4.1. Fermentation Characteristics of Ryegrass Silage
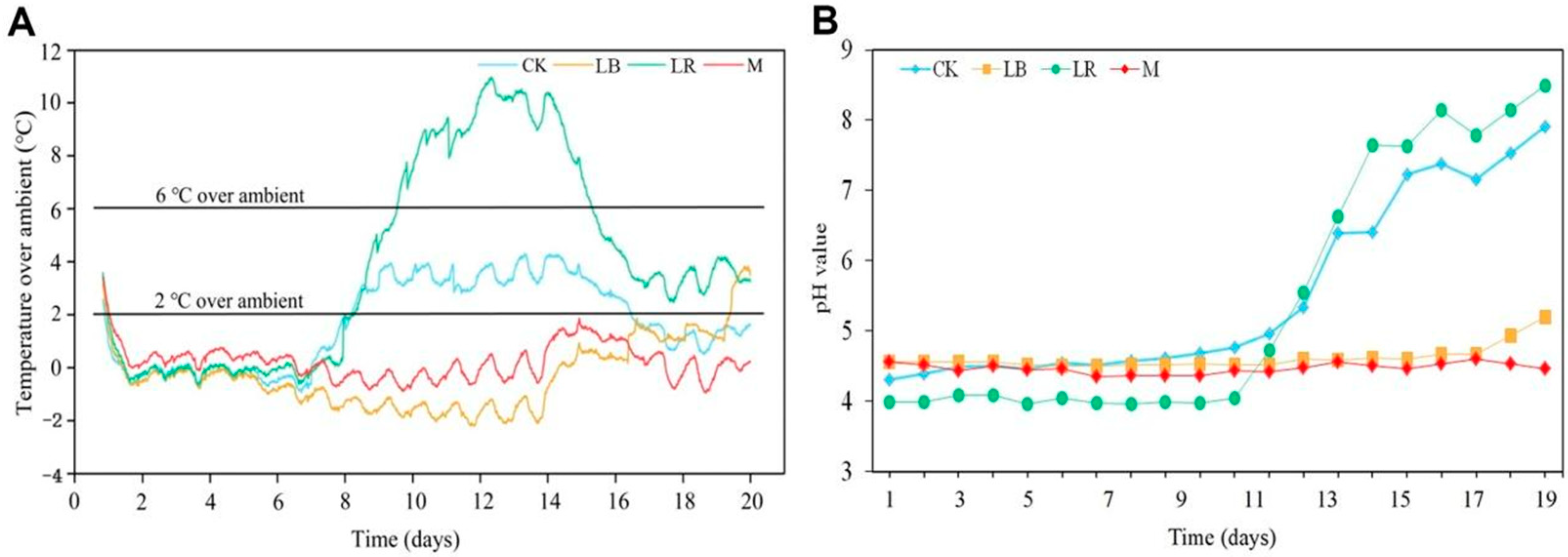
LAB inoculants are used to improve and enhance the quality of silage fermentation and may affect aerobic stability during opening and utilization. Upon the opening of the silage, the environment underwent a rapid transition from anaerobic to aerobic conditions. This resulted in the activation of yeast within the silage, which rapidly consumed LA and caused a notable increase in pH [
28]. Similarly, feed exposure to air triggers higher aerobic microbial activity, and lactic acid assimilation yeast initiates the process of aerobic spoilage. This phenomenon eventually leads to the generation of excessive heat [
29]. Following a nine-day aerobic exposure period, the pH of the CK and LR silages exhibited a marked increase, whereas the silages treated with LB and M demonstrated a more stable pH profile. This may be explained by the addition of
L. buchneri, a heterofermentative LAB, to both treatment groups, which inhibits aerobic spoilage [
18]. The pH of M and LB did not increase significantly until 19 days after aerobic exposure. The aerobic stability of the various treatments was also corroborated by these findings. The aerobic stability of the CK and LR silages was observed to persist for a period of nine days, whereas the LB and M silages demonstrated a longer duration of stability, exceeding 19 days. It has been demonstrated that the
L. buchneri strain has the capacity to enhance the aerobic stability of silage [
30], a number of experiments [
8,
31,
32] have examined this bacterium as a typical heterofermentative lactic acid bacterium (LAB) in silage, with the aim of increasing aerobic stability by producing acetic acid (AA) as a means of inhibiting yeast growth, even in silages with high water content.
L. buchneri can improve their aerobic stability [
33]. In addition,
acetobacterium has also been shown to oxidize ethanol to acetic acid, thereby increasing aerobic stability [
34].
4.2. Effects of LAB on the Microbial Community of Italian Ryegrass Silage
Generally, epiphytic bacterial communities were highly correlated with feed type [
35], and a very low natural abundance of
Lactobacillus was found in grasses [
36] and grains [
37]. It is well-documented that Lactobacillus species play a pivotal role in silage production, given their rapid growth and ability to reduce pH by producing lactic acid. Strains belonging to the Lactobacillus genus are typically employed as silage additives with the objective of enhancing the quality of the fermentation process [
38,
39]. After prolonged ensiling, exogenous LAB decreased unwanted bacteria like
Paenibacillus,
Rosenbergiella, and
Providencia while increasing the growth of Lactobacillus and ensuring their continued dominance of the bacterial community. The aforementioned findings demonstrated how exogenous LAB helps to stabilize the microbiota over extended storage [
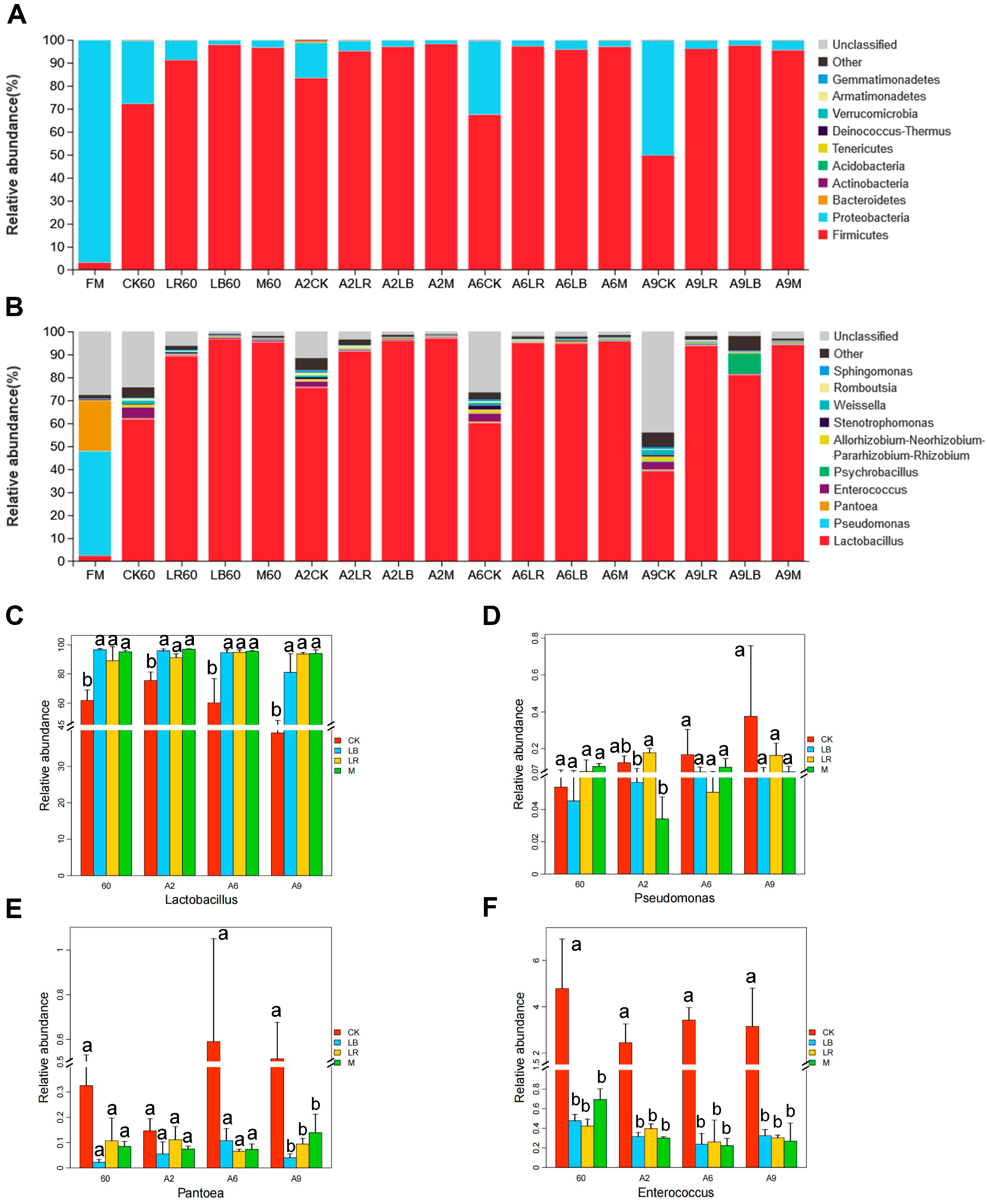
40]. As expected, in our study, LR and LB increased the abundance of
Lactobacillus (
Figure 2B). Generally,
Clostridium is undesirable in silage because it competes with LAB, leading to an increase in pH and a slowdown in nonprotein-N accumulation [
41].
Clostridium produces BA and leads to poor fermentation quality. Some species may also produce pathogenic toxins during silage that are harmful to animals [
42]. In the present study, fewer
Clostridium were observed in the LAB-treated group than in the CK group, which is consistent with the better fermentation quality of the LAB-treated silage. The bacterial diversity was consistently higher for the CK than for the other treatment groups, probably because the LAB in the CK group is less dominant than in the treatment group, hence leading to a higher diversity.
Lactobacillus was the dominant bacterial genus in all treatment groups, both at the end of fermentation and after 9 days of aerobic exposure. This may be due to the lower ambient temperature (15 °C) at the time of aerobic exposure that inhibited the activity of all microorganisms, making their structural changes very insignificant. The increase in fungal diversity at the end of fermentation may be because the particular silage environment of high-moisture Italian ryegrasses reduced the inhibition of fungi even in the microbial environment with a higher relative abundance of
Lactobacillus. In contrast, the rapid loss of water in Italian ryegrass after aerobic exposure reduced the suppression of
Lactobacillus and made the emergence of fungi more repressive in a microbial environment dominated by
Lactobacillus [
43].
The relative abundance of
Issatchenkia in the CK increased substantially at 9 days of aerobic exposure and became the dominant fungal genus, while the abundance in the
Lactobacillus-supplemented treatments increased only slightly, which is consistent with previous findings [
44] and may be due to the inhibition of
Issatchenkia growth by
Lactobacillus during aerobic exposure.
Penicillium is a vital fungus in the aerobic spoilage process of forages, producing not only various toxins but also penicillic acid and mycophenolic acid. At 9 days of aerobic exposure,
Penicillium was the dominant genus in the CK and LR groups, while its relative abundance was minimal in the LB group and the M group, probably because of the metabolic production of AA and phenyllactic acid by
L. buchneri, and these two organic acids are very effective in inhibiting the growth and Ochratoxin A production of
Penicillium [
45].
4.3. Metabonomic Analysis of Italian Ryegrass Silage
The fermentation process of silage aerobic spoilage is complex and involves interactions between metabolites and various microorganisms. The addition of LAB not only affected the composition of silage microorganisms but also changed the metabolism of silage microorganisms. Studies have revealed that LAB also produce some metabolites with antifungal properties, such as 4-hydroxybenzoic acid, 3-hydroxydecanoic acid, acetaldehyde, 3-(R)-hydroxytetradecanoic acid, vanillic acid, 2,3-butanedione, and bacteriocins [
46,
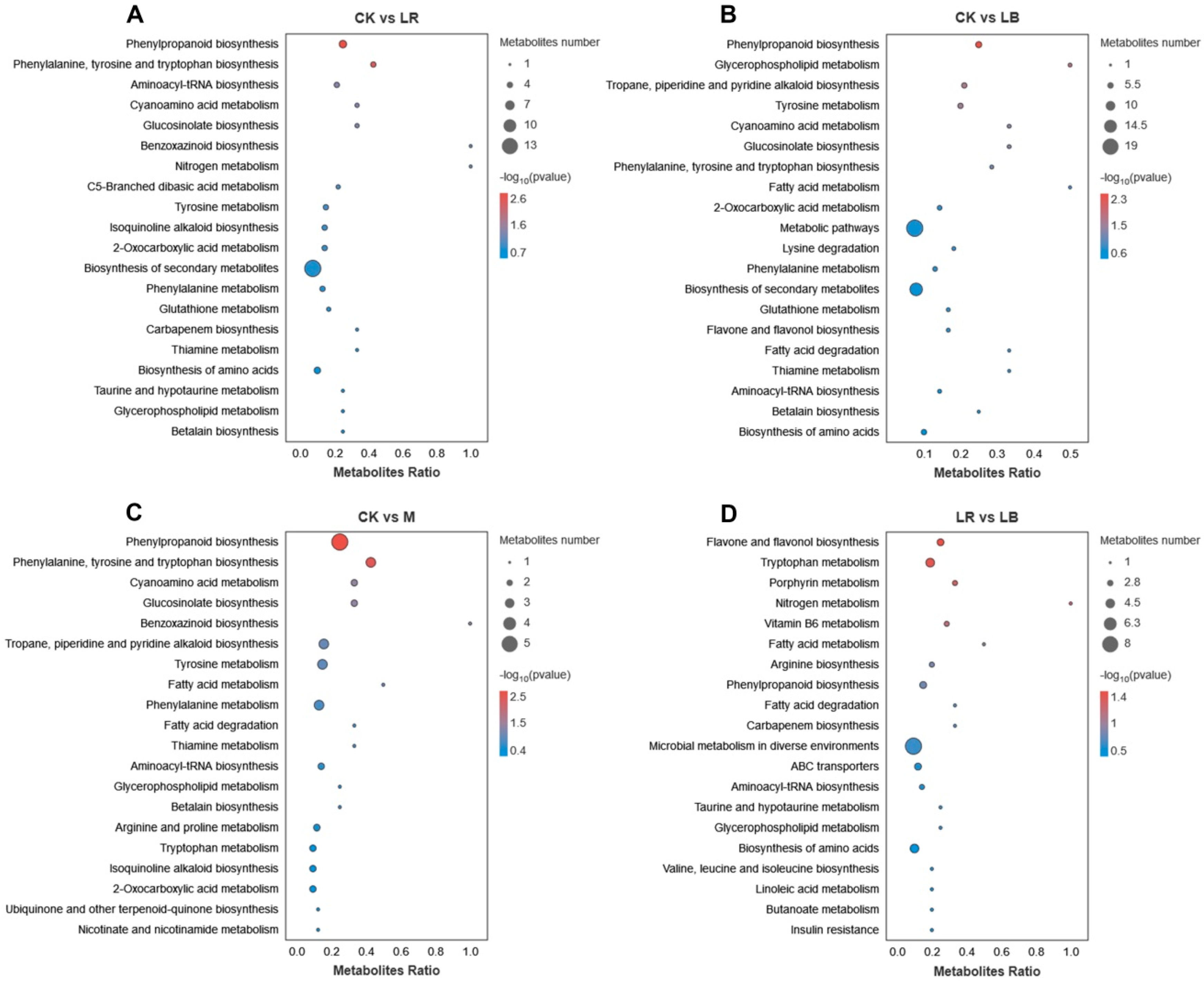
47]. Different metabolites affected various metabolic pathways, but the same differential metabolic pathway (phenylpropanoid biosynthesis) was observed in all of the LAB-treated Italian ryegrass silage compared to the CK (
Figure 5A–C). The differential metabolites in this metabolic pathway were trans-2-hydroxy-cinnamate and ferulic acid with antioxidant properties [
48] and phenylalanine, tyrosine, and cinnamaldehyde for flavor addition [
49,
50,
51]. This indicates that the use of LAB additives through the “phenylpropanoid biosynthesis” pathway increased the aerobic stability of Italian ryegrass silage. Flavonoids, a major class of plant secondary metabolites, are known to be a phenolic family with beneficial health effects due to their antioxidant, anti-inflammatory, antimicrobial, antimutagenic, and anticancer properties [
52]. Xu et al. [
53] reported that many phenolics, such as flavonoids and flavones, were identified as differential metabolites between control and inoculant sainfoin silages. The differential metabolic pathways in the LB and LR groups were “flavone and flavonol biosynthesis”, “tryptophan metabolism”, and “porphyrin and chlorophyll metabolism” (
Figure 5D). Among the differential metabolites in the “flavone and flavonol biosynthesis” metabolic pathway, including vitexin with antioxidant properties and luteolin with antimicrobial properties, we inferred that the inoculant
L. buchneri can regulate flavone and flavonol polyphenol during silage. This may be the cause of the difference between
L. buchneri and
L. rhamnosus as the main metabolite responsible for the difference in the ability of the two additives to inhibit aerobic spoilage of Italian ryegrass silage.
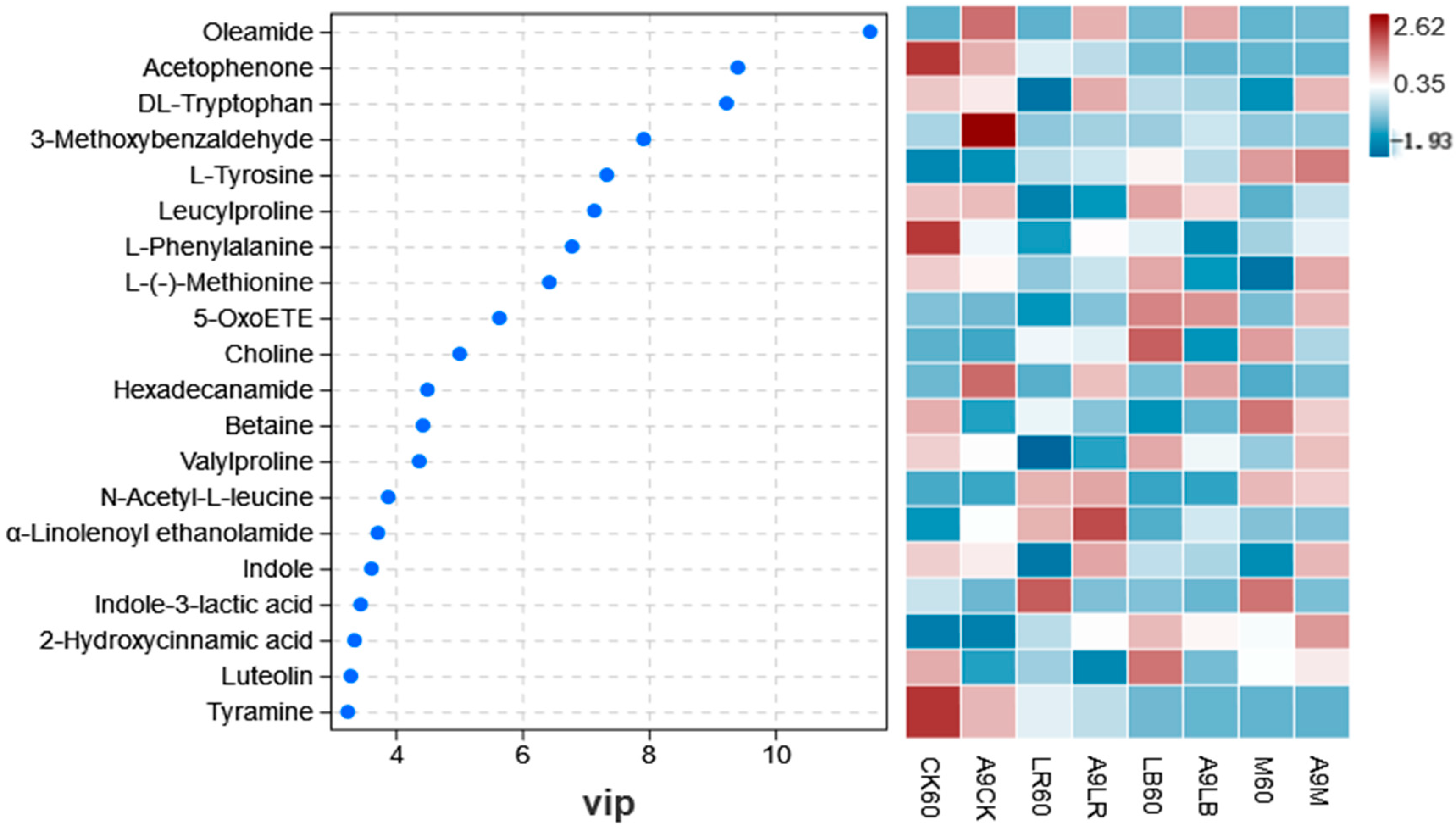
Tryptophan and phenylalanine are both aromatic amino acids and essential amino acids [
54]. In livestock feeding, low tryptophan intake will reduce appetite [
55]. Tryptophan can be used as a feed additive to regulate appetite in pigs, sheep, and cattle, thereby improving farming efficiency [
55]. Nathalie et al. [
56] produced a more severe inflammatory response in pigs fed food with low tryptophan content. Phenylalanine is a common precursor to many common precursors of many phenolic compounds, including flavonoids, polyflavonoids, lignin, and phenylpropanoids [
57]. In phycology, derivatives of phenylalanine have a wide range of physiological functions; since animals cannot synthesize phenylalanine, it has become an essential requirement in animal diets [
58]. In all additive-treated ryegrass silages, the relative abundances of DL-tryptophan and L-phenylalanine were downregulated to varying degrees at 9 days of aerobic exposure, indicating that the decrease in feed palatability after aerobic spoilage is likely to be related to these two amino acids. In addition, the downregulation of tryptophan after aerobic exposure may be because it can be metabolized by
Lactobacillus to indole-3-carboxaldehyde and by
Bifidobacterium to indole-3-lactic acid [
59,
60], whereas at 9 days of aerobic exposure, the LB group and M group still had a higher relative abundance of
Lactobacillus. This also explains the upregulation of indole-3-lactic acid in these two treatment groups after aerobic exposure.
Penicillium was positively correlated with L-phenylalanine (
Figure 7B), while the extremely high relative abundance of
Penicillium in the CK and LR groups (>50%,
Figure 3B) at 9 days of aerobic exposure may explain the upregulation of L-phenylalanine in these two treatment groups (
Figure 6). In addition,
Issatchenkia had a high relative abundance in the CK at 9 days of aerobic exposure (
Figure 3B) and was negatively correlated with the contents of 2-hydroxycinnamic acid and indole with aroma-enhancing effects, the fatty acid 5-OxoETE, and LA and the abundance of
Lactobacillus (
Figure 7B). These findings suggest that the loss of nutrients and reduced palatability of silage under aerobic conditions without the addition of
Lactobacillus are associated with
Issatchenkia.
In the present research, the only mycotoxins screened were aflatoxin, sterigmatocystin, and T-2 triol, which may be the result of the average temperature being below 15 °C, while fungi proliferation and mycotoxin production are enhanced in hot and humid environments [
61]. Aflatoxin is one of the most abundant and mutagenic mycotoxins present in forage [
62]. Aflatoxin B1 is the most carcinogenic chemical known, being strongly poisonous to humans and animals, and its toxic effects mainly cause liver damage [
63]. Several studies have shown that
L. rhamnosus can effectively resist aflatoxin B1, M1, and G1 through the adsorption mechanism [
64,
65,
66], which explains why there was a slight downregulation of aflatoxin B1, M1, and G1 in the LR group after aerobic exposure in our study. Although it is widely accepted that LA and AA produced by LAB are the main metabolites that inhibit fungal growth and mycotoxin production, it is clear that AA is the most effective antifungal metabolite of LAB [
67]. The minimum inhibitory concentration value of AA was 33 mM to inhibit 50%
Penicillium nordicum growth and 100% ochratoxin production [
45]. This may explain the fact that the LB group with the highest concentration of AA had less
Penicillium and mycotoxin than the other treatment groups (
Figure 3B and
Table 4). It has been shown that LA has no effect on the growth of several fungal species under the genus
Penicillium [
68], which explains why Italian ryegrass silage under
L. rhamnosus additions had a very high relative abundance of
Penicillium, although LA was detected at considerable levels after aerobic exposure. T-2 triol is a fungal toxin produced by the metabolism of T-2 toxin, which is less poisonous than T-2 toxin. T-2 toxin inhibits the growth of
Lactobacillus in the growth medium [
69]. In our results, the relative abundance of
Lactobacillus decreased while T-2 triol appeared significantly upregulated in the CK, LB, and LR groups as aerobic exposure proceeded (
Figure 2B and
Table 5).
To explore the complex relationships among organic acids, metabolites, microbial community composition, and the aerobic stability (temperature over ambient) of Italian ryegrass silage, we conducted an SEM analysis. We found that aerobic stability was not directly affected by metabolites or the bacterial community. This result contradicted our hypothesis that metabolites and bacterial communities were the main and direct contributors to the aerobic stability of Italian ryegrass silages. In this regard, we speculate that high moisture conditions inhibit the direct effect of bacteria on aerobic stability and instead act indirectly on aerobic stability through interactions between metabolites. Consistent with previous studies, the fungal community was a direct factor influencing the aerobic stability of silage [
70], and in our model, the fungal community was also the only direct negative influence. In addition to the indirect effects of bacteria and metabolites, a very stable fungal community structure was maintained before and after aerobic exposure, which may explain the extra-long stability of the M group after aerobic exposure. Metabolites were significantly affected directly by bacterial and fungal communities, while in
Figure 3, we see that bacteria and fungi showed similar community compositions in all additive treatment groups for 60 days of silage, which may explain the small difference in metabolites between these three treatment groups (
Figure 4). In our results, bacteria had a direct positive but not significant effect on organic acid levels. This may be because our data are in the aerobic contact phase, when the activity of the dominant bacterial genus, anaerobic
Lactobacillus, is inhibited, reducing its ability to produce LA and AA, and thus the causal relationship between the two is not significant. This can also explain the insignificant correlation between bacteria and fungi, where LAB, which receive inhibition after aerobic exposure, have a diminished inhibitory or promotional effect on fungi and lead to the regulation of fungi through interactions with metabolites. Organic acids have a negative direct effect on fungi, which is well understood because AA, PA, and BA have a good inhibitory effect on the growth and reproduction of fungi [
67].