Abstract
Grain processing produces many by-products, including wheat bran, wheat germ and rice bran, which are rich in carbohydrates, proteins and trace elements. In this study, these grain-derived by-products were used as raw materials to conduct solid-state fermentation using mixed strains of Aspergillus kawachii and Rhizopus oryzae, and the potential immunomodulatory and anti-allergic properties of fermented product were evaluated. Solid-state fermentation of a grain by-product mixture, consisting of rice bran, wheat bran, and wheat germ in a 2:1:1 weight ratio, using both A. kawachii L1 and R. oryzae L1 at 26 °C for 5 days, significantly increased the total phenolic, flavonoid, and amino acid contents. The anti-allergic activity of aqueous extract of the fermented product was evaluated in murine models of food allergy and delayed-type hypersensitivity. Oral administration of the fermented product extract (100–200 mg/kg) notably alleviated allergic symptoms such as diarrhea and histopathological changes in the intestines. Moreover, the extract effectively reduced allergen-specific serum antibodies, suppressed splenic cytokine secretion, and mitigated tissue edema and inflammation induced by allergens. Importantly, the extract induced the production of IL-10 and TGF-β, which are well-known cytokines primarily secreted by regulatory T cells. These results underscore the promising immunomodulatory effects of A. kawachii and R. oryzae fermented grain product, suggesting their potential as functional foods or additives for managing allergic disorders, with implications for future therapeutic and dietary applications.
1. Introduction
Grain processing is a significant contributor to global food by-products, accounting for approximately 12.9% of the total [1]. Among the major by-products generated in grain processing are wheat bran, wheat germ, and rice bran, which are rich in carbohydrates, proteins, and trace elements. These materials serve as valuable raw resources for the creation and development of various products, opening up possibilities for harnessing them in the transformation of fungi into both food and medicinal applications [2]. The utilization of these grain-derived by-products through solid-state fermentation has garnered attention. Solid-state fermentation is a biotechnological process in which microorganisms are cultivated on a solid substrate with low water content [3]. Commonly chosen substrates for solid-state fermentation encompass grains, such as rice, wheat, barley, and corn, along with various agricultural residues, owing to their cost-effectiveness and ready availability. Solid-state fermentation offers numerous advantages, including facile product recovery, reduced overall production costs, smaller fermenter requirements, diminished downstream processing, and lowered energy consumption for agitation and sterilization [4]. In recent years, solid-state fermentation has been utilized to enhance the recovery of bioactive compounds from various agricultural by-products, owing to its ability to increase the content and bioavailability of these compounds. For instance, a study on the fermentation of rice bran with A. oryzae and R. oryzae demonstrated significant improvements in antioxidant activities and the production of phenolics and organic acids, particularly ferulic acid and citric acid. These fermented extracts also exhibited promising skincare-related functionalities, such as tyrosinase and elastase inhibition, indicating their potential applications in cosmetic products and antioxidant-rich formulations [5]. Similarly, solid-state fermentation has been applied to sorghum, which naturally contains high levels of tannins. These tannins, though traditionally viewed as antinutritional factors, were efficiently extracted and converted into beneficial phenolic compounds through fermentation with A. oryzae and A. niger. The process not only increased the antioxidant activities of the sorghum extracts but also enriched them with diverse polyphenols, including procyanidins and catechins [6]. Moreover, solid-state fermentation followed by extrusion significantly increased the levels of phenolics, flavonoids, and γ-oryzanol. The choice of microbial strains, such as A. oryzae and Neurospora sitophila, further influenced the enhancement of bioactive components, demonstrating the versatility and effectiveness of solid-state fermentation in optimizing the health benefits of rice bran [7]. Critical factors influencing the success of solid-state fermentation include microbial species, substrate selection, water activity, temperature, aeration, and fermenter type, with the choice of microorganism playing a pivotal role. Microorganisms utilized in solid-state fermentation can exist as single pure strains, known mixed strains, or consortia of mixed indigenous microorganisms [2].
One such microorganism of interest is A. kawachii, a member of the black Aspergillus fungi group, which has traditionally been employed as an enzyme source in the production of shochu koji and rice or barley wine in Korea and Japan [8]. During fermentation, A. kawachii exhibits the capacity to produce various hydrolytic enzymes, including amylase and protease, facilitating the breakdown of grain substrates and the generation of active compounds like amino acids, peptides, and polyphenols [9]. A. kawachii solid-state fermented grain products have even demonstrated the potential to enhance the anticancer properties of silkworm larvae [10]. New research findings suggest that the aqueous solution derived from rice bran that has undergone solid-state fermentation by A. kawachii demonstrates potent antioxidant properties and effectively alleviates liver damage induced by a high-fat diet in rat models [11]. On the other hand, Rhizopus oryzae, another GRAS (generally recognized as safe) fungus, is known for its ability to produce an array of enzymes capable of breaking down carbohydrates, polymers, as well as various organic acids, alcohols, and esters. These properties render R. oryzae valuable in the food industry, biodiesel production, and pharmaceutical applications. The fermentation of rice bran by R. oryzae has been shown to enhance the content of bioactive components such as peptides, fibers, phenolics, and minerals [12]. Despite their extensive use in Asian food fermentation for centuries, there has been limited exploration of the immunomodulatory and anti-allergic potential of both A. kawachii and R. oryzae. This research seeks to address this gap.
Within the realm of immune control, there exist two distinctive categories of T helper (Th) cells, namely Th1 and Th2, which play essential roles in directing distinct facets of adaptive immunity. Th1 cells are chiefly responsible for the secretion of cytokines such as inteferon (IFN)-γ and interleukin (IL)-2, triggering responses in cytotoxic T cells and macrophages to bolster cellular immune reactions. In contrast, Th2 cells are primarily engaged in the production of cytokines like IL-4, IL-10, and IL-13, which serve to activate mast cells, eosinophils, and B cells, thus supporting the orchestration of humoral immune responses [13,14]. Dysregulation in the balance between Th1 and Th2 cells has been linked to various immune disorders, especially allergic disorders [15,16,17]. Regulatory T (Treg) cells represent another subset of T cells with suppressive capacity, pivotal in controlling the development and maintenance of allergic diseases by secreting suppressive cytokines like IL-10 and transforming growth factor (TGF)-β, facilitating the generation of immune tolerance to allergens [18].
Food allergy are prevalent immune system disorders in the pediatric population, often triggered by common allergens such as eggs, milk, peanuts, tree nuts, and seafood [19]. The gastrointestinal system frequently serves as the primary site of action for food allergens. Mast cells, armed with allergen-specific immunoglobulin (Ig)E antibodies, function as effector cells, initiating hypersensitivity reactions. Given the prominent role of Th2-associated cytokines in IgE isotype switching, strategies aimed at inhibiting Th2 polarization have been explored as therapeutic avenues for managing food allergy [20]. Delayed-type hypersensitivity (DTH), an antigen-induced immune response, serves as a valuable tool for assessing cell-mediated immune responses associated with Th1 reactivity. Activation of antigen-presenting cells leads to the release of cytokines and chemokines, promoting vascular endothelial cell permeability, phagocyte infiltration, and fluid accumulation at DTH-responsive sites [21]. Unlike food allergy, DTH symptoms, such as tissue edema and swelling, typically peak 24–48 h after allergen exposure. Current pharmacotherapy for both food allergy and DTH primarily focuses on symptom relief, with allergic management primarily revolving around allergen avoidance.
The aim of this study was to investigate the immunomodulatory and anti-allergenic potential of solid-state fermentation products using a grain by-product mixture consisting of rice bran, wheat bran and wheat germ fermented with mixed strains of A. kawachii and R. oryzae. The assessment was conducted through murine models of food allergy and DTH, shedding light on their suitability as functional foods or additives for alleviating allergic disorders.
2. Materials and Methods
2.1. Culture, Chemicals and Reagents
Aspergillus kawachii L1 was purchased from the Bioresources Collection and Research Center (Hsinchu, Taiwan), and Rhizopus oryaze L1 was provided by Chian-E Biomedical Technology Corporation (Taipei, Taiwan). Rice bran was purchased from Guan shan township agricultural association (Taitung, Taiwan), and wheat bran and wheat germ were purchased from Lien Hwa Milling Corporation (Taoyuan, Taiwan). All chemical compounds and reagents used in this study were acquired from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and Panreac (Castellar del Vallès, Barcelona, Spain), unless explicitly specified otherwise. The cell culture materials and reagents were procured from GE Healthcare Life Sciences (Marlborough, MA, USA). The media and culture supplies for microbial growth were sourced from Difco Laboratories (Detroit, MI, USA). Furthermore, enzyme-linked immunosorbent assay (ELISA) kits employed for the measurement of cytokines and immunoglobulins (Ig) were obtained from eBioscience, Inc. (San Diego, CA, USA).
2.2. Solid-State Fermentation of Grain-Derived by Products by A. kawachii L1 and R. oryzae L1
The fermentation process followed the methodology reported in a previous study, with some modifications [5]. R. oryaze L1 and A. kawachi L1 were inoculated on MEA plates and cultured at 26 °C for 3 and 4 days, respectively. The colonies of these two strains were isolated and inoculated individually on MEA slants, then cultured at 26 °C for 3–4 days. 150 g of a grain by-product mixture of rice bran, wheat bran and wheat germ with a weight ratio of 2:1:1 were added with 100 mL of distilled water, sterilized at 121 °C for 30 min and spread in a stainless steel box (20 × 16 × 4 cm, length: depth: height). After cooling, the grain by-product mixture was inoculated with 10 mL spore suspensions (containing 1 × 105 spores/mL for each strain), and incubated at 26 °C for 5 days. The fermented product was extracted with sterile distilled water (w/v = 1/6) and stirred at 200 rpm at 60 °C for 1 h. After cooling and centrifugation, the supernatants were harvested and lyophilized for further experiments.
2.3. Determination of Total Phenolics, Flavonoids and Amino Acids Contents
The quantification of total soluble phenolics in aqueous extracts of both fermented and unfermented products was accomplished using the Folin–Ciocalteu reagent, with gallic acid serving as the reference standard phenolic compound, consistent with the methodology outlined in the preceding investigation [22]. The procedure involved mixing 1 mg of lyophilized samples with 45 mL of distilled water, followed by the addition of 1 mL of Folin–Ciocalteu reagent. After thorough mixing for 3 min, 3 mL of a 2% Na2CO3 solution was introduced. Subsequently, the mixture was left to stand in the dark for 2 h with intermittent shaking, and the optical density was measured at 760 nm. The concentration of total phenolic content was quantified in μg of gallic acid equivalent (GAE) by referencing a calibration curve of gallic acid standards. Furthermore, lyophilized extract samples were dissolved in distilled water, and the diluted solutions or rutin (0.5 mL) were mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. After a 40 min incubation at room temperature, the optical density was measured at 415 nm to determine the concentration of flavonoid content in μg of rutin equivalent (RE) based on the calibration curve of rutin standards [22]. Quantitative amino acid analysis was conducted using a Beckman System 6300 amino acid analyzer (Beckman Instruments, Inc., Brea, CA, USA).
2.4. Ethics Statement and Conduct of Animal Experiments
Female BALB/c mice, 5 weeks old and free from specific pathogens, were procured from the National Laboratory Animal Center of Taiwan. The mice were accommodated within the laboratory animal facility at the National Taiwan Ocean University (NTOU) for a one-week acclimation period before the commencement of any treatments. Throughout this acclimation period, the mice had unrestricted access to both diet and water. It is important to note that all experimental procedures adhered to the protocols and principles outlined in the National Research Council’s Guide for the Care and Use of Laboratory Animals. Furthermore, these experiments were subject to approval by the NTOU Institutional Animal Care and Use Committee (NTOU IACUC-110023).
The murine models for food allergy and DTH were established following the procedures detailed in prior research studies [23,24]. In the murine model of food allergy, the mice were randomly divided into 4 groups: the naïve group (NA), the vehicle group (VH), and the treatment groups (L and H groups), each consisting of 5 mice. The VH group received daily oral administration of 0.1 mL of phosphate-buffered saline (PBS) by gavage. Meanwhile, mice in the treatment groups were orally administered either 100 mg/kg or 200 mg/kg of lyophilized extract (L and H groups, respectively) dissolved in 0.1 mL of PBS via gavage. Except for the mice in the NA group, all other mice were subjected to intraperitoneal injections. On the 3rd day, they received a 0.1 mL injection of a PBS solution containing 50 μg ovalbumin (OVA) and 1 mg of aluminum hydroxide, and on the 17th day, they were sensitized through an intraperitoneal injection of a double dose of OVA and aluminum hydroxide. Subsequent OVA (50 mg/mouse) challenges by gavage were carried out every other day from day 31 to day 39. Prior to challenges, mice were fasted for 3 h. Allergic diarrhea, characterized by stool form severity scored from 0 to 3, was observed 30–60 min after the challenge: 0, no fecal matter or solid state; 1, funicular form; 2, slurry; 3, watery state. On day 40, the mice were euthanized by CO2 inhalation, and their duodenal tissues were collected for histopathological examination. Villus/crypt ratio, number of mast cells observed in hematoxylin and Eosin-stained or toluidine blue-stained sections were determined by ImageJ software 1.53v as described in the previous studies [25]. The serum samples were harvested for the detection of antibodies, and the spleen samples were collected individually for the preparation of splenocyte suspensions.
In the murine model of DTH, the mice were distributed into four groups the same as that of the food allergy model, with each group comprising 5 mice. In the VH group, mice received daily oral administrations of 0.1 mL of PBS via gavage. Conversely, mice in the treatment groups (L and H groups) were orally administered 100 or 200 mg/kg of lyophilized extract, respectively. With the exception of the mice in the NA group, the other mice were sensitized through an intraperitoneal injection of 0.1 mL of a PBS solution containing 100 μg of OVA and 2 mg of aluminum hydroxide on the 3rd day. On day 9, mice were anesthetized with isoflurane at a maintenance concentration of 2.5% and oxygen pressurized at 4 kg/cm2 using the XGI-8 Anesthesia System, and then subcutaneously injected with 200 μg OVA (dissolved in 20 μL PBS) into the footpads to cause local swelling [23]. After 24 h, the change in thickness of mouse footpads were measured by electronic digital caliper (CD-15APX). Following the experimental procedures, the mice were humanely euthanized by CO2 inhalation, and their footpad tissues, serum, and spleen were collected for subsequent investigations. For histopathological examination, tissue sections were meticulously prepared and stained by the National Laboratory Animal Center (Taipei, Taiwan).
2.5. Cultivation of Splenocytes and Quantification of Cytokines and Immunoglobulins
Splenocytes, at a concentration of 5 × 106 cells per mL, were plated in 96-well culture plates with 0.1 mL per well. These plates were incubated for 44 h in the presence of OVA at a concentration of 10 μg/per mL. Subsequently, 10 μL of a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) stock solution (5 mg/mL in PBS) were added to each well and incubated for 4 h. After the incubation period, formazan formed was dissolved using a 10% dimethyl sulfoxide solution, and the absorbance of the plates was measured at 570 nm [25]. In addition, the concentrations of IL-4, IFN-γ, IL-17, IL-10, and TGF-β in the supernatants from the cultured splenocytes and the levels of total IgG, IgE, OVA-specific IgG and IgE in serum were quantified using ELISA following the supplier’s instructions [25].
2.6. Statistical Analysis
A comparison between the two groups was carried out using the Student’s t-test, conducted with SigmaPlot V14 from Systat Software Inc. (San Jose, CA, USA). Statistical significance was determined at a threshold of p < 0.05.
3. Results
3.1. Appearance and Contents of Total Phenolics, Flavonoids and Amino Acids of the Solid-State Fermented Grain Product Extract
A mixture of grain- derived substrate composed of rice bran, wheat bran, and wheat germ, were added into stainless steel containers. After thorough mixing, sterilization, and cooling, single strains and mixed cultures of A. kawachii and R. oryzae were added, respectively. After cultivation at 26 °C for 24 h, A. kawachii mycelium grows on the substrate surface and adheres tightly to the grain substrate. The mycelium of R. oryzae protrudes from the grain substrate and appears foggy white. In the mixed strain, more obvious foggy white R. oryzae mycelia were observed, while A. kawachii mycelium grew white dot, and the amount of A. kawachii mycelium was lower than that of R. oryzae mycelium, indicating that the growth rate of A. kawachii was slower than that of R. oryzae (Figure 1A).
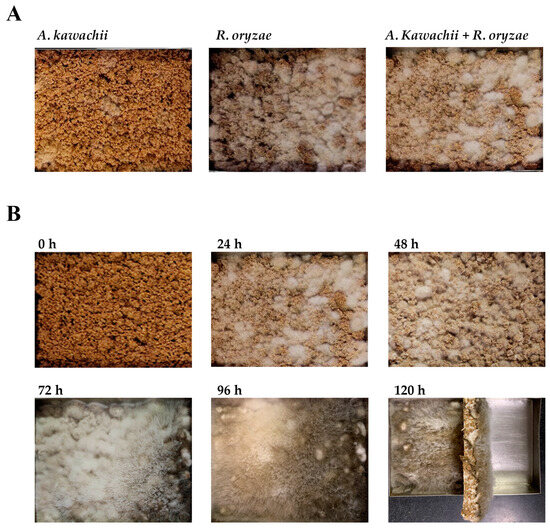
Figure 1.
Appearance of the solid-state fermented grain product. (A) The appearance of the grain substrate after solid-state fermentation with a single strain of A. kawachii, R. oryzae, or a mixed strain of A. kawachii and R. oryzae for 24 h. (B) The appearance of the grain substrate after solid-state fermentation with a mixed strain of A. kawachii and R. oryzae for 0–120 h.
The growth of the mixed strains on the substrate was monitored from 0 to 120 h in culture, and changes in mycelium growth and appearance of the grain substrate were observed, as shown in Figure 1B. As the fermentation time increases, the mycelium of R. oryzae covers the entire surface of the grain substrate and black spores begin to develop. In addition, the number of white spots of A. kawachii mycelium also increased. Because R. oryzae grows in the upper layer, covering A. kawachii and culture substrate, after fermentation for 72 h, the mycelium of R. oryzae grows taller and thicker, and its black spores make some areas appear black. After 120 h of fermentation, it can be observed that A. kawachii mycelium and R. oryzae mycelium form a thick and solid structure (Figure 1B).
Given previous studies demonstrating the immunomodulatory properties of amino acids, total phenolics, and flavonoids in grains and fermented products [26,27,28], we conducted a comparative analysis of these components between aqueous extracts obtained from solid-state fermented grain product and the unfermented one. With the exception of ornithine and hydroxyproline, the levels of other amino acids in the extract of fermented product were markedly higher than those in unfermented one. Concurrently, the concentrations of total phenolics and flavonoids in the extract of fermented product exhibited a significant increase, measuring approximately 2.53- and 2.66-fold higher, respectively (Table S1).
3.2. Effects of Solid-State Fermented Grain Product Extract on Attenuating Allergen-Induced Enteritis and Modulating Cytokine Production
In the pilot study, serial doses were tested, and it was found that the lowest effective dose of extract, 100 mg/kg, statistically mitigated allergic diarrhea and footpad swelling. To adhere to the 3Rs principle of animal experimentation, the extract was administered daily via gavage to OVA-sensitized and challenged mice at doses of 100 and 200 mg/kg. Over the course of the research, except for diarrhea following challenge, no unusual signs or deviations in activity were noted in any of the mice. This suggests that the administered doses of fermented product extract did not exert any evident adverse effects on growth. We assessed the severity of diarrhea using a diarrhea score as detailed in Section 2. Except for the non-allergic (NA) group, the diarrhea scores of mice in all remaining groups exhibited a progressive increase with each successive challenge. Significantly, it is noteworthy that the treatment groups displayed statistically lower diarrhea scores compared to the VH group during both the 4th and 5th challenges (Figure 2A).
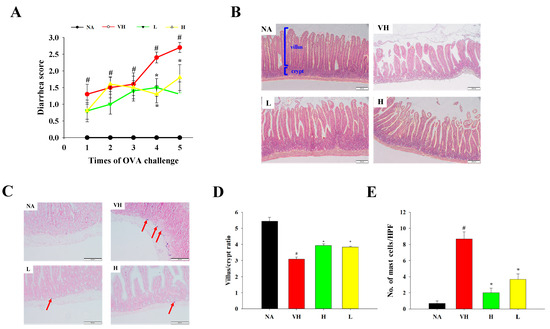
Figure 2.
Effects of fermented product extract on modulating body weight gains, diarrhea scores, histopathological changes in duodenal tissues and mast cell infiltration in mice with food allergy. BALB/c mice were sensitized and challenged with OVA to induce allergic diarrhea, and diarrhea severity was monitored visually for 1 h after each challenge as described in Section 2. (A) Diarrhea scores were recorded throughout the animal experiment. After sacrifice, the duodenal tissue sections were prepared for staining. Representative (B) hematoxylin and eosin-stained and (C) toluidine blue-stained sections are shown (original magnification, ×200). Red arrows indicate mast cell infiltration. (D) Villus/crypt ratio and (E) the number of mast cells were determined by ImageJ software 1.53v. Data are expressed as the mean ± SEM (n = 5). Results are representative of two independent experiments. # p < 0.05 compared to the NA group. * p < 0.05 compared to the VH group.
In order to obtain a more comprehensive understanding of the effects of fermented product extract on the mitigation of allergic enteritis, we carried out histopathological assessments using duodenal tissue sections stained with hematoxylin and eosin and toluidine blue. Within the VH group, conspicuous tissue damage, villus edema, crypt hyperplasia, and mast cell infiltration were observed (Figure 2B,C). In contrast, the treatment groups displayed less severe histopathological alterations when compared to the VH group (Figure 2C,D). Additionally, we noted a higher villus/crypt ratio and a lower number of mast cells in the treatment groups compared to the VH group (Figure 2D,E).
Considering the pivotal functions of allergen-specific IgE and IgG in both triggering and restraining allergen-induced cross-linking of high-affinity IgE receptors, leading to mast cell degranulation, we examined the levels of total and OVA-specific IgE and IgG. Except for total IgG, OVA sensitization and challenge significantly increased the production of total IgE, OVA-specific IgG, and IgE, thus confirming the successful induction of humoral immunity. (Figure 3A–D). While the mice in the H group displayed reduced levels of OVA-specific IgG and IgE in comparison to those in the VH group, no statistically significant disparities in the overall production of total and OVA-specific IgE and IgG were noted when comparing the treatment groups to the VH group (Figure 3A–D).
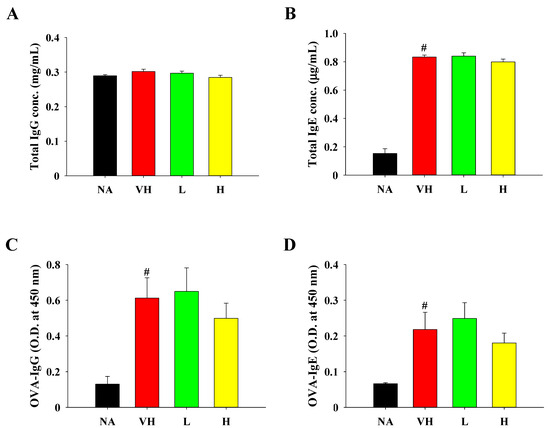
Figure 3.
Effects of fermented product extract on modulating serum antibody production in mice with food allergy. Serum samples were collected before sacrifice for measurement of (A) total IgG, (B) total IgE, (C) OVA-specific IgG and (D) OVA-specific IgE by ELISA. Data are expressed as the mean ± SEM (n = 5). Results are representative of two independent experiments. # p < 0.05 compared to the NA group.
Given that the food allergy model simulates a Th2-skewed immune dysfunction, we additionally evaluated the production of OVA-induced T cell-associated cytokines. Notably, the viability of splenocytes derived from the VH group was greater in comparison to those from the NA group following culture in the presence of OVA (Figure 4A). However, the viability of splenocytes was comparable between the treatment groups and the VH group (Figure 4A). Levels of IL-4, IFN-γ, IL-17, and IL-10 were elevated in the VH group, whereas the treatment groups exhibited lower levels of IL-4, IFN-γ, and IL-17 compared to those in the VH group (Figure 4B–D). We also measured IL-10 and TGF-β levels, known as suppressive cytokines produced by Treg cells that dampen immune responses. Nevertheless, the extract treatment had limited effects on IL-10 production (Figure 4E), and TGF-β levels were below the limit of quantification in all groups.
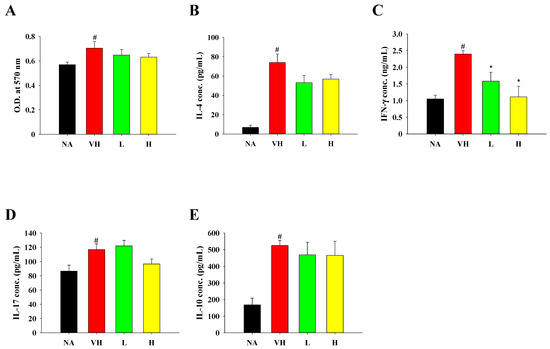
Figure 4.
Effects of fermented product extract on modulating splenic cell viability and cytokine production in mice with food allergy. After the last OVA challenge, mice were sacrificed and their spleens were isolated. Splenocytes were prepared and re-stimulated with OVA for 48 h as described in Section 2. (A) Cell viability was measured by MTT assay. The concentrations of (B) IL-4, (C) IFN-γ, (D) IL-17 and (E) IL-10 in supernatants were quantified by ELISA. Data are expressed as the mean ± SEM (n = 5). Results are representative of two independent experiments. # p < 0.05 compared to the NA group. * p < 0.05 compared to the VH group.
3.3. Effects of Solid-State Fermented Grain Product Extract on Ameliorating Allergen-Induced DTH and Modulating Cytokine Production
To investigate the potential of fermented product extract in mitigating DTH, mice were administered daily doses of the extracts via gavage at 100 and 200 mg/kg. These mice were then sensitized and injected with OVA at the footpads to induce DTH. Crucially, the body weights of mice across various groups displayed consistent comparability throughout this study (Figure 5A). There were no indications of abnormal signs or deviations in activity observed in any of the mice, underscoring the safety of the administered doses. The severity of DTH was initially assessed by measuring the edema of footpads injected with OVA. The footpad thickness of mice in the vehicle (VH) group exhibited a marked increase 24 h after OVA challenge (Figure 5B). Nevertheless, a notable reduction in footpad thickness was evident in the treatment groups when compared to the VH group (Figure 5B). Additionally, pronounced cell infiltration at the OVA injection site was noted in the VH group, while less severe cell infiltration was observed in the treatment groups (Figure 5C).
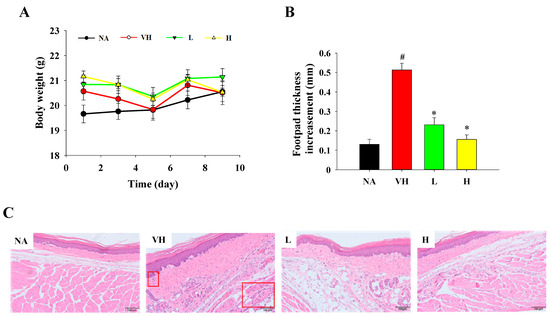
Figure 5.
Effects of fermented product extract on modulating body weight gains and histopathological changes in footpads challenged with OVA in mice with DTH. BALB/c mice were sensitized and challenged with OVA at footpads to induce DTH. (A) Body weight gains were recorded throughout the animal experiment. (B) The thickness of footpads was measured at 24 h after challenge. After sacrifice, the footpads tissue sections were prepared for staining. (C) Representative hematoxylin and eosin-stained sections are shown (original magnification, ×200). Red boxes indicate cell infiltration at subcutaneous and muscular tissues. # p < 0.05 compared to the NA group. * p < 0.05 compared to the VH group.
To ascertain whether fermented product extract exerted similar immunomodulatory effects in different allergy models, we measured the levels of serum antibodies. In line with our findings in the food allergy model, treatment with the extract had limited effects on total and OVA-specific IgG and IgE production in serum (Figure 6A–D).
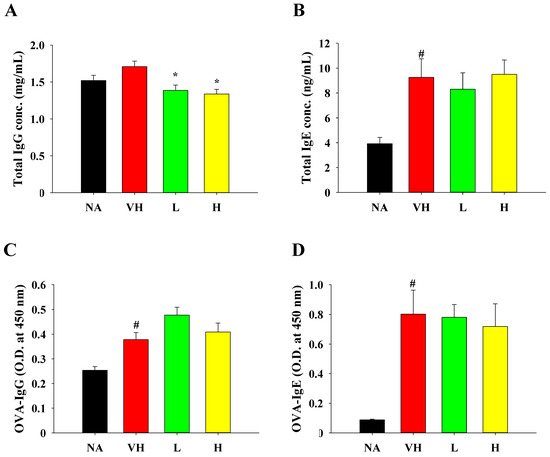
Figure 6.
Effects of fermented product extract on modulating serum antibody production in mice with DTH. Serum samples were collected before sacrifice for measurement of (A) total IgG, (B) total IgE, (C) OVA-specific IgG and (D) OVA-specific IgE by ELISA. Data are expressed as the mean ± SEM (n = 5). Results are representative of two independent experiments. # p < 0.05 compared to the NA group. * p < 0.05 compared to the VH group.
Moreover, the profiles of splenocyte viability and IL-4, IFN-γ, and IL-17 production mirrored those observed in the food allergy model (Figure 7A–D). Notably, treatment with fermented product extract led to an increase in the expression of IL-10 and TGF-β, indicating an enhancement of Treg cell activity (Figure 7E,F).
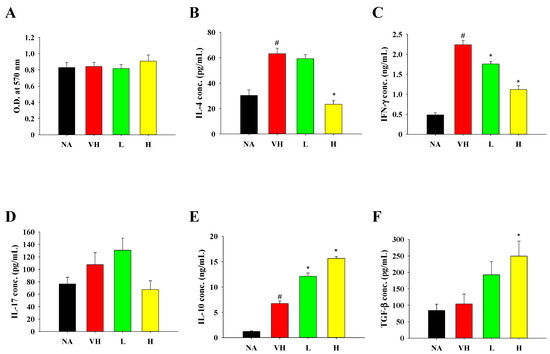
Figure 7.
Effects of fermented product extract on modulating splenic cell viability and cytokine production in mice with DTH. After the OVA challenge, mice were sacrificed and their spleens were isolated. Splenocytes were prepared and re-stimulated with OVA for 48 h as described in Section 2. (A) Cell viability was measured by MTT assay. The concentrations of (B) IL-4, (C) IFN-γ, (D) IL-17, (E) IL-10 and (F) TGF-β in supernatants were quantified by ELISA. Data are expressed as the mean ± SEM (n = 5). Results are representative of two independent experiments. # p < 0.05 compared to the NA group. * p < 0.05 compared to the VH group.
4. Discussion
Grain processing produces by-products resources such as wheat bran and rice bran. Based on the concept of resource recycling, this study shows that these grain-derived by-products can be used as culture substrates to encourage both A. kawachii and R. oryzae to grow vigorously. In addition, this research also opens new frontiers by investigating the immunomodulatory and anti-allergenic properties of grain-derived by-products fermented by A. kawachii and R. oryzae, which have never been explored before. In this study, the anti-allergic effects of the fermented product extract are closely linked to the modulation of key cytokine production, as follows. IL-4 is a critical cytokine in the promotion of Th2 responses, which are heavily implicated in allergic disorders. It plays a pivotal role in the differentiation of naïve T cells into Th2 cells, which then produce more IL-4, IL-5, and IL-13, driving the allergic inflammation process [29]. IFN-γ, a key Th1 cytokine, counterbalances Th2 responses by inhibiting the proliferation of Th2 cells and reducing IgE production [29]. However, in some autoimmune diseases, such as multiple sclerosis, excessive IFN-γ can exacerbate inflammation by promoting the activation of macrophages and other immune cells [30]. IL-17, primarily produced by Th17 cells, has been associated with chronic inflammatory diseases, including psoriasis and rheumatoid arthritis, where it contributes to tissue damage by recruiting neutrophils and other pro-inflammatory cells. In the context of allergies, IL-17 can exacerbate the severity of airway inflammation, particularly in severe asthma [31]. IL-10 is an anti-inflammatory cytokine that plays a crucial role in suppressing immune responses and maintaining immune homeostasis. It is involved in controlling excessive immune responses in allergic conditions and autoimmune diseases, preventing tissue damage by inhibiting the activation and proliferation of T cells, and reducing cytokine production [32]. TGF-β is another key regulatory cytokine with dual roles. It can promote the differentiation of Treg cells, which suppress immune responses and maintain tolerance, but it can also contribute to fibrosis in chronic inflammatory conditions, such as in the lungs of asthma patients or in systemic sclerosis [33].
Fermentation with these fungal strains enhanced the nutritional content of the grains and effectively reduced allergic symptoms and inflammation in murine models, suggesting potential applications of these fermented products as functional foods or additives for managing allergic disorders. So far, no information pertaining to the immunomodulatory activities of A. kawachii and R. oryzae is available. In addition, only a few studies have reported the immunomodulatory potential of rice bran, wheat bran, and wheat germ. It has documented that rice bran is a rich source of potent antioxidants and immune-enhancing compounds. These include phytosterols, polysaccharides, and essential minerals and trace minerals such as magnesium, selenium, and zinc, along with vitamin E, omega-3 fatty acids, and various phytonutrients [34]. Dietary fibers from rice bran and wheat bran, along with their metabolites, such as short-chain fatty acids, have also been reported to have beneficial effects on both the gut and immune systems [35]. Moreover, wheat germ extract can significantly enhance the blastic transformation of peripheral blood T lymphocytes when stimulated by concanavalin A [36]. Avemar, a product derived from wheat germ, has demonstrated notable immune restorative effects in thymectomized mice. Avemar has also shown promise in ameliorating clinical manifestations of experimental systemic lupus erythematosus by modulating the Th1/Th2 network and inhibiting the Th2 response [37]. Additionally, wheat germ-derived bioactive peptides have been shown to reduce cell proliferation and the production of pro-inflammatory cytokines IFN-γ and IL-17 in human peripheral blood mononuclear cells [38]. These peptides also increase the number of antibody-producing cells and serum hemolysin, stimulate the proliferation of spleen lymphocytes in mice, and enhance the phagocytic function of macrophages [39].
Numerous early studies have consistently demonstrated the capacity of microorganisms, such as Aspergillus spp. and Rhizopus spp., to significantly augment the phenolic content and antioxidant activity of various grains and their processing by-products through solid-state fermentation processes [3,40,41,42,43]. Our current investigation aligns with these findings, as we observed a substantial increase in total phenolic and flavonoid content in grains subjected to fermentation by two distinct molds, surpassing the levels found in raw materials. It is worth noting that grains contain both free and bound phenolic compounds, with the latter being tightly associated with the cell wall matrix. Microorganisms possess enzymatic capabilities that facilitate the breakdown of these bonds, thus liberating bound phenolics. This phenomenon underscores the efficacy of microbial fermentation as an effective process for enhancing phenolic content [44].
Phenolics and flavonoids have been identified as potential modulators of allergic responses. For instance, polyphenols exhibit the ability to interfere with the presentation of food allergens to allergen-specific T cells by antigen-presenting cells. Additionally, they promote a reduction in the release of mast cell proteases in the intestinal environment, mitigate the expression of Th2-associated and pro-inflammatory genes, and influence IgE production by B cells [26,45,46]. Several mechanisms involving polyphenols appear effective in preventing allergic sensitization, such as their ability to bind with allergenic proteins or IgE, act as anti-inflammatory and antioxidant compounds, and modulate cell functions, including the regulation of Treg cells [47,48,49]. Dietary polyphenols have also been linked to alterations in gut microbiota composition and function, with implications for Treg generation [50,51]. Flavonoids, through their interference with T helper cell activation, represent a primary mechanism for inhibiting allergic responses. Literature reports have highlighted the role of flavonoids in inhibiting the activation and proliferation of Th2 cells and Th2 cytokines, such as IL-4, IL-5, and IL-13, which are pivotal in allergy-related processes [28]. Flavonoids also exhibit the potential to attenuate allergic reactions by hindering the adhesion and migration of peripheral basophil cells, suppressing IgE and IgG1 levels, and inhibiting Th2 cytokines [52]. Specific flavonoids, including quercetin, myricetin, luteolin, and kaempferol, have been identified for their antioxidant, anti-allergic, and anti-inflammatory properties [53,54,55,56,57,58,59].
Studies have demonstrated that amino acid-based formulas are a relevant dietary strategy for pediatric patients affected by allergies [60,61]. Furthermore, research by Ma et al. established a strong correlation between gut proline levels and serum IgE, suggesting proline as a promising biomarker for IgE-mediated allergies. Proline also exhibits the potential to suppress Th2 cells and activate Tregs, presenting a potential therapeutic avenue for allergic diseases [62]. In vivo and in vitro experiments have revealed that histidine supplementation can suppress IgE-mediated allergic responses by inhibiting mast cell degranulation, the secretion of inflammatory lipids, and the release of inflammatory cytokines [63]. Moreover, lysine and glutamic acid have been shown to act as effective stabilizers for pollen allergen extracts, preventing their degradation over time [64]. However, other bioactive metabolites produced during the fermentation process still need to be clarified. Recently, Lin et al. have reported that the optimization of koji production using puffed rice, rather than traditional rice, led to remarkable improvements in enzyme activities critical for miso fermentation. The application of response surface methodology resulted in an increase in β-glucosidase activity and substantial enhancements in β-amylase and protease activities. These improvements directly influenced the functional properties of the miso, increasing its polyphenol content, antioxidant activity, and aglycone conversion [65]. Accordingly, further research using response surface methodology could optimize the fermentation process and the selection of grain-derived by-products. On the other hand, Hsieh et al. have demonstrated that treatment with fermented Djulis extract restored cell viability and reduced reactive oxygen species and p-NF-κB levels significantly in PM2.5-stimulated alveolar macrophages. The in silico analysis showed that interactions between these bioactive metabolites in the extract and NF-κB play a crucial role in alleviating oxidative stress and inflammation [66]. The interaction between the immune system and metabolites produced by fermented strains presents an intriguing area of study that warrants further investigation using metabolomics approaches. By thoroughly evaluating the action mechanisms at both cellular and molecular levels, we can uncover the potential of these fermented grain-derived by-products as therapeutic phytomedicines for alleviating allergic disorders.
5. Conclusions
This study introduced a novel approach for the solid-state fermentation of grain-derived by products utilizing a blend of A. kawachii L1 and R. oryzae L1 strains. The significance of this work was underscored by the compelling evidence of anti-allergic activity observed in both murine models of food allergy and DTH following the administration of aqueous extract derived from these fermented products. Additionally, our findings highlighted the distinct modulatory effects exerted by these extracts on the production of T cell-associated cytokines. Nonetheless, it is important to acknowledge that further investigations are warranted to elucidate the precise mechanisms through which these fermented product extracts enhance the functionality of immunosuppressive populations, particularly Treg cells, in the context of various allergic diseases. This avenue of research holds promise for advancing our understanding of the therapeutic potential of fermented grain products and their applications in the management of allergic conditions. Ultimately, the insights gleaned from future studies will contribute to the development of more effective treatments for a wide range of allergic disorders.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation10090457/s1. Table S1: Contents of total phenolics, flavonoids, and amino acids of the fermented and unfermented product extracts.
Author Contributions
Conceptualization, data curation and writing, C.-H.H.; investigation and methodology, Y.-M.L.; conceptualization, funding acquisition, supervision, writing—review and editing, project administration, G.-J.T. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of the National Science and Technology Council, Taiwan (NSTC 111-2221-E-019-006) is gratefully acknowledged.
Institutional Review Board Statement
All animal experiments were conducted in accordance with the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the NTOU Institutional Animal Care and Use Committee (NTOU IACUC-110023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets utilized or examined in the present study can be obtained.
Conflicts of Interest
Author Yu-Ming Liao was employed by the Chian-E Biomedical Technology Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An update regarding the bioactive compound of cereal by-products: Health benefits and potential applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Wang, W.; Wang, W.; Wei, D. Construction of enhanced transcriptional activators for improving cellulase production in Trichoderma reesei RUT C30. Bioresour. Bioprocess. 2018, 5, 40. [Google Scholar] [CrossRef]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q. 2008, 22, 49–70. [Google Scholar]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Abd Kahar, A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef]
- Espitia-Hernández, P.; Ruelas-Chacón, X.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Flores-Naveda, A.; Sepúlveda-Torre, L. Solid-state fermentation of Sorghum by Aspergillus oryzae and Aspergillus niger: Effects on tannin content, phenolic profile, and antioxidant activity. Foods 2022, 11, 3121. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Chen, B.; Wang, X.; Qiao, Y.; Chen, J. Combined treatment of rice bran by solid-state fermentation and extrusion: Effect of processing sequence and microbial strains. Food Chem. X 2024, 23, 101549. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Takeshima, N.; Ohba, H.; Omori, T.; Shimoda, M.; Wada, H. Production of acid-stable α-amylase by Aspergillus kawachii during barley Shochu-Koji production. J. Ferment. Bioeng. 1997, 84, 224–227. [Google Scholar] [CrossRef]
- Kum, S.-J.; Yang, S.-O.; Lee, S.M.; Chang, P.-S.; Choi, Y.H.; Lee, J.J.; Hurh, B.S.; Kim, Y.-S. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J. Agric. Food Chem. 2015, 63, 1401–1418. [Google Scholar] [CrossRef]
- Cho, H.-D.; Min, H.-J.; Won, Y.-S.; Ahn, H.-Y.; Cho, Y.-S.; Seo, K.-I. Solid state fermentation process with Aspergillus kawachii enhances the cancer-suppressive potential of silkworm larva in hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2019, 19, 241. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Chen, Y.-L.; Chiu, W.-C.; Yeh, C.-L.; Tung, Y.-T.; Shirakawa, H.; Liao, W.-T.; Yang, S.-C. Effects of the water extract of fermented rice bran on liver damage and intestinal injury in aged rats with high-fat diet feeding. Plants 2022, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; da Silva Graça, C.; Chiattoni, L.M.; Massarolo, K.C.; Duarte, F.A.; Mellado, M.d.L.S.; de Souza Soares, L.A. Fermentation process in the availability of nutrients in rice bran. Res. Rev. J. Microbiol. Biotechnol. 2017, 6, 45–52. [Google Scholar]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar] [PubMed]
- Crow, M.K.; Kirou, K.A. Interferon-α in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2004, 16, 541–547. [Google Scholar] [CrossRef]
- Gordon, J.N.; Di Sabatino, A.; MacDonald, T.T. The pathophysiologic rationale for biological therapies in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2005, 21, 431–437. [Google Scholar]
- Wu, C.; Yang, G.; Bermúdez-Humarán, L.G.; Pang, Q.; Zeng, Y.; Wang, J.; Gao, X. Immunomodulatory effects of IL-12 secreted by Lactococcus lactis on Th1/Th2 balance in ovalbumin (OVA)-induced asthma model mice. Int. Immunopharmacol. 2006, 6, 610–615. [Google Scholar] [CrossRef]
- Palomares, O.; Martin-Fontecha, M.; Lauener, R.; Traidl-Hoffmann, C.; Cavkaytar, O.; Akdis, M.; Akdis, C. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes Immun. 2014, 15, 511–520. [Google Scholar] [CrossRef]
- Elghoudi, A.; Narchi, H. Food allergy in children—The current status and the way forward. World J. Clin. Pediatr. 2022, 11, 253. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.-Y.; Davis, C.M. IgE-mediated food allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Kraneveld, A.; Buckley, T.; van Heuven-Nolsen, D.; Van Schaik, Y.; Koster, A.S.; Nijkamp, F. Delayed-type hypersensitivity-induced increase in vascular permeability in the mouse small intestine: Inhibition by depletion of sensory neuropeptides and NK1 receptor blockade. Br. J. Pharmacol. 1995, 114, 1483–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.-H.; Chen, T.-Y.; Tsai, G.-J. Hypouricemic Effect of Submerged Culture of Ganoderma lucidum in Potassium Oxonate-Induced Hyperuricemic Rats. Metabolites 2022, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Hu, C.-M.; Huang, C.-H.; Wey, S.-P.; Jan, T.-R. Cannabidiol attenuates delayed-type hypersensitivity reactions via suppressing T-cell and macrophage reactivity. Acta Pharmacol. Sin. 2010, 31, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Pan, C.-L.; Tsai, G.-J.; Chang, C.-J.; Tsai, W.-C.; Lu, S.-Y. Anti-allergic diarrhea effect of diosgenin occurs via improving gut dysbiosis in a murine model of food allergy. Molecules 2021, 26, 2471. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, Y.-C.; Jan, T.-R. Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J. Funct. Foods 2017, 31, 44–51. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.; Nijhuis, M.M.O.; Harthoorn, L.F.; Langford, J.E. Tolerance development in cow’s milk–allergic infants receiving amino acid–based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Ngoc, L.P.; Gold, D.R.; Tzianabos, A.O.; Weiss, S.T.; Celedon, J.C. Cytokines, allergy, and asthma. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 161–166. [Google Scholar] [CrossRef]
- Pollard, K.M.; Cauvi, D.M.; Toomey, C.B.; Morris, K.V.; Kono, D.H. Interferon-γ and systemic autoimmunity. Discov. Med. 2013, 16, 123. [Google Scholar]
- Pène, J.R.M.; Chevalier, S.; Preisser, L.; Vénéreau, E.; Guilleux, M.-H.L.N.; Ghannam, S.; Molès, J.-P.; Danger, Y.; Ravon, E.; Lesaux, S. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008, 180, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Gorelik, L.; Flavell, R.A. Transforming growth factor-β in T-cell biology. Nat. Rev. Immunol. 2002, 2, 46–53. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lee, K.-W.; Choi, H.-D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Hidvégi, M.; Rásó, E.; Farkas, R.T.; Lapis, K.; Szende, B. Effect of MSC on the immune response of mice. Immunopharmacology 1999, 41, 183–186. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Rossi, E. Oxidative stress and nutritional prevention in autoimmune rheumatic diseases. Autoimmun. Rev. 2004, 3, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Santos-Sánchez, G.; Pedroche, J.; Fernández-Pachón, M.-S.; Millán, F.; Millán-Linares, M.C.; Lardone, P.J.; Bejarano, I.; Guerrero, J.M. Immunomodulatory and antioxidant properties of wheat gluten protein hydrolysates in human peripheral blood mononuclear cells. Nutrients 2020, 12, 1673. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Shi, Y.; Han, F.; Sun, J.; Le, G. Study on Wheat Peptides on Immune Modulating and Antioxidant Effect. Nat. Prod. Res. Dev. 2009, 21, 473. [Google Scholar]
- Moore, J.; Cheng, Z.; Hao, J.; Guo, G.; Liu, J.-G.; Lin, C.; Yu, L. Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J. Agric. Food Chem. 2007, 55, 10173–10182. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D.; Liu, J.; Cheng, Q. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of subfractions from oats (Avena sativa L.). J. Agric. Food Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Kimoto-Nira, H.; Moriya, N.; Nogata, Y.; Sekiyama, Y.; Toguchi, Y. Fermentation of Shiikuwasha (Citrus depressa Hayata) pomace by lactic acid bacteria to generate new functional materials. Int. J. Food Sci. Technol. 2019, 54, 688–695. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Zuercher, A.; Holvoet, S.; Weiss, M.; Mercenier, A. Polyphenol-enriched apple extract attenuates food allergy in mice. Clin. Exp. Allergy 2010, 40, 942–950. [Google Scholar] [CrossRef]
- Li, Y.; Mattison, C.P. Polyphenol-rich pomegranate juice reduces IgE binding to cashew nut allergens. J. Sci. Food Agric. 2018, 98, 1632–1638. [Google Scholar] [CrossRef]
- Bansode, R.R.; Randolph, P.D.; Plundrich, N.J.; Lila, M.A.; Williams, L.L. Peanut protein-polyphenol aggregate complexation suppresses allergic sensitization to peanut by reducing peanut-specific IgE in C3H/HeJ mice. Food Chem. 2019, 299, 125025. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, T.; Hou, J.; Li, M. Ferulic acid alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Immunopharmacol. Immunotoxicol. 2020, 42, 156–164. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Lugli, G.A.; Bernasconi, S.; Margolles, A.; Di Pierro, F.; Van Sinderen, D.; Ventura, M. The infant gut microbiome as a microbial organ influencing host well-being. Ital. J. Pediatr. 2020, 46, 16. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. Influence of polyphenols on allergic immune reactions: Mechanisms of action. Proc. Nutr. Soc. 2012, 71, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.-Y.; Kyung, T.-S.; Kim, D.-Y.; Hwang, J.-H.; Kim, Y.H. Anti-allergic effect of luteolin in mice with allergic asthma and rhinitis. Cent. Eur. J. Immunol. 2017, 42, 24–29. [Google Scholar] [CrossRef]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264. 7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [PubMed]
- Vo, T.S.; Le, T.T.; Kim, S.Y.; Ngo, D.H. The role of myricetin from Rhodomyrtus tomentosa (Aiton) Hassk fruits on downregulation of FcɛRI-mediated mast cell activation. J. Food Biochem. 2020, 44, e13143. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeong, G.S. Therapeutic effect of kaempferol on atopic dermatitis by attenuation of T cell activity via interaction with multidrug resistance-associated protein 1. Br. J. Pharmacol. 2021, 178, 1772–1788. [Google Scholar] [CrossRef]
- Okumo, T.; Furuta, A.; Kimura, T.; Yusa, K.; Asano, K.; Sunagawa, M. Inhibition of angiogenic factor productions by quercetin in vitro and in vivo. Medicines 2021, 8, 22. [Google Scholar] [CrossRef]
- Yuan, A.; Zeng, J.; Zhou, H.; Liu, Q.; Rao, Z.; Gao, M.; Liu, R.; Zeng, N. Anti-type I allergic effects of Jing-Fang powder extracts via PI3K/Akt pathway in vitro and in vivo. Mol. Immunol. 2021, 135, 408–420. [Google Scholar] [CrossRef]
- Candy, D.C.; Van Ampting, M.T.; Oude Nijhuis, M.M.; Wopereis, H.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; Shah, N.; West, C.E. A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants. Pediatr. Res. 2018, 83, 677–686. [Google Scholar] [CrossRef]
- Nocerino, R.; Di Scala, C.; Coppola, S.; Giglio, V.; Carucci, L.; Cosenza, L.; Voto, L.; Iannicelli, A.M.; Luzzetti, A.; Berni Canani, R. Tolerability of a new amino acid-based formula for children with IgE-mediated cow’s milk allergy. Ital. J. Pediatr. 2021, 47, 151. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; Wang, F.; Zhang, Y.; Liu, Y.; Zhang, J.; Gao, Z.; Zhang, Y.; Xie, H.; Wang, Y. Intestinal proline is a potential anti-allergy factor for allergy diagnosis and therapy. Front. Nutr. 2022, 9, 1036536. [Google Scholar] [CrossRef] [PubMed]
- Silwal, P.; Choi, S.; Shin, K.; Lee, C.Y.; Park, J.I.; Heo, J.-Y.; Lim, K.; Park, S.-K. Histidine Suppresses IgE-Mediated Allergic Responses. J. Korean Soc. Food Sci. Nutr. 2018, 47, 697–702. [Google Scholar] [CrossRef]
- Ariaee, N.; Sankian, M.; Varasteh, A.; Hosseinpour, M.; Jabbari, F. Determining the Effect of Amino Acids on the Allergenic Activity of Pollen Extracts. Rep. Biochem. Mol. Biol. 2020, 8, 394. [Google Scholar]
- Lin, S.-P.; Chou, Y.-C.; Chen, C.-E.; Lai, Y.-T.; Hsieh, C.-C.; Tsai, T.-Y.; Lin, Y.-H.; Santoso, S.P.; Lin, H.-W.; Cheng, K.-C. Enhancing Miso Production with Optimized Puffed-Rice Koji and its Biological Function Evaluation. Food Biosci. 2024, 60, 104463. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yu, S.-H.; Kuo, H.-C.; Cheng, K.-W.; Hsu, C.-C.; Lin, Y.-P.; Khumsupan, D.; Lin, S.-P.; Angkawijaya, A.E.; Cheng, K.-C. Alleviation of PM2. 5-induced alveolar macrophage inflammation using extract of fermented Chenopodium formosanum Koidz sprouts via regulation of NF-κB pathway. J. Ethnopharmacol. 2024, 318, 116980. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).