β-Glucan from Highland Barley Spent Grain: Yield, Molecular Weight, Physicochemical Properties, Antioxidant Capacity, and Gel Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Strain
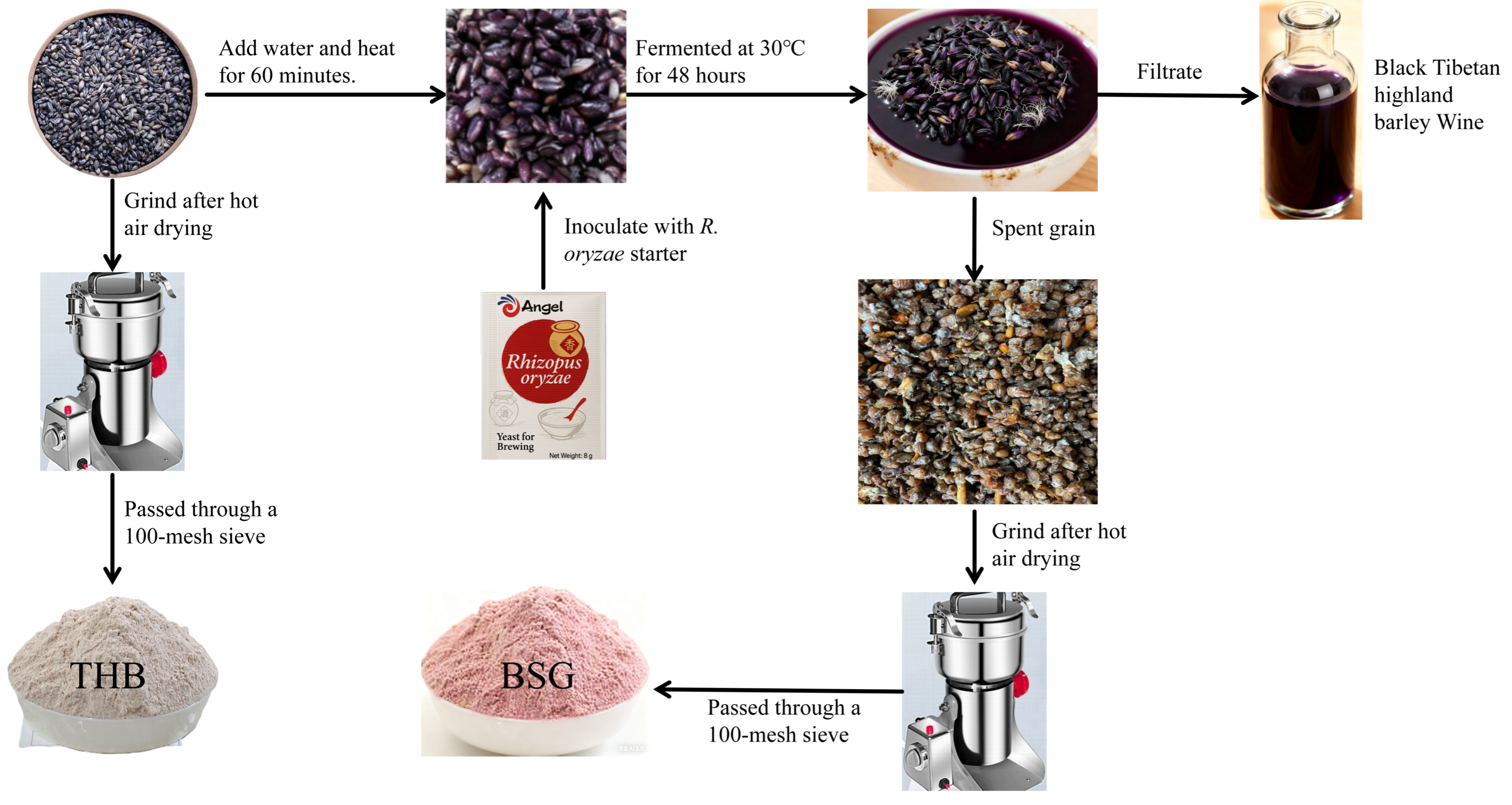
2.2. Extraction of β-Glucan
2.3. Analysis of Extracted β-Glucan Composition and Content
2.3.1. Determination of Total Polysaccharide
2.3.2. Determination of β-Glucan Content
2.3.3. Determination of Protein Content
2.4. Determination of Molecular Weight of β-Glucan
2.5. Morphological Characterization of β-Glucan
2.5.1. SEM Analysis
2.5.2. AFM Analysis
2.6. Physicochemical Characterization of β-Glucan
2.6.1. Monosaccharide Composition Analysis
2.6.2. FT-IR Measurement
2.7. Determination of Antioxidant Activity In Vitro
2.7.1. Determination of Total Reducing Power
2.7.2. NO Radical Scavenging Activity
2.7.3. DPPH Radical Scavenging Activity
2.7.4. ABTS Radical Scavenging Activity
2.8. Gel Characteristics and Functional Properties
2.8.1. Determination of Apparent Viscosity
2.8.2. Determination of Oil-Holding Capacity
2.9. Statistical Analysis
3. Results and Discussion
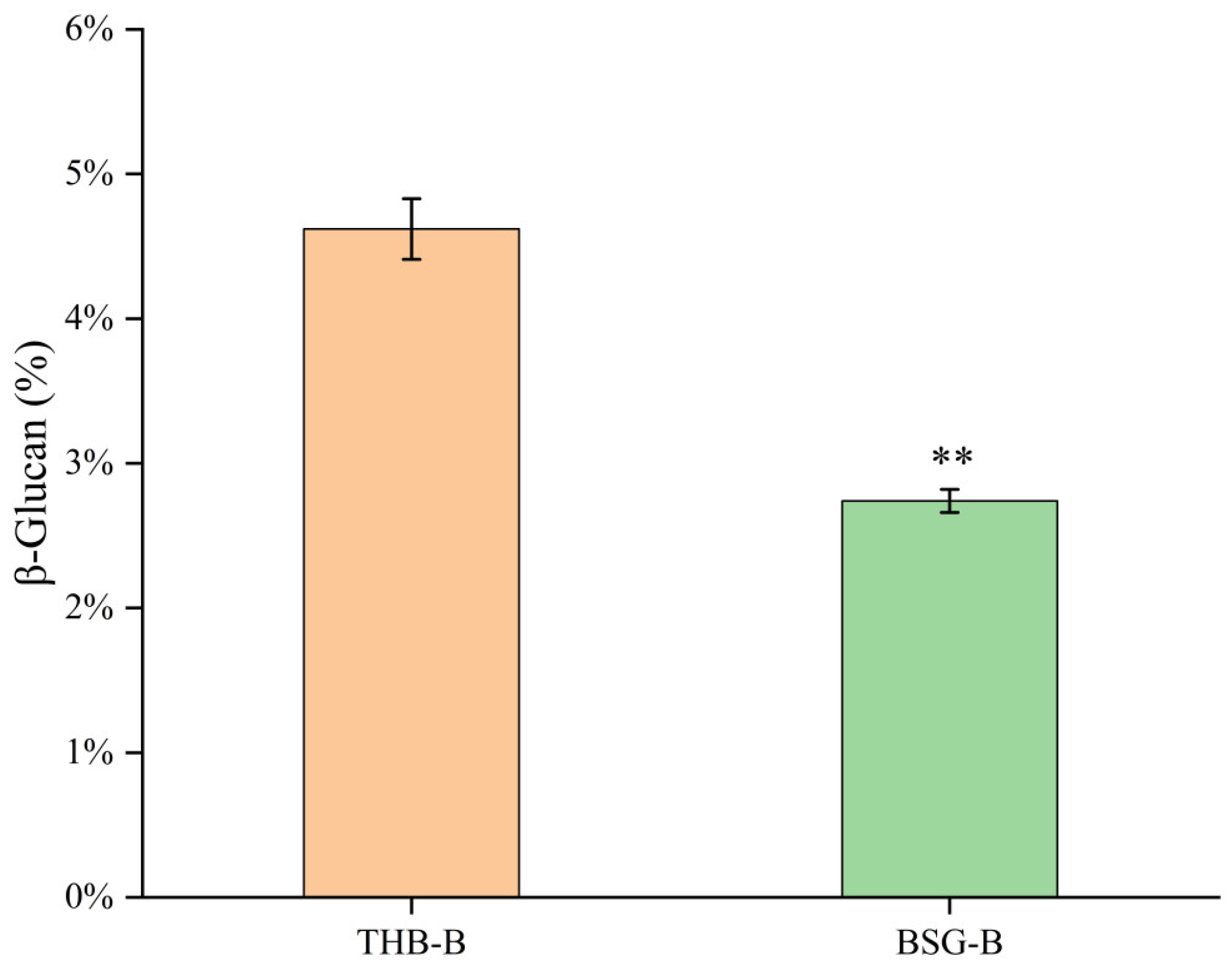
3.1. Yield of β-Glucan from BSG
3.2. Molecular Weight of β-Glucan from BSG
3.3. Physicochemical Properties of β-Glucan from BSG
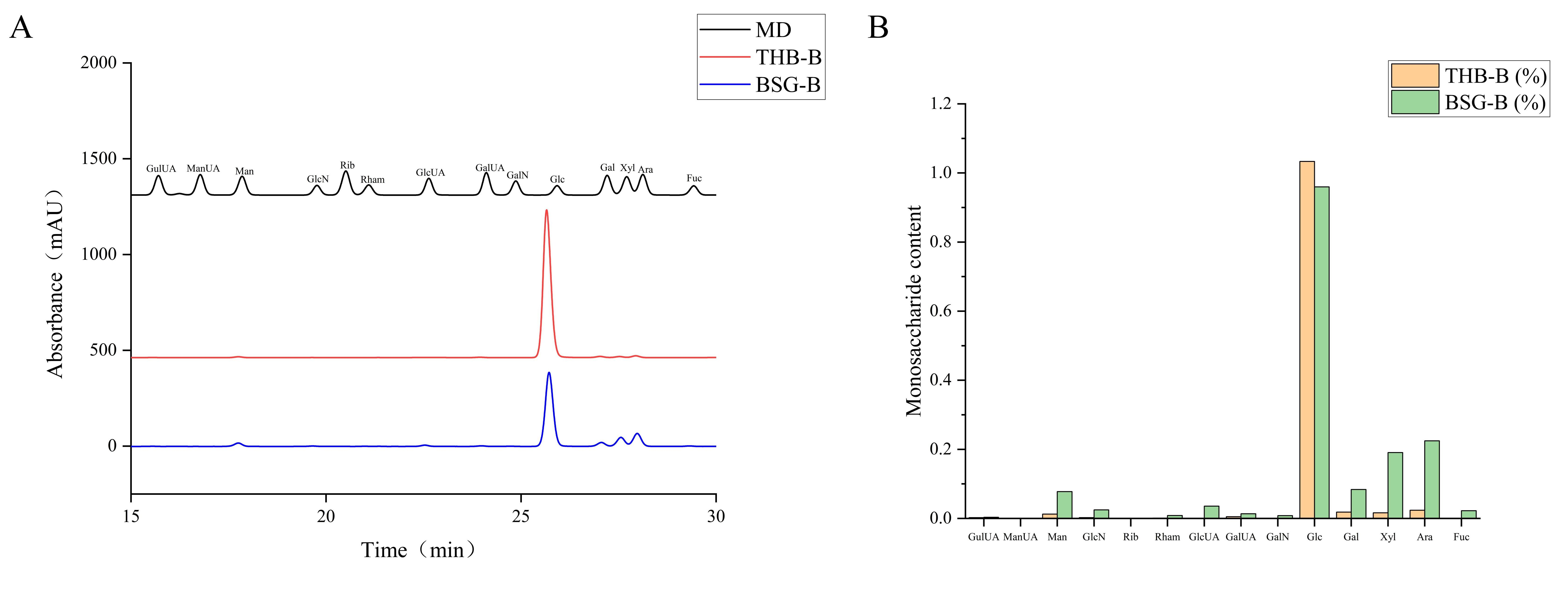
3.3.1. Monosaccharide Composition
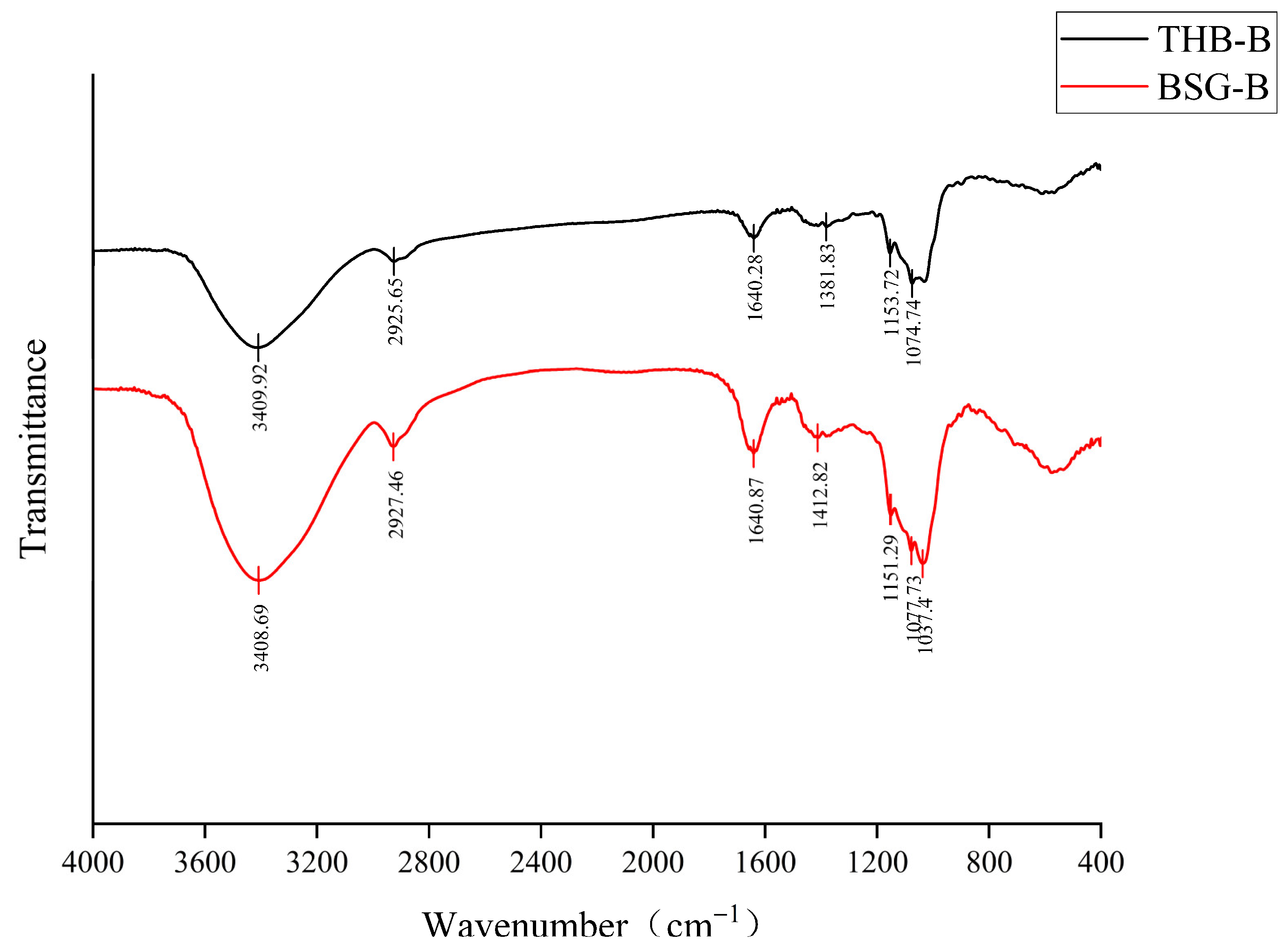
3.3.2. FT-IR Analysis
3.4. Morphologies of β-Glucan from BSG
3.4.1. Macroscopic Morphology
3.4.2. Microscopic Morphology by SEM Analysis
3.4.3. Nanostructural Morphology by AFM Analysis
3.5. Antioxidant Activity of β-Glucan from BSG
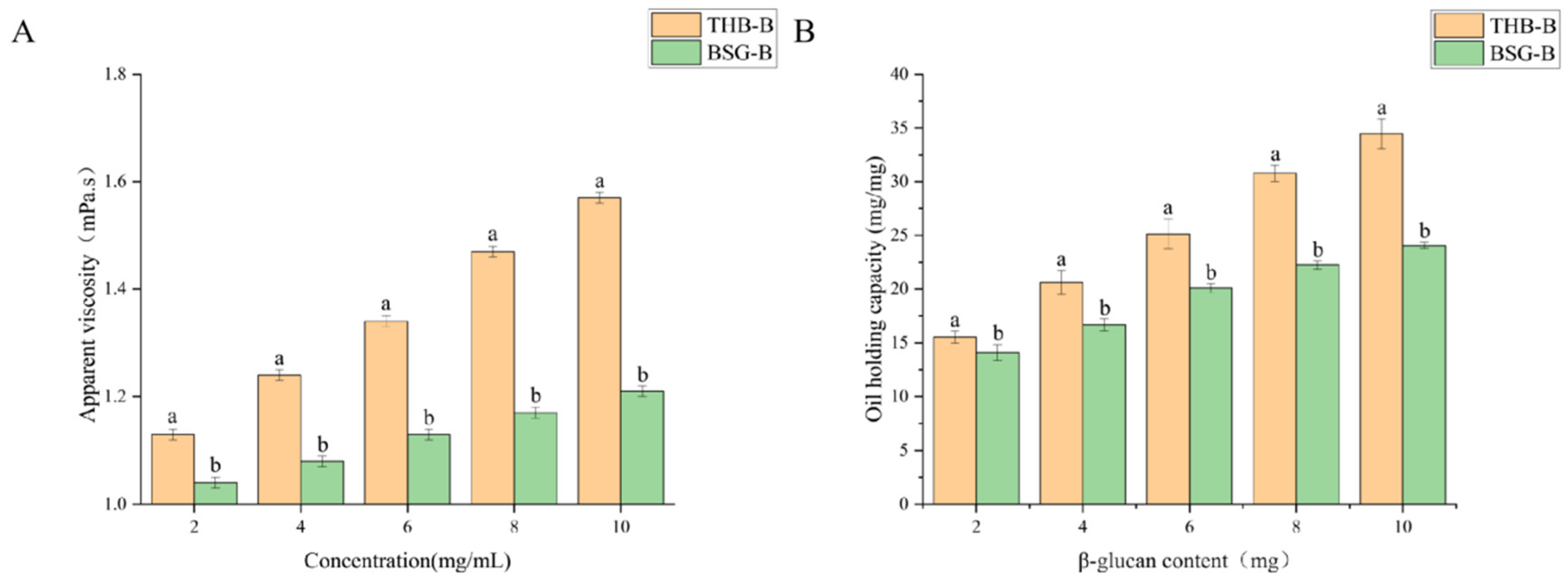
3.6. Gel Characteristics of β-Glucan from BSG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obadi, M.; Sun, J.; Xu, B. Highland barley: Chemical composition, bioactive compounds, health effects, and applications. Food Res. Int. 2021, 140, 110065. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Zhang, M.; Fang, Y.; Chen, Z.; Huan, M. Fecal microbiota transplantation: Whole grain highland barley improves glucose metabolism by changing gut microbiota. Food Sci. Hum. Wellness 2024, 13, 2014–2024. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Mushtaq, B.S.; Mahdi, A.A.; Al-Maqtari, Q.A.; Al-Adeeb, A.; Ahmed, A.; Fan, M.; Li, Y.; Qian, H.; Jinxin, L.; et al. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansi, W.; Mahdi, A.A.; Al-Maqtari, Q.A.; Sajid, B.M.; Al-Adeeb, A.; Ahmed, A.; Fan, M.; Li, Y.; Qian, H.; Jinxin, L.; et al. Characterization of molecular, physicochemical, and morphological properties of starch isolated from germinated highland barley. Food Biosci. 2021, 42, 101052. [Google Scholar] [CrossRef]
- Al-Ansi, W.; Zhang, Y.; Alkawry, T.A.A.; Al-Adeeb, A.; Mahdi, A.A.; Al-Maqtari, Q.A.; Ahmed, A.; Mushtaq, B.S.; Fan, M.; Li, Y.; et al. Influence of germination on bread-making behaviors, functional and shelf-life properties, and overall quality of highland barley bread. LWT 2022, 159, 113200. [Google Scholar] [CrossRef]
- Ruirong, P.; Hamadou, A.H.; Xu, B. Germination in improving the nutrition, health benefits and processing of highland barley. Int. J. Food Sci. Technol. 2024, 59, 2162–2171. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Dong, Y.; Xu, T.; Wu, F. Dietary supplementation with Lactobacillus plantarum dy-1 fermented barley suppresses body weight gain in high-fat diet-induced obese rats. J. Sci. Food Agric. 2016, 96, 4907–4917. [Google Scholar] [CrossRef]
- Yan, J.-K.; Chen, T.-T.; Wang, Z.-W.; Wang, C.; Liu, C.; Li, L. Comparison of physicochemical characteristics and biological activities of polysaccharides from barley (Hordeum vulgare L.) grass at different growth stages. Food Chem. 2022, 389, 133083. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Tan, C.; Zhao, Y.; Zhu, Y.; Bai, J.; Xiao, X.; Zhang, L.; Teng, D.; Tian, J.; et al. Effects of L. plantarum dy-1 fermentation time on the characteristic structure and antioxidant activity of barley β-glucan in vitro. Curr. Res. Food Sci. 2022, 5, 125–130. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Bai, J.; Li, J.; Cheng, K.; Zhou, X.; Dong, Y.; Xiao, X. Fermented barley extracts with Lactobacillus plantarum dy-1 decreased fat accumulation of Caenorhabditis elegans in a daf-2 -dependent mechanism. J. Food Biochem. 2020, 44, e13459. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Dong, Y.; Zhou, X. Fermented barley extracts with Lactobacillus plantarum dy-1 changes serum metabolomic profiles in rats with high-fat diet-induced obesity. Int. J. Food Sci. Nutr. 2019, 70, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Zhang, J.; Cui, L.; Lu, H.; Zhu, Y.; Zhao, Y.; Fan, S.; Xiao, X. Barley β-glucan inhibits digestion of soybean oil in vitro and lipid-lowering effects of digested products in cell co-culture model. Food Res. Int. 2023, 164, 112378. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Duan, R.; Yu, H.; Liu, S.; Bao, Y. Research Progress in the Extraction, Structural Characteristics, Bioactivity, and Commercial Applications of Oat β-Glucan: A Review. Foods 2024, 13, 4160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Tan, C.; Sun, X.; Zhao, Y.; Zhang, J.; Zhu, Y.; Bai, J.; Dong, Y.; Zhou, X. Fermented barley β-glucan regulates fat deposition in Caenorhabditis elegans. J. Sci. Food Agric. 2020, 100, 3408–3417. [Google Scholar] [CrossRef]
- Guo, T.; Horvath, C.; Chen, L.; Chen, J.; Zheng, B. Understanding the nutrient composition and nutritional functions of highland barley (Qingke): A review. Trends Food Sci. Technol. 2020, 103, 109–117. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of non-starchy polysaccharides on barley milling behavior and evaluating bioactive composition of milled fractions. Food Chem. 2017, 218, 137–143. [Google Scholar] [CrossRef]
- Tufail, T.; Saeed, F.; Tufail, T.; Ain, H.B.U.; Hussain, M.; Noreen, S.; Shah, M.A. Exploring the cholesterol-lowering effects of cereal bran cell wall-enriched diets. Food Sci. Nutr. 2024, 12, 4944–4951. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, M.; Wang, S.; Wang, Z.; Wen, H.; Sun, Z.; Zhang, Y. Characterization and discrimination of the taste and aroma of Tibetan Qingke baijiu using electronic tongue, electronic nose and gas chromatography–mass spectrometry. Food Chem. X 2024, 22, 101443. [Google Scholar] [CrossRef]
- Guo, L.; Luo, Y.; Zhou, Y.; Bianba, C.; Guo, H.; Zhao, Y.; Fu, H. Exploring microbial dynamics associated with flavours production during highland barley wine fermentation. Food Res. Int. 2020, 130, 108971. [Google Scholar] [CrossRef]
- Ma, W.; Geng, X.; Jia, F.; Zhang, X.; Zhang, Y.; Xue, J. Investigation of microbial composition and functional characterization of Zangqu using high throughput sequencing. LWT 2021, 136, 110342. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Qiao, Z.; Cao, X.; Luo, Q.; Zhao, J.; Wang, F.; Zhang, W. Assessment of β-glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of China. Food Chem. 2019, 293, 32–40. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Q.; Liu, B.; Zhang, Y.; Feng, S. Study on the dynamic changes of nutrient components and flavor compounds during the fermentation process of high-quality highland barley wine. Int. J. Gastron. Food Sci. 2024, 35, 100860. [Google Scholar] [CrossRef]
- Bai, J.; He, L.; Zhang, J.; Gu, X.; Wu, B.; Wang, A.; Zhu, Y.; Zhang, J.; Zhao, Y.; Yuan, J.; et al. Influences of Lactiplantibacillus plantarum and Saccharomyces cerevisiae fermentation on the nutritional components, flavor property and lipid-lowering effect of highland barley. J. Futur. Foods 2024, 4, 258–266. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Makia, R.S.; Ainyanbhor, I.E.; Yousif, E.; Gaaz, T.S.; Zainulabdeen, K.; Jikah, A.N.; Opiti, R.A.; Akpoghelie, P.O.; et al. Beta-glucan: An overview in biological activities, derivatives, properties, modifications and current advancements in food, health and industrial applications. Process Biochem. 2024, 147, 347–370. [Google Scholar] [CrossRef]
- Singla, A.; Gupta, O.P.; Sagwal, V.; Kumar, A.; Patwa, N.; Mohan, N.; Ankush; Kumar, D.; Vir, O.; Singh, J.; et al. Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients 2024, 16, 900. [Google Scholar] [CrossRef]
- Fan, S.; Ding, Y.; Hu, Z.; Zhang, Z.; Fu, L.; Zhang, J.; Zhu, Y.; Bai, J.; Xiao, X. Inter-individual variation in human microbiota drives differential impacts on the fermentability of insoluble bran by soluble β-glucans from whole barley. Food Hydrocoll. 2025, 162, 111034. [Google Scholar] [CrossRef]
- Laitinen, M.; Mäkelä-Salmi, N.; Maina, N.H. Gelation of cereal β-glucan after partial dissolution at physiological temperature: Effect of molecular structure. Food Hydrocoll. 2023, 141, 108722. [Google Scholar] [CrossRef]
- Cui, Y.; Han, X.; Hu, X.; Li, T.; Li, S. Distinctions in structure, rheology, antioxidation, and α-glucosidase inhibitory activity of β-glucans from different species. Int. J. Biol. Macromol. 2023, 253, 127684. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Sujithra, S.; Arthanareeswaran, G.; Ismail, A.; Taweepreda, W. Isolation, purification and characterization of β-glucan from cereals—A review. Int. J. Biol. Macromol. 2024, 256, 128255. [Google Scholar] [CrossRef]
- Chen, W.; Gao, L.; Song, L.; Sommerfeld, M.; Hu, Q. An improved phenol-sulfuric acid method for the quantitative measurement of total carbohydrates in algal biomass. Algal Res. 2023, 70, 102986. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-L.; Wu, D.-T.; Zhao, J.; Li, S.-P. A rapid and accurate method for the quantitative estimation of natural polysaccharides and their fractions using high performance size exclusion chromatography coupled with multi-angle laser light scattering and refractive index detector. J. Chromatogr. A 2015, 1400, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Yang, M.; Shen, Q.; Li, L.-Q.; Huang, Y.-Q.; Cheung, H.-Y. Phytochemical profiles, antioxidant activities of functional herb Abrus cantoniensis and Abrus mollis. Food Chem. 2015, 177, 304–312. [Google Scholar] [CrossRef]
- Lee, H.-G.; Nagahawatta, D.; Amarasiri, R.; Jeon, Y.-J.; Kang, M.-C. Physico-chemical and DPPH-hydroxyl radical scavenging characteristics of crude polysaccharides from Sargassum thunbergii. Algal Res. 2023, 73, 103152. [Google Scholar] [CrossRef]
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural characterization and DPPH· radical scavenging activity of a polysaccharide from Guara fruits. Carbohydr. Polym. 2014, 103, 143–147. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, J.; Liu, X.; Li, X.; Sun, X.; Kou, Y.; Li, D.; Liu, Y.; Zhang, H.; Wu, Y. Pectic polysaccharides from Biluochun Tea: A comparative study in macromolecular characteristics, fine structures and radical scavenging activities in vitro. Int. J. Biol. Macromol. 2022, 195, 598–608. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Ma, Y.-L.; Thakur, K.; Wang, C.-H.; Wang, H.; Ren, Y.-F.; Zhang, J.-G.; Wei, Z.-J. Effects of extraction methods on the rheological properties of polysaccharides from onion (Allium cepa L.). Int. J. Biol. Macromol. 2018, 112, 22–32. [Google Scholar] [CrossRef]
- Jin, Q.; Yu, H.; Wang, X.; Li, K.; Li, P. Effect of the molecular weight of water-soluble chitosan on its fat-/cholesterol-binding capacities and inhibitory activities to pancreatic lipase. PeerJ 2017, 5, e3279. [Google Scholar] [CrossRef]
- Li, Y.; You, M.; Liu, H.; Liu, X. Comparison of distribution and physicochemical properties of β-glucan extracted from different fractions of highland barley grains. Int. J. Biol. Macromol. 2021, 189, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cui, S.W.; Wang, Q.; Yada, R.Y. Studies of aggregation behaviours of cereal β-glucans in dilute aqueous solutions by light scattering: Part I. Structure effects. Food Hydrocoll. 2011, 25, 189–195. [Google Scholar] [CrossRef]
- Sun, T.; Li, J.; Qin, Y.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Shao, Z. Rheological and functional properties of oat β-glucan with different molecular weight. J. Mol. Struct. 2020, 1209, 127944. [Google Scholar] [CrossRef]
- Nie, X.-R.; Li, H.-Y.; Du, G.; Lin, S.; Hu, R.; Li, H.-Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.-T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Wan, M.; Chen, J.; Li, S.; Cao, M.; Zhang, D. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef]
- Wang, R.; Chen, P.; Jia, F.; Tang, J.; Ma, F. Optimization of polysaccharides from Panax japonicus C.A. Meyer by RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2012, 50, 331–336. [Google Scholar] [CrossRef]
- Sourki, A.H.; Koocheki, A.; Elahi, M. Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017, 95, 462–475. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Bai, Y.-Y.; Tang, J.; Chen, W. Antioxidation and α-glucosidase inhibitory activities of barley polysaccharides modified with sulfation. LWT 2015, 64, 104–111. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Liu, F.; Gao, H.; Sun, H.; Meng, M.; Zhang, Y.-M. Synthesis, characterization and antioxidant activity of selenium polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2016, 93, 1090–1099. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Ma, C.; Li, X.; Li, D.; Yang, Y.; Xu, Y.; Wang, L. Characterization and inhibitory activities on α-amylase and α-glucosidase of the polysaccharide from blue honeysuckle berries. Int. J. Biol. Macromol. 2020, 163, 414–422. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, S.; Wang, Q.; Lu, X.; Lin, L.; Tian, Y.; Xiao, J.; Zheng, B. Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohydr. Polym. 2016, 144, 438–446. [Google Scholar] [CrossRef]
- Shen, S.-G.; Jia, S.-R.; Wu, Y.-K.; Yan, R.-R.; Lin, Y.-H.; Zhao, D.-X.; Han, P.-P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Dong, H.; Lin, S.; Zhang, Q.; Chen, H.; Lan, W.; Li, H.; He, J.; Qin, W. Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides. Int. J. Biol. Macromol. 2016, 93, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Xiong, Z.; Wang, G.; Xia, Y.; Lai, P.; Ai, L. Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.). Phytochemistry 2018, 155, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, X.; Bai, J.; Fan, S.; Daglia, M.; Li, J.; Ding, Y.; Zhang, Y.; Zhao, Y. Changes in the structural, physicochemical and functional properties and in vitro fecal fermentation characteristics of barley dietary fiber fermented by Lactiplantibacillus plantarum dy-1. Food Funct. 2024, 15, 4276–4291. [Google Scholar] [CrossRef]
- Li, S.; Bai, J.; Yuan, J.; Yang, Z.; Du, Q.; Pan, B.; Zhu, Y.; Xiao, X.; Li, Y.; Li, C. Comparing in vitro digestion-fermentation characteristics and in vivo lipid-lowering effects of hot water and alkali-extracted polysaccharides from Volvariella volvacea. J. Food Sci. 2025, 90, e70175. [Google Scholar] [CrossRef]



| Content | THB-B | BSG-B |
|---|---|---|
| Extracted yield (%) | 5.11 ± 0.21 a | 4.13 ± 0.10 b |
| Total sugar (%) | 92.69 ± 1.79 a | 81.79 ± 1.39 b |
| β-Glucan (%) | 90.46 ± 1.74 a | 66.35 ± 1.13 b |
| Protein (%) | 2.22 ± 0.34 a | 1.96 ± 0.26 a |
| THB-B | BSG-B | |
|---|---|---|
| Mw (Da) | 2.33 × 106 (±2.34%) b | 5.24 × 106 (±6.34%) a |
| Mw/Mn | 5.40 (±2.98%) | 4.72 (±9.84%) |
| Mz/Mn | 39.16 (±5.74%) | 129.92 (±13.47%) |
| Reducing Monosaccharide | THB-B (%) | BSG-B (%) |
|---|---|---|
| Gulonic acid | 0.058 | 0.080 |
| Mannuronic acid | 0.000 | 0.000 |
| Mannose | 0.294 | 1.957 |
| Glucosamine | 0.056 | 0.587 |
| Ribose | 0.000 | 0.000 |
| Rhamnose | 0.023 | 0.194 |
| Glucuronic acid | 0.011 | 0.858 |
| Galacturonic acid | 0.119 | 0.315 |
| Galactosamine | 0.000 | 0.188 |
| Glucose | 97.959 | 81.128 |
| Galactose | 0.434 | 2.188 |
| Xylose | 0.385 | 5.153 |
| Arabinose | 0.562 | 6.770 |
| Fucose | 0.000 | 0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Namgyal, L.; Chen, S.; Zhou, Y.; Nayab, A.; Qin, Z.; Dondup, D.; Sun, L. β-Glucan from Highland Barley Spent Grain: Yield, Molecular Weight, Physicochemical Properties, Antioxidant Capacity, and Gel Characteristics. Fermentation 2025, 11, 600. https://doi.org/10.3390/fermentation11100600
Li S, Namgyal L, Chen S, Zhou Y, Nayab A, Qin Z, Dondup D, Sun L. β-Glucan from Highland Barley Spent Grain: Yield, Molecular Weight, Physicochemical Properties, Antioxidant Capacity, and Gel Characteristics. Fermentation. 2025; 11(10):600. https://doi.org/10.3390/fermentation11100600
Chicago/Turabian StyleLi, Suyang, Lhundrup Namgyal, Shiyi Chen, Yong Zhou, Afira Nayab, Zhou Qin, Dawa Dondup, and Ling Sun. 2025. "β-Glucan from Highland Barley Spent Grain: Yield, Molecular Weight, Physicochemical Properties, Antioxidant Capacity, and Gel Characteristics" Fermentation 11, no. 10: 600. https://doi.org/10.3390/fermentation11100600
APA StyleLi, S., Namgyal, L., Chen, S., Zhou, Y., Nayab, A., Qin, Z., Dondup, D., & Sun, L. (2025). β-Glucan from Highland Barley Spent Grain: Yield, Molecular Weight, Physicochemical Properties, Antioxidant Capacity, and Gel Characteristics. Fermentation, 11(10), 600. https://doi.org/10.3390/fermentation11100600







