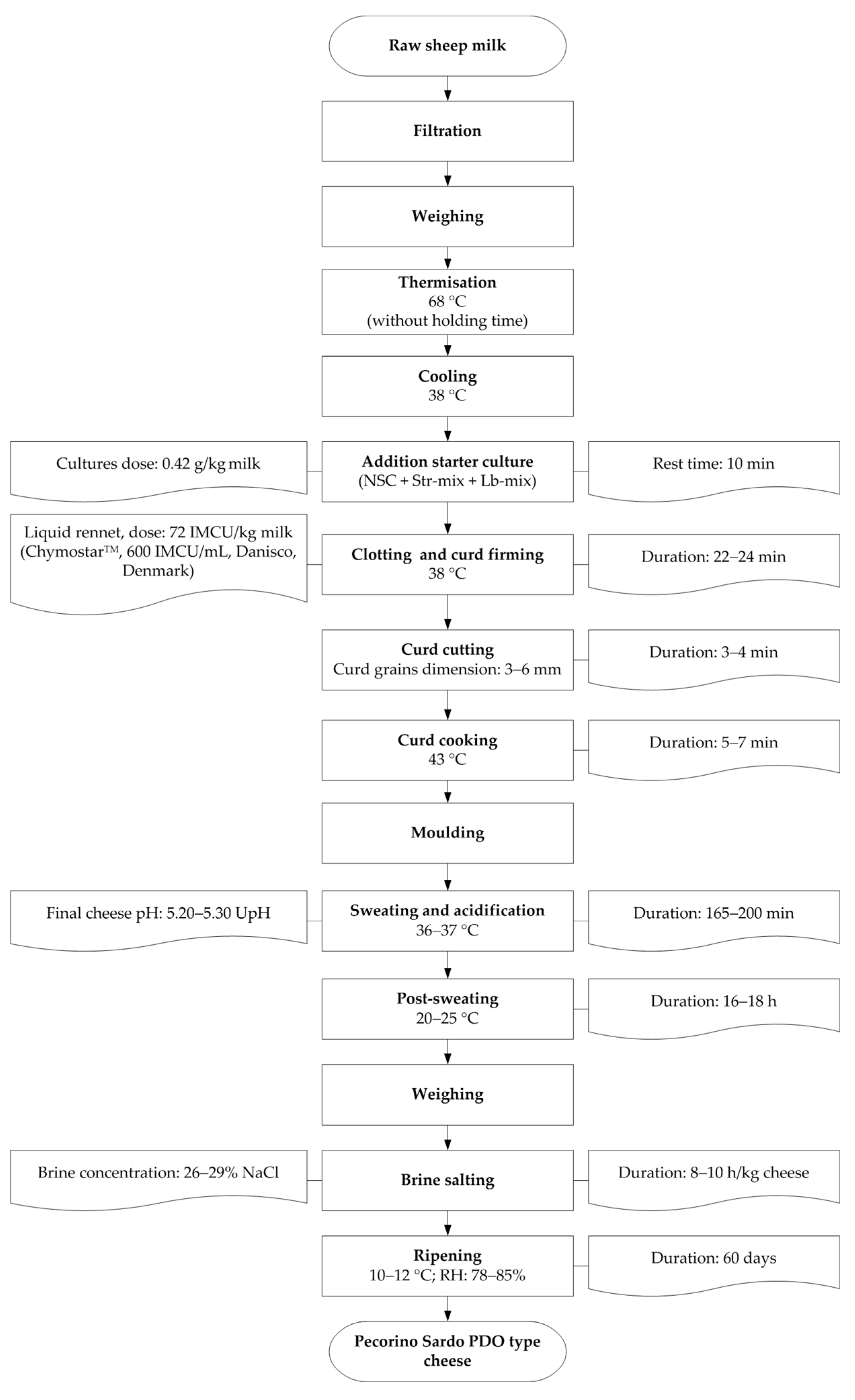
2.3. Starter Culture Preparation and Cheesemaking
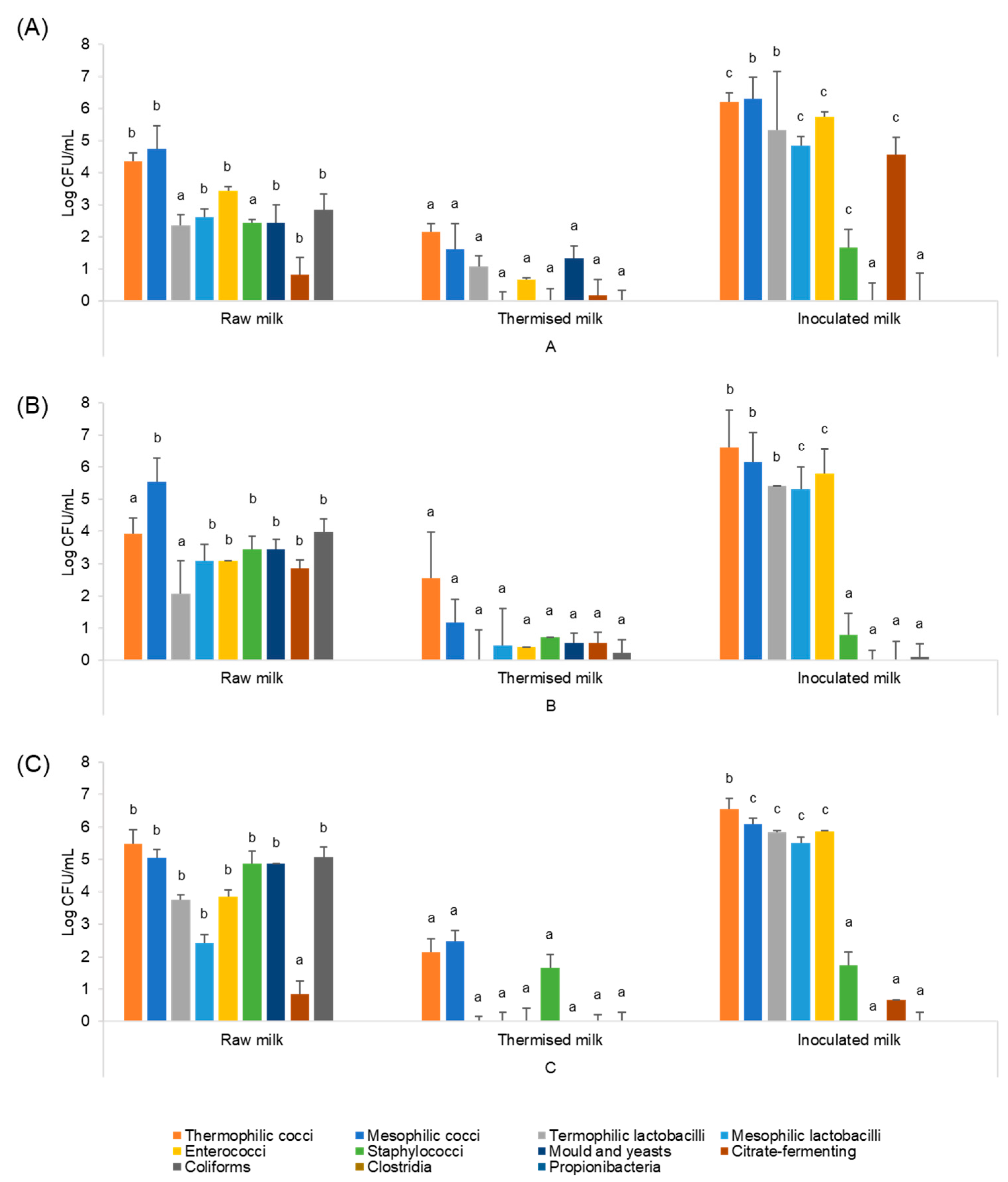
2.4. Microbial Analysis
2.5. Chemical Analysis
2.5.1. Milk
2.5.2. Cheeses
2.6. Consumer Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Identification and Biodiversity Evaluation of the Bacterial Isolates for Str-Mix and Lb-Mix Preparation
3.2. Technological Parameters of Cheesemaking
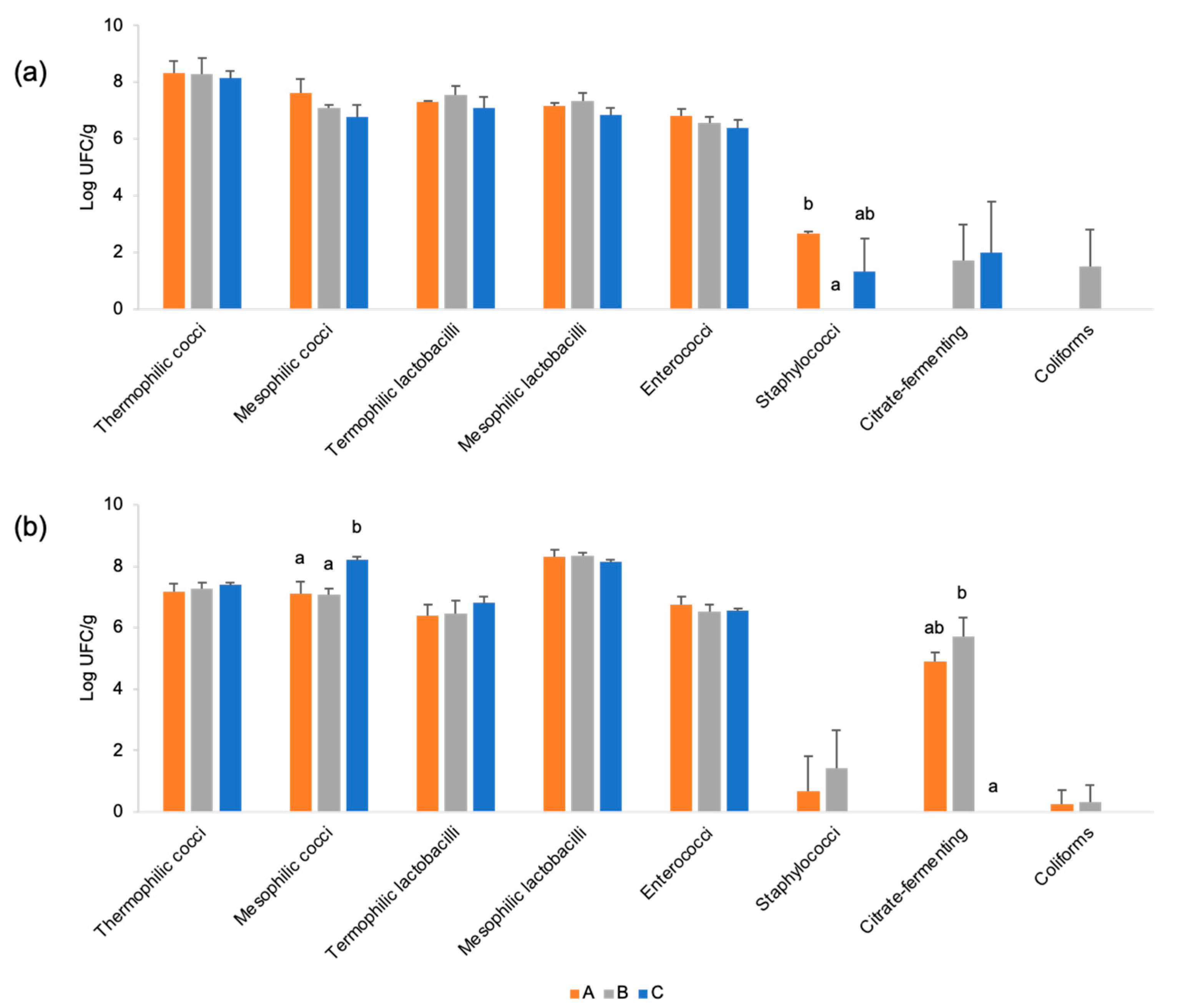
3.3. Microbial Characterisation
3.4. Chemical Characterisation
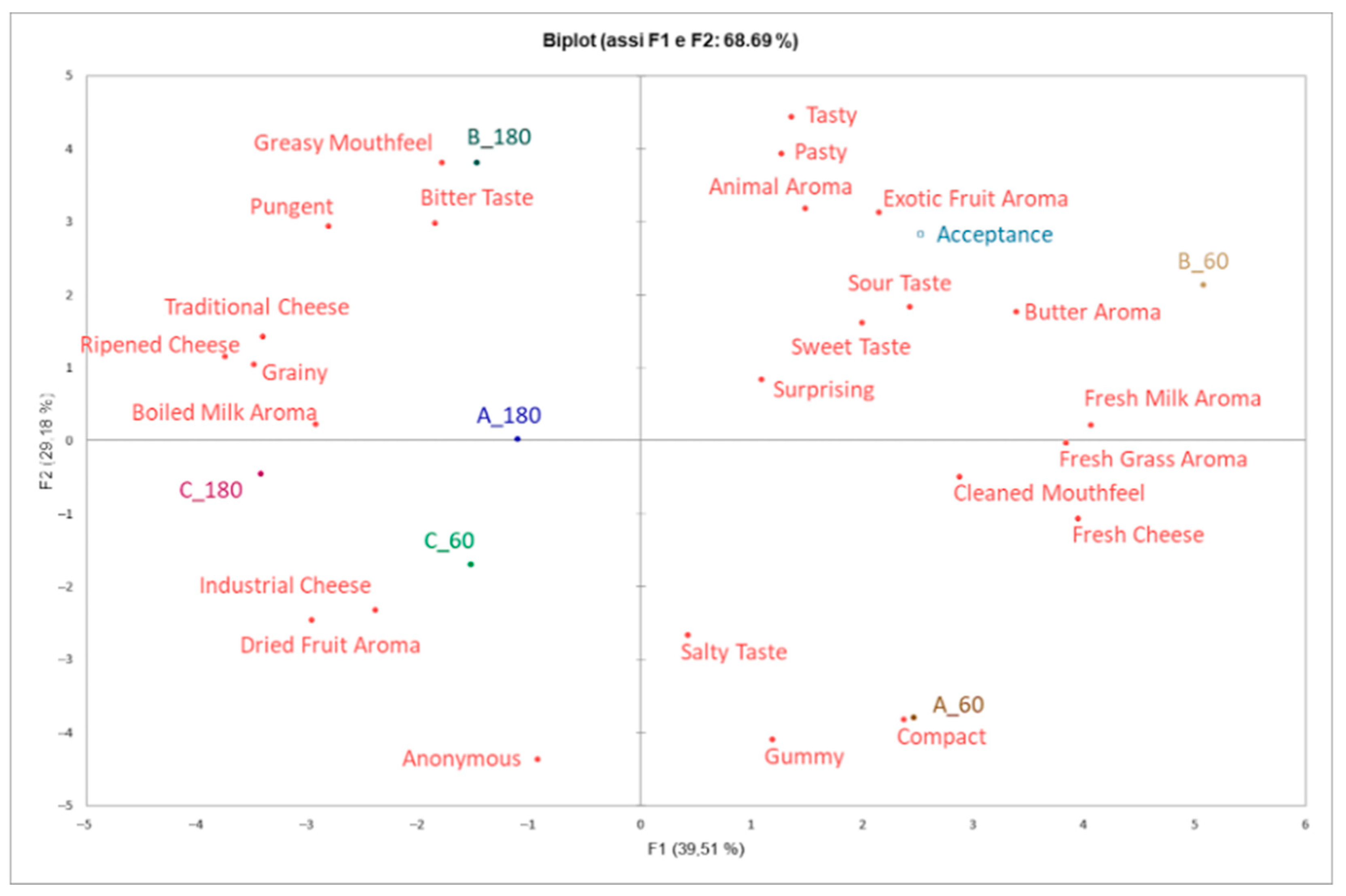
3.5. Consumer Liking and Perception
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carminati, D.; Giraffa, G.; Quiberoni, A.; Binetti, A.; Suárez, V.; Reinheimer, J. Advances and trends in starter cultures for dairy fermentations. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2010; pp. 177–192. [Google Scholar] [CrossRef]
- Cogan, T.M.; Beresford, T.P.; Steele, J.; Broadbent, J.; Shah, N.P.; Ustunol, Z. Invited review: Advances in starter cultures and cultured foods. J. Dairy. Sci. 2007, 90, 4005–4021. [Google Scholar] [CrossRef]
- Ercolini, D.; Frisso, G.; Mauriello, G.; Salvatore, F.; Coppola, S. Microbial diversity in natural whey cultures used for the production of Caciocavallo Silano PDO cheese. Int. J. Food Microbiol. 2008, 124, 164–170. [Google Scholar] [CrossRef]
- Kelleher, P.; Murphy, J.; Mahony, J.; van Sinderen, D. Next-generation sequencing as an approach to dairy starter selection. Dairy Sci. Technol. 2015, 95, 545–568. [Google Scholar] [CrossRef]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef]
- Parente, E.; Cogan, T.M.; Powell, I. Starter Cultures: General Aspects; Academic Press: Cambridge, MA, USA, 2017; pp. 201–226. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Chessa, L.; Paba, A.; Daga, E.; Dupré, I.; Comunian, R. Biodiversity and safety assessment of half-century preserved natural starter cultures for Pecorino Romano PDO cheese. Microorganisms 2021, 9, 1363. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Daga, E.; Dupré, I.; Piga, C.; Di Salvo, R.; Mura, M.; Addis, M.; Comunian, R. Autochthonous natural starter cultures: A chance to preserve biodiversity and quality of Pecorino Romano PDO cheese. Sustainability 2021, 13, 8214. [Google Scholar] [CrossRef]
- Montel, M.C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef]
- García-Díez, J.; Saraiva, C. Use of starter cultures in foods from animal origin to improve their safety. Int. J. Environ. Res. Public Health 2021, 18, 2544. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic acid bacteria in raw-milk cheeses: From starter cultures to probiotic functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Andrighetto, C.; Marcazzan, G.; Lombardi, A. Use of RAPD-PCR and TTGE for the evaluation of biodiversity of whey cultures for Grana Padano cheese. Lett. Appl. Microbiol. 2004, 38, 400–405. [Google Scholar] [CrossRef]
- Panesar, P.S. Fermented dairy products: Starter cultures and potential nutritional benefits. Food Nutr. Sci. 2011, 2, 5. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Torriani, S.; Akkermans, A.D.L.; De Vos, W.M.; Vaughan, E.E. Diversity, dynamics, and activity of bacterial communities during production of an artisanal sicilian cheese as evaluated by 16S rRNA analysis. Appl. Env. Microbiol. 2002, 68, 1882–1892. [Google Scholar] [CrossRef]
- Chessa, L.; Daga, E.; Dupré, I.; Paba, A.; Fozzi, M.C.; Dedola, D.G.; Comunian, R. Biodiversity and safety: Cohabitation experimentation in undefined starter cultures for traditional dairy products. Fermentation 2024, 10, 29. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Dupré, I.; Daga, E.; Fozzi, M.C.; Comunian, R. A strategy for the recovery of raw ewe’s milk microbiodiversity to develop natural starter cultures for traditional foods. Microorganisms 2023, 11, 823. [Google Scholar] [CrossRef]
- Cremonesi, P.; Vanoni, L.; Morandi, S.; Silvetti, T.; Castiglioni, B.; Brasca, M. Development of a pentaplex PCR assay for the simultaneous detection of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. delbrueckii subsp. lactis, L. helveticus, L. fermentum in whey starter for Grana Padano cheese. Int. J. Food Microbiol. 2011, 146, 207–211. [Google Scholar] [CrossRef]
- Chessa, L.; Paba, A.; Daga, E.; Comunian, R. Effect of growth media on natural starter culture composition and performance evaluated with a polyphasic approach. Int. J. Dairy. Technol. 2019, 72, 152–158. [Google Scholar] [CrossRef]
- Hardy, J. Le fromage. In Paris: Techniques et Documents, 2nd ed.; Eck, A., Ed.; Lavoisier: Paris, France, 1987; pp. 37–60. [Google Scholar]
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of milk protein standardization using different methods on the composition and yields of Cheddar cheese. J. Dairy. Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Pirisi, A.; Murgia, A.; Scintu, M.F. Estimate of Pecorino Romano and Pecorino Sardo cheese yield from the protein and fat contents in sheep milk. Sci. Tec. Latt. Casear 1994, 45, 476–483. [Google Scholar]
- Isolini, D.; Grand, M.; Glâttli, H. Selektivmedien zum Nachweis von obligat und fakultativ heterofermentativen Laklobazillen. Schweiz. Milchw. Forsch. 1990, 19, 57–59. [Google Scholar]
- Bottazzi, V.; Ledda, A.; Arrizza, S. Bacteries fermentant les citrates et gonflement du fromage “Pecorino Romano”. Lait 1971, 51, 328–331. [Google Scholar] [CrossRef]
- ISO 6731:2010 [IDF 21:2010]; Milk, Cream and Evaporated Milk—Determination of Total Solids Content. International Organisation for Standardization (ISO): Geneva, Switzerland, 2010.
- ISO 5534:2004 [IDF 4:2004]; Cheese and Processed Cheese—Determination of the Total Solids Content. International Organisation for Standardization (ISO): Geneva, Switzerland, 2004.
- Soxhlet, F. Die gewichtsanalytische Bestimmung des Milchfettes. Dinglers Polyt. J. 1879, 232, 461–465. [Google Scholar]
- ISO 8968-1:2014 [IDF 20-1:2014]; Milk and Milk Products—Determination of Nitrogen Content. Part 1: Kjeldahl Principle and Crude Protein Calculation. International Organization for Standardization (ISO): Geneva, Switzerland, 2014.
- ISO 5943 [IDF 88:2006]; Cheese and Processed Cheese Products—Determination of Chloride Content—Potentiometric Titration Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- Idda, I.; Spano, N.; Addis, M.; Galistu, G.; Ibba, I.; Nurchi, V.M.; Pilo, M.I.; Scintu, M.F.; Piredda, G.; Sanna, G. Optimization of a newly established gas-chromatographic method for determining lactose and galactose traces: Application to Pecorino Romano cheese. J. Food Compos. Anal. 2018, 74, 89–94. [Google Scholar] [CrossRef]
- Jiang, J.; Bjoerck, L.; Fondén, R.; Emanuelson, M. Occurrence of conjugated cis-9,trans-11-octadecadienoic acid in bovine milk: Effects of feed and dietary regimen. J. Dairy Sci. 1996, 79, 438–445. [Google Scholar] [CrossRef] [PubMed]
- ISO 15884:2002 [IDF 182:2002]; Milk Fat Preparation of Fatty Acid Methyl Esters. International Organization for Standardization (ISO): Geneva, Switzerland, 2002.
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of fatty acid content in sheep milk by Mid-Infrared spectrometry with a selection of wavelengths by Genetic Algorithms. LWT 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Panfili, G.; Manzi, P.; Pizzoferrato, L. High-performance liquid chromatographic method for the simultaneous determination of tocopherols, carotenes, and retinol and its geometric isomers in Italian cheeses. Analyst 1994, 119, 1161–1165. [Google Scholar] [CrossRef]
- Manzi, P.; Panfili, G.; Pizzoferrato, L. Normal and reversed-phase HPLC for more complete evaluation of tocopherols, retinols, carotenes and sterols in dairy products. Chromatographia 1996, 43, 89–93. [Google Scholar] [CrossRef]
- Addis, M.; Piredda, G.; Pes, M.; Di Salvo, R.; Scintu, M.F.; Pirisi, A. Effect of the use of three different lamb paste rennets on lipolysis of the PDO Pecorino Romano Cheese. Int. Dairy J. 2005, 15, 563–569. [Google Scholar] [CrossRef]
- Gripon, J.C.; Desmazeaud, M.J.; Le Bars, D.; Bergere, J.L. Etude du rôle des micro-organismes et des enzymes au cours de la maturation des fromages. II.-Influence de la présure commerciale. Lait 1975, 55, 502–516. [Google Scholar] [CrossRef]
- Institute of Food Science & Technology (IFST). Guidelines for Ethical and Professional Practices for the Sensory Analysis of Foods. Available online: https://www.ifst.org (accessed on 12 November 2025).
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95/46/EC (General Data Protection Regulation); Official Journal of the European Union: Strasbourg, France, 2016.
- Fiszman, S.; Salgado, N.; Orrego, C.E.; Ares, G. Comparison of methods for generating sensory vocabulary with consumers: A case study with two types of satiating foods. Food Qual. Prefer. 2015, 44, 111–118. [Google Scholar] [CrossRef]
- Thomson, D.M.H.; McEwan, J.A. An application of the repertory grid method to investigate consumer perceptions of foods. Appetite 1988, 10, 181–193. [Google Scholar] [CrossRef]
- Ares, G.; Reis, F.; Oliveira, D.; Antúnez, L.; Vidal, L.; Giménez, A.; Chheang, S.L.; Hunter, D.C.; Kam, K.; Roigard, C.M.; et al. Recommendations for use of balanced presentation order of terms in CATA questions. Food Qual. Prefer. 2015, 46, 137–141. [Google Scholar] [CrossRef]
- ISO 11136:2014; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. International Organisation for Standardization (ISO): Geneva, Switzerland, 2014.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organisation for Standardization (ISO): Geneva, Switzerland, 2007.
- ISO 22935-2:2023 [IDF 99-2:2023]; Milk and Milk Products—Sensory Analysis—Part 2: Methods for Sensory Evaluation. International Organisation for Standardization (ISO): Geneva, Switzerland, 2023.
- Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on Clinical Trials on Medicinal Products for Human Use, and Repealing Directive 2001/20/EC Text with EEA Relevance; Official Journal of the European Union: Strasbourg, France, 2014.
- Italian Ministry of Health. Orientamenti per i Comitati Etici in Italia; Presidenza del Consiglio dei Ministri: Rome, Italy, 2001.
- Ares, G.; Jaeger, S.R. 11—Check-All-That-Apply (CATA) Questions with Consumers in Practice: Experimental Considerations and Impact on Outcome. In Rapid Sens Prof Tech; Delarue, J., Lawlor, J.B., Rogeaux, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 227–245. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Marshall, V.M. Starter cultures for milk fermentation and their characteristics. Int. J. Dairy. Technol. 1993, 46, 49–56. [Google Scholar] [CrossRef]
- González-González, F.; Delgado, S.; Ruiz, L.; Margolles, A.; Ruas-Madiedo, P. Functional bacterial cultures for dairy applications: Towards improving safety, quality, nutritional and health benefit aspects. J. Appl. Microbiol. 2022, 133, 212–229. [Google Scholar] [CrossRef]
- Comunian, R.; Chessa, L. Development and application of starter cultures. Fermentation 2024, 10, 512. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards; Allende, A.; Alvarez-Ordóñez, A.; Bortolaia, V.; Bover-Cid, S.; De Cesare, A.; Dohmen, W.; Guillier, L.; Jacxsens, L.; Nauta, M.; et al. Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 21: Suitability of taxonomic units notified to EFSA until September 2024. EFSA J. 2025, 23, e9169. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Atzori, A.S.; Dimauro, C.; Ibba, I.; Gaias, G.F.; Correddu, F.; Nudda, A. The milk fingerprint of Sardinian dairy sheep: Quality and yield of milk used for Pecorino Romano P.D.O. cheese production on population-based 5-year survey. Ital. J. Anim. Sci. 2021, 20, 171–180. [Google Scholar] [CrossRef]
- Pirisi, A.; Pes, M. Formaggi Ovi-Caprini. In Manuale Caseario; Tecniche Nuove Milano: Milano, Italy, 2011. [Google Scholar]
- Ekstrand, B. Antimicrobial factors in milk—A review. Food Biotechnol. 1989, 3, 105–126. [Google Scholar] [CrossRef]
- Tanaka, T. Antimicrobial Activity of Lactoferrin and Lactoperoxidase in Milk; Nova Science Publisher Inc.: New York, NY, USA, 2007. [Google Scholar]
- Guinee, T.P.; Mulholland, E.O.; Kelly, J.; Callaghan, D.J.O. Effect of protein-to-fat ratio of milk on the composition, manufacturing efficiency, and yield of Cheddar cheese. J. Dairy. Sci. 2007, 90, 110–123. [Google Scholar] [CrossRef]
- Klijn, N.; Nieuwenhof, F.F.; Hoolwerf, J.D.; van der Waals, C.B.; Weerkamp, A.H. Identification of Clostridium tyrobutyricum as the causative agent of late blowing in cheese by species-specific PCR amplification. Appl. Env. Microbiol. 1995, 61, 2919–2924. [Google Scholar] [CrossRef]
- Sousa, M.J.; Ardö, Y.; McSweeney, P.L.H. Advances in the study of proteolysis during cheese ripening. Int. Dairy J. 2001, 11, 327–345. [Google Scholar] [CrossRef]
- Afshari, R.; Pillidge, C.J.; Dias, D.A.; Osborn, A.M.; Gill, H. Cheesomics: The future pathway to understanding cheese flavour and quality. Crit. Rev. Food Sci. Nutr. 2020, 60, 33–47. [Google Scholar] [CrossRef]
- Moreno, M.R.F.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Deplano, M.; Corda, A.; Cosentino, S. Microbiological and chemical characterization of Fiore Sardo, a traditional Sardinian cheese made from ewe’s milk. Int. J. Dairy Technol. 2006, 59, 171–179. [Google Scholar] [CrossRef]
- Ruaro, A.; Andrighetto, C.; Torriani, S.; Lombardi, A. Biodiversity and characterization of indigenous coagulase-negative staphylococci isolated from raw milk and cheese of North Italy. Food Microbiol. 2013, 34, 106–111. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- Beresford, T.P. Lactic Acid Bacteria|Citrate Fermentation by Lactic Acid Bacteria. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 166–172. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Sheehan, J.J. Cheese: Avoidance of Gas Blowing. In Encyclopedia of Dairy Sciences, 3rd ed.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Ravyts, F.; Vuyst, L.D.; Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng. Life Sci. 2012, 12, 356–367. [Google Scholar] [CrossRef]
- Carr, F.J.; Don, C.; Maida, N. The lactic acid bacteria: A literature survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef] [PubMed]
- Pavunc, A.; Novak, J.; Kos, B.; Uroić, K.; Blažić, M.; Šušković, J. Characterization and application of autochthonous starter cultures for fresh cheese production. Food Technol. Biotechnol. 2012, 50, 141. [Google Scholar]
- Italian Ministry of Health 0024708-P-16/06/2016. Aggiornamenti su Integratori Alimentari, Tolleranze Analitiche Applicabili in fase di Controllo e Indicazioni Sull’assenza o la Ridotta Presenza di Lattosio nei Prodotti Lattiero-Caseari. Available online: https://www.agrar.it/upload/documenti/8-Circolare%20Ministero%20della%20Salute%20Giugno%202016%20-%20Estratti%20e%20titolazioni.pdf (accessed on 18 November 2025).
- Arrizza, S. Caratteristiche microbiologiche della scotta fermento. Sci. Tec. Latt. Casear 1972, 23, 226–230. [Google Scholar]
- EU No 1169/2011 of the European Parliament and of the Council L. 304/18 22.11.2011; Official Journal of the European Union: Strasbourg, France, 2011.
- CREA—Consiglio per la Ricerca in Agricoltura e l’Analisi dell’Economia Agraria. Centro di Ricerca Alimenti e Nutrizione. Linee Guida per una Sana Alimentazione; CREA: Roma, Italy, 2018. [Google Scholar]
- Regulation EC No 1924/2006 of the European Parliament and of the Council L. 404/9 30.12.2006; Official Journal of the European Union: Strasbourg, France, 2006.
- Ares, G.; Barreiro, C.; Deliza, R.; Giménez, A.; Gámbaro, A. Application of a check-all-that-apply question to the development of chocolate milk desserts. J. Sens. Stud. 2010, 25, 67–86. [Google Scholar] [CrossRef]
- Ares, G.; de Andrade, J.C.; Antúnez, L.; Alcaire, F.; Swaney-Stueve, M.; Gordon, S.; Jaeger, S.R. Hedonic product optimisation: CATA questions as alternatives to JAR scales. Food Qual. Prefer. 2017, 55, 67–78. [Google Scholar] [CrossRef]
- Han, P.; Fark, T.; de Wijk, R.A.; Roudnitzky, N.; Iannilli, E.; Seo, H.-S.; Hummel, T. Modulation of sensory perception of cheese attributes intensity and texture liking via ortho- and retro-nasal odors. Food Qual. Prefer. 2019, 73, 1–7. [Google Scholar] [CrossRef]
- Pirc, M.; Mu, S.; Frissen, G.; Stieger, M.; Boesveldt, S. Smells like fat: A systematic scoping review on the contribution of olfaction to fat perception in humans and rodents. Food Qual. Prefer. 2023, 107, 104847. [Google Scholar] [CrossRef]
- Silva, L.F.; Sunakozawa, T.N.; Monteiro, D.A.; Casella, T.; Conti, A.C.; Todorov, S.D.; Barretto Penna, A.L. Potential of cheese-associated lactic acid bacteria to metabolize citrate and produce organic acids and acetoin. Metabolites 2023, 13, 1134. [Google Scholar] [CrossRef]
- Libudzisz, Z.; Galewska, E. Citrate metabolism in Lactococcus lactis subsp. lactis var. diacetylactis strains. Food 1991, 35, 611–618. [Google Scholar] [CrossRef]
| Dairies | |||
|---|---|---|---|
| A | B | C | |
| Amount of milk processed (kg) | 103.8 ± 0.02 a | 62.4 ± 0.04 b | 60 ± 0.00 c |
| pH of raw milk (UpH) | 6.62 ± 0.07 | 6.71 ± 0.04 | 6.68 ± 0.00 |
| Thermisation temperature (°C) | 68.3 ± 0.3 | 68.2 ± 0.2 | 68.6 ± 0.4 |
| Clotting temperature (°C) | 37.8 ± 0.3 | 37.8 ± 0.8 | 38.1 ± 0.1 |
| Rennet dose (IMCU/kg) | 72 | 72 | 72 |
| Clotting and curd firming time (min) | 22 ± 1.2 | 22 ± 1.0 | 24 ± 1.2 |
| Curd cutting time (min) | 4.0 ± 0.0 a | 4.3 ± 0.6 a | 3.0 ± 0.0 b |
| Semi-cooking at 43 °C time (min) | 7.3 ± 0.6 a | 6.3 ± 0.6 a | 5.0 ± 0.0 b |
| pH of cheese at the start sweating (UpH) | 6.38 ± 0.12 | 6.36 ± 0.05 | 6.38 ± 0.02 |
| Sweating duration at 36–37 °C (min) | 220 ± 27 | 165 ± 21 | 185 ± 24 |
| pH of cheese at the end of sweating (UpH) | 5.29 ± 0.03 a | 5.27 ± 0.02 a | 5.19 ± 0.02 b |
| pH of 1-day cheese (UpH) | 5.20 ± 0.12 ab | 5.07 ± 0.02 b | 5.27 ± 0.02 a |
| Cheese wheels produced (n.) | 5 | 3 | 3 |
| Weight of single cheese wheel (kg) | 3.1 ± 0.02 b | 3.5 ± 0.3 a | 2.7 ± 0.1 b |
| Weight cheese produced (kg) | 15.4 ± 0.1 a | 10.6 ± 0.8 b | 8.2 ± 0.2 c |
| Brine concentration (%) | 26 | 29 | 28 |
| Salting time (h) | 34 | 30 | 28 |
| Dairies | |||
|---|---|---|---|
| A | B | C | |
| Cheese yield 2 | |||
| YA (kg/100 kg) | 14.9 ± 0.1 b | 17.0 ± 1.2 a | 13.6 ± 0.3 c |
| YAM (kg/100 kg) | 14.9 ± 0.4 b | 17.3 ± 1.1 a | 13.2 ± 0.2 c |
| Yp (kg/100 kg) | 14.6 ± 0.2 b | 17.0 ± 0.8 a | 13.1 ± 0.01 c |
| YAN (kg/100 kg) | 15.5 ± 0.2 ab | 14.9 ± 0.3 b | 16.0 ± 0.4 a |
| YANM (kg/100 kg) | 15.5 ± 0.2 | 15.2 ± 0.3 | 15.4 ± 0.3 |
| Recoveries 3 | |||
| FR (%) | 77.5 ± 2.5 b | 77.1 ± 1.8 b | 82.5 ± 1.2 a |
| PR (%) | 79.9 ± 1.1 | 79.4 ± 1.5 | 77.2 ± 0.4 |
| Dairies | |||
|---|---|---|---|
| A | B | C | |
| Total Solids (g/100 g) | 15.9 ± 0.1 b | 18 ± 1 a | 14.7 ± 0.1 b |
| Fat (g/100 g) | 5.0 ± 0.1 b | 6.5 ± 0.6 a | 4.01 ± 0.01 b |
| Protein (g/100 g) | 5.09 ± 0.05 b | 5.5 ± 0.1 a | 4.93 ± 0.02 b |
| Casein (g/100 g) | 3.83 ± 0.04 b | 4.2 ± 0.1 a | 3.75 ± 0.01 b |
| Fat/Protein | 0.98 ± 0.04 b | 1.2 ± 0.1 a | 0.813 ± 0.002 c |
| Lactose (g/100 g) | 4.79 ± 0.04 | 4.8 ± 0.1 | 4.91 ± 0.04 |
| Dairies | |||
|---|---|---|---|
| A | B | C | |
| Moisture (g/100 g) | 43 ± 1 ab | 42 ± 1 b | 45 ± 1 a |
| Fat/Dry Matter (g/100 g) | 46 ± 1 b | 51 ± 1 a | 44.1 ± 0.4 b |
| Protein/Dry Matter (g/100 g) | 44 ± 1 a | 41 ± 1 b | 46 ± 1 a |
| Fat/Protein | 1.0 ± 0.1 b | 1.2 ± 0.1 a | 0.95 ± 0.01 b |
| Lactose (g/100 g) | 0.007 ± 0.002 | 0.009 ± 0.003 | 0.004 ± 0.001 |
| Dairies | |||
|---|---|---|---|
| A | B | C | |
| Moisture (g/100 g) | 38 ± 2 | 36.2 ± 0.4 | 37.1 ± 0.4 |
| Fat/Dry Matter (g/100 g) | 45 ± 2 b | 50 ± 1 a | 42.5 ± 0.4 b |
| Protein/Dry Matter (g/100 g) | 44 ± 1 b | 38 ± 1 c | 46.2 ± 0.5 a |
| Fat/Protein | 1.0 ± 0.1 b | 1.32 ± 0.02 a | 0.92 ± 0.02 c |
| Lactose (g/100 g) | 0.0012 ± 0.0002 ab | 0.003 ± 0.001 a | 0.001 ± 0.001 b |
| NaCl (g/100 g) | 1.6 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| NaCl/Moisture (g/100 g) | 4.3 ± 0.1 | 4.1 ± 0.3 | 4.1 ± 0.4 |
| Per 100 g of Cheese | Dairy | RI (%) 1 | ||
|---|---|---|---|---|
| A | B | C | ||
| Energy (kcal) | 364 | 384 | 359 | 18 ÷ 19 |
| Fat (g) | 28 b | 32 a | 27 b | 39 ÷ 46 |
| of which: | ||||
| 19 ab | 21 a | 18 b | 90 ÷ 105 |
| 5.2 a | 5.1 a | 4.0 b | |
| 09 | 1.1 | 0.9 | |
| Carbohydrate (g) | 0 | 0 | 0 | |
| of which: | ||||
| 0 | 0 | 0 | |
| Proteins (g) | 28 b | 24 c | 29 a | 48 ÷ 58 |
| Salt (NaCl) (g) | 1.6 | 1.5 | 1.5 | 25 ÷ 27 |
| Vitamin A (μg) | 326 | 326 | 310 | 39 ÷ 41 |
| Calcium (mg) | 1093 | 1042 | 1111 | 130 ÷ 139 |
| Zinc (mg) | 3.42 a | 2.74 b | 2.99 ab | 27 ÷ 34 |
| Copper (mg) | 0.24 | 0.22 | 0.12 | 12 ÷ 24 |
| Magnesium (mg) | 58.5 a | 50.6 b | 56.3 a | 13 ÷ 16 |
| 60 Days | 180 Days | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |||
| Acceptance | p-values | mean scores | p-values | mean scores | ||||
| 0.0264 | 6.2 ± 1.4 | 6.4 ± 1.7 | 6.0 ± 1.7 | 0.9241 | 6.3 ± 1.7 | 6.3 ± 1.7 | 6.2 ± 1.7 | |
| CATA Descriptors | p-values | relative frequencies | p-values | relative frequencies | ||||
| Bitter Taste | 0.260 | 0.071 | 0.079 | 0.119 | 0.128 | 0.117 | 0.169 | 0.078 |
| Sour Taste | <0.0001 | 0.159 a | 0.310 b | 0.143 a | 0.337 | 0.156 | 0.156 | 0.221 |
| Salty Taste | 1.000 | 0.175 | 0.175 | 0.175 | 0.003 | 0.247 b | 0.065 a | 0.182 ab |
| Sweet Taste | 0.034 | 0.198 a | 0.167 ab | 0.103 a | 0.025 | 0.143 ab | 0.221 b | 0.104 a |
| Pungent | 0.004 | 0.032 a | 0.095 ab | 0.151 a | 0.882 | 0.156 | 0.169 | 0.143 |
| Exotic Fruit Aroma | 0.135 | 0.008 | 0.040 | 0.024 | 0.368 | 0.026 | 0.026 | 0.000 |
| Dried Fruit Aroma | 0.097 | 0.040 | 0 a | 0.032 | 0.846 | 0.039 | 0.026 | 0.039 |
| Fresh Milk Aroma | 0.020 | 0.175 ab | 0.238 b | 0.127 a | 0.486 | 0.104 | 0.117 | 0.065 |
| Boiled Milk Aroma | 0.245 | 0.111 | 0.127 a | 0.175 | 0.943 | 0.156 | 0.143 | 0.156 |
| Butter Aroma | 0.061 | 0.190 | 0.230 a | 0.127 | 0.827 | 0.18 | 0.182 | 0.156 |
| Fresh Grass Aroma | 0.027 | 0.056 a | 0.056 a | 0.008 b | 0.368 | 0.026 | 0.026 | 0.000 |
| Animal | 0.104 | 0.087 | 0.159 | 0.127 | 0.662 | 0.143 | 0.130 | 0.104 |
| Gummy | 0.169 | 0.190 | 0.127 | 0.198 | 0.047 | 0.143 b | 0.039 a | 0.104 ab |
| Compact/Cohesive | 0.075 | 0.492 | 0.373 | 0.405 | 0.039 | 0.338 b | 0.195 a | 0.260 ab |
| Grainy | <0.0001 | 0.095 a | 0.087 a | 0.278 b | 0.008 | 0.130 a | 0.286 b | 0.312 b |
| Pasty | 0.042 | 0.302 a | 0.429 b | 0.373 ab | 0.310 | 0.364 | 0.416 | 0.312 |
| Traditional Cheese | 0.677 | 0.190 | 0.159 | 0.190 | 0.674 | 0.273 | 0.286 | 0.325 |
| Industrial Cheese | <0.0001 | 0.175 a | 0.095 a | 0.278 b | 0.862 | 0.195 | 0.169 | 0.169 |
| Ripened Cheese | 0.001 | 0.040 a | 0.048 a | 0.143 b | 0.179 | 0.208 | 0.195 | 0.286 |
| Fresh Cheese | <0.0001 | 0.317 b | 0.333 b | 0.143 a | 0.121 | 0.065 | 0.091 | 0.026 |
| Tasty | 0.002 | 0.373 a | 0.532 b | 0.357 a | 0.368 | 0.442 | 0.532 | 0.429 |
| Cleaned Mouthfeel | 0.861 | 0.079 | 0.095 | 0.087 | 0.039 | 0.000 a | 0.052 b | 0.013 ab |
| Greasy Mouthfeel | 0.918 | 0.167 | 0.175 | 0.183 | 0.738 | 0.182 | 0.221 | 0.195 |
| Surprising | 0.018 | 0.024 ab | 0.071 b | 0.008 a | 0.045 | 0.091 b | 0.013 a | 0.039 ab |
| Anonymous | 0.000 | 0.222 b | 0.048 a | 0.151 b | 0.483 | 0.143 | 0.091 | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chessa, L.; Paba, A.; Dupré, I.; Addis, M.; Piga, C.; Pes, M.; Comunian, R. From Raw Milk Microbiome to Cheese: The Challenge of Indigenous Natural Starter Culture Exploitation. Fermentation 2025, 11, 660. https://doi.org/10.3390/fermentation11120660
Chessa L, Paba A, Dupré I, Addis M, Piga C, Pes M, Comunian R. From Raw Milk Microbiome to Cheese: The Challenge of Indigenous Natural Starter Culture Exploitation. Fermentation. 2025; 11(12):660. https://doi.org/10.3390/fermentation11120660
Chicago/Turabian StyleChessa, Luigi, Antonio Paba, Ilaria Dupré, Margherita Addis, Carlo Piga, Massimo Pes, and Roberta Comunian. 2025. "From Raw Milk Microbiome to Cheese: The Challenge of Indigenous Natural Starter Culture Exploitation" Fermentation 11, no. 12: 660. https://doi.org/10.3390/fermentation11120660
APA StyleChessa, L., Paba, A., Dupré, I., Addis, M., Piga, C., Pes, M., & Comunian, R. (2025). From Raw Milk Microbiome to Cheese: The Challenge of Indigenous Natural Starter Culture Exploitation. Fermentation, 11(12), 660. https://doi.org/10.3390/fermentation11120660







