Abstract
Astragali Radix, a traditional Chinese herbal medicine widely used for its medicinal properties, is known to be rich in active components that possess various pharmacological effects. However, the effectiveness of microbial fermentation in enhancing the content of these active substances remains unclear. In this study, a microflora of lactic acid bacteria was used to ferment Astragali Radix, and the promoting effect of Chlorella Growth Factor (CGF) on the fermentation process was investigated so as to clarify the changes in major active compound content in the fermented Astragali Radix broth. Non-targeted metabolomic analysis based on ultra-high-performance liquid chromatography–mass spectrometry was conducted to analyze the differences in metabolites before and after fermentation. The results showed that the total polysaccharide, total flavonoid, and total saponin content in the fermented Astragali Radix broth increased by up to 51.42%, 97.76%, and 72.81% under the optimized conditions, respectively. Streptococcus lutetiensis was the dominant bacterial species during the fermentation process. There were significant differences in metabolites in the fermentation broth before and after fermentation, among which amino acids (such as L-Aspartyl-L-Phenylalanine, etc.) and saponin compounds (such as Cloversaponin I, Goyasaponin I, etc.) were the main upregulated metabolites, which can enhance the physiological functions of Astragali Radix fermentation broth. The CGF exhibited the ability to promote the increase of active substance content in the fermented Astragali Radix broth.
1. Introduction
Astragali Radix is the dried root of the leguminous plant Astragalus mongolicus or Astragalus membranaceus, which mainly grows in China, Korea, and Mongolia [1]. It has been used medicinally for over 2000 years due to its antimicrobial and anti-inflammatory properties, enhancement of body immunity, and effectiveness in treating cardiovascular and cerebrovascular diseases [2,3]. Astragali Radix contains a diverse range of chemical components, including polysaccharides, flavonoids, saponins, amino acids, trace elements, and other substances. Among them, polysaccharides, flavonoids, and saponins are regarded as the primary active components and medicinal constituents [4]. The abundance of compounds contained in Astragali Radix gives it good medicinal value and natural health functions. However, the major bioactive components of the plant material, such as flavonoids, are usually present in a covalently esterified or insoluble bound state or bound to sugar residues and cell wall constituents, making them difficult to extract [5]. As a result, their bioaccessibility, bioavailability, and health benefit effects are seriously compromised [5].
Microbial fermentation is a proficient method for converting and releasing plant constituents, which can generate diverse fermentation products with biological functions [6]. During microbial fermentation, specific hydrolytic enzymes may catalyze the decomposition of the cell walls of the substrate, which can subsequently release bioactive compounds [7]. This process of solubilization and transformation results in the release of active ingredients within the plant material, which then increases the overall content and availability of medicinal ingredients [8].
Lactic acid bacteria are currently receiving considerable attention owing to their positive impact and are frequently utilized as fermenters to transform natural items. Fermenting natural plant resources with lactic acid bacteria can enhance the concentration of active ingredients, improve the efficacy of medicinal compounds, stimulate the production of new bioactive components, enhance flavor, and reduce the toxicity of botanicals [9]. There has been an increasing utilization of lactic acid bacteria as the primary fermentation strain for phytopharmaceutical production. For instance, Weon et al. fermented Codonopsis pilosula with three mixed lactic acid bacteria strains and found that the levels of caffeic acid, vanillic acid, and caffeine were increased in the fermented Codonopsis pilosula [10]; Yim et al. analyzed the constituents of Lactobacillus plantarum during the fermentation of Sijunzi decoction and observed an increase in the content of glycyrrhizinic acid, liquirtigenin, and atractylenolide [11]. Furthermore, multi-strain fermentation exhibited synergistic and interactive effects between strains, enhancing substrate utilization efficiency and increasing the release of bioactive components during fermentation compared to single-strain fermentation [5,6]. The utilization of microbial fermentation methods to make plant fermentation broth can promote the extraction of active ingredients from plants and provide a new way for the deep processing and diversification of medicinal plants [12].
Chlorella sp. is a kind of green microalgae renowned for its high nutritional value and diverse range of active substances. It has been classified as a green nutritional source of health food for human consumption in the 21st century [13,14]. Among its many components, one particular factor that attracts significant attention is the Chlorella Growth Factor (CGF). CGF is a unique and active cellular substance found in Chlorella sp., consisting of 56.6% proteins, 42.59% amino acids, and 6.8% nucleotides, as well as vitamins A, B, biotin, and others [15]. It is known for its “hormone-like” properties and has been shown to contain nuclear proteins that promote cell renewal, repair, and growth [16,17]. This distinctive feature allows for accelerated bacteria cell division, enhanced cell growth, and increased bacteria survivability in a high-salt environment [18]. With its broad application scope in the food, beverage, nutraceutical, and pharmaceutical industries, CGF is predicted to be highly valuable.
The utilization of a fermentation process for the organic amalgamation of lactic acid bacteria together with natural plants has the potential to enhance the dissolution and conversion efficiency of active natural plant ingredients, thereby augmenting their potential pharmacological value. However, there is currently limited research on utilizing lactic acid bacteria transformation to increase the active ingredient content in Astragali Radix, and the changes in composition before and after fermentation remain uncertain. Additionally, there is a lack of relevant research reports regarding the utilization of CGF to stimulate plant fermentation with lactic acid bacteria. In this study, a microflora of lactic acid bacteria consisting of six species was employed as a fermentation agent to investigate its impact on elevating the levels of active compounds such as polysaccharides, flavonoids, and saponins in Astragali Radix fermentation broth. This study aimed to analyze the changes in metabolites before and after fermentation and explore the enhanced effect of CGF on the fermentation process of the microflora. This research provides a new methodology for increasing the content of active ingredients in medicinal plants through microbial fermentation.
2. Materials and Methods
2.1. Bacteria Strains and Reagents
Bacteria strains: Six strains of lactic acid bacteria, Streptococcus lutetiensis, Lactobacillus plantarum, Streptococcus salivarius subsp. thermophilus, Bifidobacterium animalis subsp., Lactobacillus rhamnosus GG, and Lactobacillus delbrueckii subsp. bulgaricus, were purchased from CICC (China Industrial Microbial Strain Conservation and Management Centre, Beijing, China). Chlorella sp. was purchased from FACHB (Freshwater Algae Culture Collection at the Institute of Hydrobiology, Wuhan, China).
Reagents: Astragali Radix was purchased from Minxian Hetai Traditional Chinese Medicines Co. (Lanzhou, China). Glucose, rutin, and astragaloside controls were purchased from Shanghai MacLin Biochemical Co., Ltd. (Shanghai, China). All other materials were analytical grade.
2.2. Preparation of Fermentation Medium and CGF Extract
- (1)
- Fermentation medium: Astragali Radix was crushed to powder and sieved through an 80-mesh sieve. A total of 2 g of dried Astragali Radix powder was taken and mixed in 20 mL of distilled water and then sterilized at 121 °C for 25 min and cooled for later use.
- (2)
- Fermentation lactic acid bacteria biomass: a total of 1 mL was taken from each of the aforementioned six preserved lactic acid bacteria biomass (with a colony count of 2.0 × 106 CFU/mL for each) and were inoculated into 100 mL of NB medium (beef extract 0.3 g, peptone 1.0 g, sodium chloride 0.5 g, distilled water 100 mL, pH 7.2–7.5). Then, the solution was incubated at 37 °C for 24 h to obtain the fermentation lactic acid bacteria biomass. (The total colony count of lactic acid bacteria in the solution was counted as 2.3 × 107 CFU/mL).
- (3)
- Chlorella Growth Factor (CGF) extract: 50 mL fresh Chlorella sp. culture medium (counting 3.5 × 107/mL microalgal cells) was subjected to centrifugation at 4000 rpm for 10 min. The resulting precipitate was collected and washed three times with sterile water [19]. Subsequently, it was dissolved in another 50 mL of sterile water and heated in an autoclaved cooker at 121 °C for 15 min to obtain the CGF extract [20].
2.3. Fermentation of Astragali Radix and Determination of Active Compounds
2.3.1. Fermentation of Astragali Radix
Time, temperature, substrate concentration, pH, inoculum volume, and CGF addition were set as univariate variables for Astragali Radix fermentation. The fermentation time was set at 0, 6, 12, 18, 24, 30, and 36 h, respectively. The temperature ranged from 25, 30, 35, 40, to 45 °C. The substrate concentration varied between 0.1, 0.07, 0.05, and 0.04 g/mL. The initial pH values were set at 3, 5, 7, and 9. The inoculum volume was 1, 2, 3, 4, and 5 mL, and the amount of CGF added was 1, 2, 3, 4, and 5 mL, respectively. The initial fermentation conditions were as follows: fermentation time of 24 h, fermentation temperature of 30 °C, substrate concentration of 0.1 g/mL, initial pH values of 5, inoculum volume of 1 mL, and CGF extract addition of 2 mL. After each single-factor experiment, the determined optimal conditions were used for the subsequent single-factor experiment. Each group underwent three parallel experiments to determine the total polysaccharide, total flavonoid, and total saponin contents in the fermentation broths according to the methods outlined in Section 2.3.2.
2.3.2. Treatment of Fermentation Broth and Determination of Active Compounds
The fermentation broth was decanted and transferred to a centrifuge tube and then centrifuged at 4000 rpm for 10 min. The supernatant was concentrated under reduced pressure to about 5 mL. Subsequently, 10 mL of 80% ethanol was added, and the mixture was left at a low temperature overnight. Following ultrasonication for 80 min, the solution was again centrifuged at 4000 rpm for 10 min. The obtained precipitate was collected, dissolved in water, and then subjected to centrifugation and filtration. Ethanol (95%) was added to the filtrate to reach an ethanol content of 80% and then left to stand overnight at a low temperature. The precipitate after overnight standing was washed with ethanol and dried under vacuum to obtain a powder, which was used for the determination of total polysaccharide in Astragali Radix. The ethanol extract of the extractum was concentrated under reduced pressure to an extractum of approximately 5 mL. The extractum was extracted three times with water-saturated ethyl aceate (in ratios of 20:15:10). The ethyl acetate layer was collected, and ethyl acetate was recovered. The precipitate obtained was used for the determination of total flavonoid content. The remaining aqueous layer was further extracted three times with water-saturated nbutanol (in ratios of 20:15:10). The nbutanol layer was collected, and n-butanol was recovered. The precipitate obtained was used for the determination of total saponin.
- (1)
- Determination of total polysaccharide content
In the crude sample used for the determination of total polysaccharide, 40 mL of sterile water at 80 °C was added and sonicated for 80 min to dissolve it, after which it was centrifuged at 2000 r/min for 10 min, and the supernatant was then taken for the determination of total polysaccharide. Glucose was used as a control for the determination of total polysaccharide using an ultraviolet spectrophotometer (UV-Vis) at 620 nm.
- (2)
- Determination of total flavonoid content
The sample employed for the determination of total flavonoid was dissolved in 3 mL of slightly warmed anhydrous ethanol. The volume was then adjusted to 10 mL with sterile water. Next, the mixture underwent three extractions with petroleum ether (20:15:10). The aqueous layer obtained from the extraction was used to determine the total flavonoid content. Rutin was used as a control for the determination of total flavonoid using UV-Vis at 510 nm.
- (3)
- Determination of total saponin content
The samples used for the total saponin assay were adjusted to a volume of 10 mL with methanol. Astragaloside was used as a control for the determination of total saponin using UV-Vis at 590 nm. The absorbance values were substituted into the standard curve equation to determine the concentration of the substance in the dilution solution. Using Formula (1), the content of the substance in the original fermentation broth was calculated based on this concentration.
where: X—total polysaccharide (flavonoid, saponin) content (mg/g) in Astragali Radix fermentation broth; C—concentration of glucose (rutin, astragaloside) in the dilution solution (mg/mL); N—dilution times of the extract; V1—total volume of the extract (mL); V2—volume of dilution solution used for determination of aspiration (mL); m—weight of original powdered sample (g).
X = (C × N × V1)/(V2 m)
2.4. Analysis of Microbial Community Structure
Based on the results of the single-factor experiments of Astragali Radix fermentation, the structural changes of the lactic acid bacteria community before and after fermentation were studied under optimal conditions (temperature: 37 °C, substrate concentration: 0.07 g/mL, pH: 6, inoculum dosage: 2 mL, CGF addition: 3 mL). A total of 3 treatment groups were set up, with 3 parallels in each group. The first group was the CK group, i.e., the inoculated lactic acid bacteria biomass was immediately stored in a −80 °C refrigerator. The second group was the LB group, i.e., the inoculated solution was fermented for 24 h. The third group was the LC group, i.e., inoculated with lactic acid bacteria biomass and CGF fermentation for 24 h. After fermentation, all samples were centrifuged, and the resulting precipitate was used to determine the microbial diversity of the samples (analyzed by Shanghai Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China).
For the obtained sequence data, the representative sequences of OTUs with a 97% similarity level were taxonomically analyzed using the RDP classifier Bayesian algorithm, and the representative sequences of each OTU were aligned to the Silva database (http://www.arb-silva.de, accessed on 5 June 2023) using the mothur 1.45.3 software to complete the species-level annotation of the OTUs. The bacteria community structure was then statistically analyzed for alpha diversity and species abundance at each taxonomic level. The differences and significance of OTU diversity between groups were analyzed using the Wilcox rank sum test and the Tukey test. *, **, and *** indicate statistical significance, i.e., p < 0.05, p < 0.01, and p < 0.001, respectively.
2.5. Non-Targeted Metabolomics Analysis
The same three treatment groups as described in Section 2.4 were set up, with four replicates for each group. The first group was the blank group (CK group), i.e., not inoculated with the lactic acid bacteria biomass and CGF extract. The second group was the lactic acid bacteria fermentation group (LB group), and the third group was the lactic acid bacteria + CGF fermentation group (LC group). The samples were then fermented according to the fermentation conditions and procedure described in Section 2.4. The supernatant of the samples after fermentation was centrifuged for non-targeted metabolomics using ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) (analyzed by Shanghai Majorbio Bio-pharm Technology Co., Ltd.).
Metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/, accessed on 22 June 2023) and the HMDB database (https://www.hmdb.ca/, accessed on 5 June 2023). The metabolomic data were analyzed using a principal component analysis (PCA), the PCA scores of each subgroup were plotted, and the metabolites with a VIP value ≥ 1, p < 0.05, and fold change (FC) ≥ 1 were selected as differential metabolites, and metabolite difference volcano plots were drawn.
2.6. Data Processing
The experimental data were analyzed using one-way ANOVA using SPSS 26.0 (International Business Machines 169 Corp., Armonk, NY, USA) software, and all the data were expressed as mean ± standard deviation, p < 0.05 denoted statistical significance, and the graphs were plotted using Origin 2023 (OriginLab Corp., Northampton, MA, USA).
3. Results and Discussion
3.1. Effect of Fermentation Conditions on the Content of Active Substances in Astragali Radix Fermentation Broth
Microbial fermentation is a biotransformation process involving a series of enzymatic reactions, and it is subject to many constraints such as fermentation time, temperature, substrate concentration, pH, and inoculum dosage, all of which influence the fermentation results. Therefore, exploring the optimal fermentation conditions is crucial to increasing the content of active ingredients in Astragali Radix fermentation broth.
3.1.1. Effect of Fermentation Time
As shown in Figure 1, during the initial 24 h, the content of total polysaccharide in the fermentation broth of Astragali Radix increased with prolonged fermentation time, peaking at 659.83 mg/g after 24 h, after which it showed a declining trend. The content of total flavonoid exhibited an initial increase over time during the initial 30 h of fermentation, reaching a peak value of 9.73 mg/g at 30 h. Thereafter, a gradual decline commenced. The total saponin content demonstrated a pattern of increasing and then decreasing, reaching a maximum value of 15.19 mg/g at a fermentation time of 18 h. Total polysaccharide, total flavonoid, and total saponin were increased by up to 224.05 mg/g, 4.81 mg/g, and 6.40 mg/g, respectively, after fermentation compared to unfermented. On the whole, the total polysaccharide, total flavonoid, and total saponin contents were observed to be higher within the 18–30 h fermentation time.
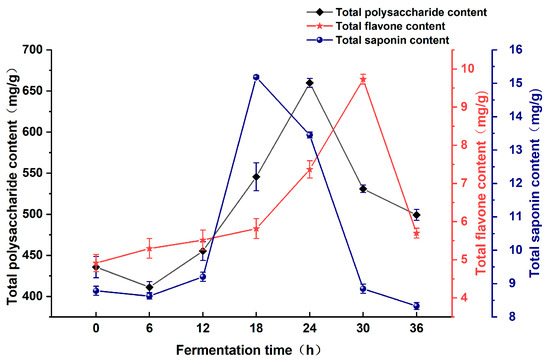
Figure 1.
Effect of fermentation time on the active substance content of Astragali Radix fermentation broth.
The duration of fermentation plays a significant role in the outcome of microbial fermentation. A shorter fermentation time leads to relatively low production of enzymes such as cellulase, hemicellulase, and protease. On the other hand, an excessively long fermentation duration results in insufficient nutrients provided by the medium, which can potentially reduce the enzyme-producing performance of lactobacilli [21]. This, in turn, can affect the release of active ingredients present in the fermentation broth of Astragali Radix [22]. At the same time, with the prolongation of the fermentation time, the readily available carbon sources in the medium are gradually depleted. Initially, lactic acid bacteria efficiently degrade polysaccharides and other complex carbon sources to support their growth and metabolism. However, as the fermentation progresses, the degradation of polysaccharides becomes more challenging due to the depletion of simpler carbon sources and potential changes in enzyme expression and activity. This, combined with the potential involvement of other microbial species and environmental factors, leads to a decrease in the content of total polysaccharide, total saponin, and total flavonoid in the Astragali Radix fermentation broth at the late stage of fermentation [23,24].
3.1.2. Effect of Fermentation Temperature
As shown in Figure 2, the total polysaccharide content of Astragali Radix fermentation broth initially increased with the increase in fermentation temperature, with the highest content reaching 630.53 mg/g at the fermentation temperature of 35 °C, and then a decreasing trend was observed with the increase in fermentation temperature. The total flavonoid content exhibited a slight increase at fermentation temperatures of 30–40 °C, reaching a maximum value of 8.26 mg/g at 40 °C. However, this trend reversed as the temperature decreased. The total saponin content demonstrated an upward trajectory within the temperature range of 25 to 40 °C, exhibiting its maximum concentration of 13.25 mg/g at 40 °C. This was followed by a discernible decline. The total polysaccharide, flavonoid, and saponin concentrations were found to be higher at temperatures between 35 and 40 °C.
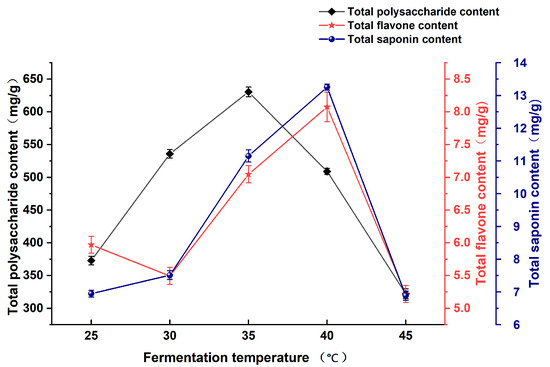
Figure 2.
Effect of fermentation temperature on the content of active substances in Astragali Radix fermentation broth.
The results demonstrate that the concentrations of the three active components in the fermentation broth of Astragali Radix exhibited an upward trend with increasing temperature, reaching a peak at 35–40 °C. This can be attributed to the fact that high temperatures facilitate the effective release of active ingredients from plant cell walls in Astragali Radix [22]. Meanwhile, the optimal temperature range for the growth of lactic acid bacteria is also between 35–40 °C, which makes it more conducive for lactic acid bacteria to decompose plant cell walls and release more active substances. Any deviation from this temperature range, whether below or above the optimal range, can impede the transport and exchange of intracellular and extracellular enzymes, as well as other substances, thereby reducing the conducive environment for fermentation processes [25]. Furthermore, elevated temperatures induce alterations in the cell membrane structure and protein hydrolysis metabolism, which may ultimately culminate in cell death within the bacterial population. Consequently, the presence of active substances in the fermentation broth of Astragalus fermentum is adversely affected [26].
3.1.3. Effect of Substrate Concentration
As shown in Figure 3, the variation in substrate concentration significantly impacted the content of active components present in the fermentation broth. The content of total polysaccharides in Astragali Radix fermentation broth decreased with decreasing substrate concentration, and the highest content was 664.10 mg/g at a substrate concentration of 0.1 g/mL. The highest values of total flavonoid and total saponin were observed at a substrate concentration of 0.07 mg/mL, with values of 9.84 mg/g and 9.57 mg/g, respectively. Thereafter, both the flavonoid and saponin exhibited a decreasing trend. The total polysaccharide, flavonoid, and saponin contents were found to be higher in the range of substrate concentrations between 0.01 and 0.07 mg/mL.
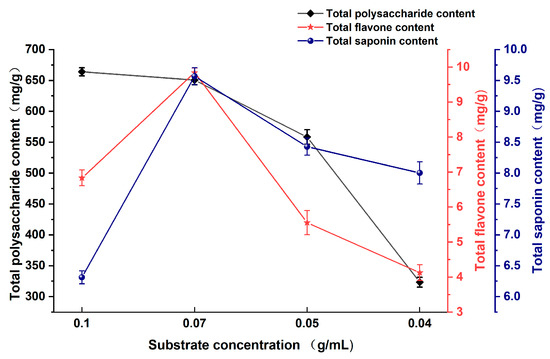
Figure 3.
Effect of substrate concentration on the content of active substances in Astragali Radix fermentation broth.
An optimal concentration of Astragali Radix substrate plays a crucial role in promoting the fermentation of lactic acid bacteria. When the substrate concentration is excessively high, it exerts osmotic stress on the lactic acid bacteria cells. This stress impedes normal bacteria growth and metabolism, subsequently leading to diminished enzyme production [27]. Additionally, the high substrate concentration affects the expansion of the Astragali Radix powder particles, thereby hindering water absorption and impeding the solubilization and transfer of nutrients. Conversely, a low substrate concentration results in the formation of agglomerated Astragali Radix particles, reducing medium permeability and impeding oxygen transport and heat diffusion. These conditions are unfavorable for the proliferation of lactic acid bacteria, ultimately leading to lower enzyme production and a reduced content of active substances within the fermentation broth [28].
3.1.4. Effect of pH
pH serves as a comprehensive indicator of microbial metabolic activity under certain environmental conditions and is an important parameter. As shown in Figure 4, the total polysaccharide and total flavonoid contents in the fermentation broth of Astragali Radix exhibited an increasing trend between pH 3 and 7, reaching the highest values of 666.55 mg/g and 10.14 mg/g, respectively, at pH 7. Thereafter, a decrease in the contents was observed. The total saponin content was observed to reach a maximum at pH 5, with a value of 14.76 mg/g. Thereafter, a decline in the content was noted. The total polysaccharide, total flavonoid, and total saponin concentrations were found to be higher in the pH range of 5–7.
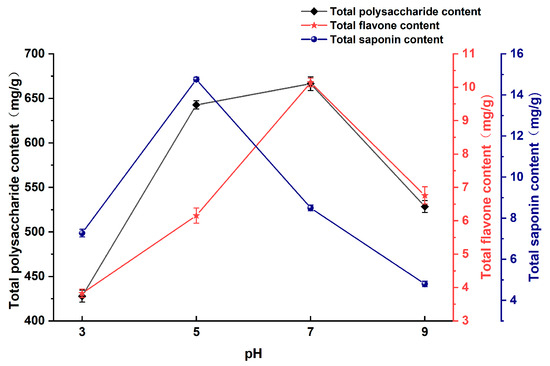
Figure 4.
Effect of pH on the content of active substances in Astragali Radix fermentation broth.
The pH of the fermentation medium affects enzyme activity, which needs to be within a certain pH range in order to function, beyond which the structure of the enzyme can be inhibited or disrupted, reducing enzyme activity and hindering the metabolism of the bacteria [29]. A lower initial pH is favorable for the dissolution of some of the active compounds in Astragali Radix, while too high a pH is unfavorable for the growth and reproduction of the bacteria and the secretion of extracellular polysaccharides. Therefore, choosing an appropriate pH level is essential for facilitating the dissolution of active ingredients in Astragali Radix while creating a suitable environment for bacteria survival and normal metabolic activities [30].
3.1.5. Effect of Bacteria Inoculum Dosage
As shown in Figure 5, the total polysaccharide content in the Astragali Radix fermentation broth significantly increased after lactic acid bacteria fermentation, reaching a maximum value of 684.86 mg/g at an inoculum dosage of 2 mL. This enhancement can be attributed to the enzymatic degradation and transformation of polysaccharides in Astragali Radix by the lactic acid bacteria during fermentation, making the polysaccharides more bioavailable and thus increasing their measured concentration. However, as the inoculum dosage further increased, the polysaccharide content did not continue to rise, possibly due to intensified competition among the lactic acid bacteria at high densities, leading to a deterioration of the growth environment and consequently affecting the efficiency of polysaccharide degradation and transformation. Regarding total flavonoid content, the highest value of 6.91 mg/g was observed at an inoculum dosage of 1 mL, followed by a decreasing trend with increasing dosages. This trend may stem from the metabolic pathways of lactic acid bacteria towards flavonoids, where low inoculum dosages may favor the utilization of flavonoids as carbon sources or cofactors, while higher dosages may activate alternative metabolic pathways, slowing down the consumption rate of flavonoids or converting them into other compounds [31]. As for total saponin content, it initially increased with the inoculum dosage, peaking at 7.64 mg/g at 3 mL, and then declined. This trend suggests a complex interaction mechanism between lactic acid bacteria and saponin compounds, including degradation, transformation, and possible synergistic effects. Nevertheless, the decline at higher inoculum dosages may be related to changes in the growth environment and adjustments in the metabolic pathways of the lactic acid bacteria [32]. Compared to the uninoculated group, the total polysaccharide, total flavonoid, and total saponin contents in the Astragali Radix fermentation broth significantly increased by 322.96 mg/g, 2.26 mg/g, and 1.87 mg/g, respectively, after lactic acid bacteria fermentation. This further underscores the effectiveness of lactic acid bacteria fermentation in extracting and transforming the active components of Astragali Radix. Additionally, within the inoculum dosage range of 1–3 mL, the fermentation broth exhibits higher levels of total polysaccharide, total flavonoid, and total saponin, suggesting that optimizing the inoculum dosage could enhance the medicinal value and economic benefits of the Astragali Radix fermentation broth in practical applications [33].
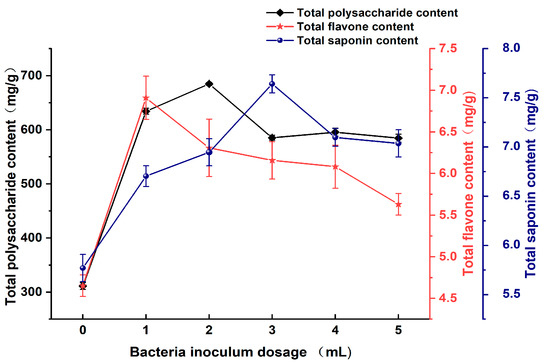
Figure 5.
Effect of bacteria inoculum dosage on the content of active substances in Astragali Radix fermentation broth.
The appropriate inoculum dosage can shorten the bacteria’s stagnation period, allowing them to quickly enter the logarithmic growth phase, which, in turn, reduces the fermentation cycle [22]. When a small inoculum dosage is used, the bacteria colony experiences slower growth with a prolonged metabolic cycle, ultimately impacting enzyme vitality. Conversely, when too much inoculum is used, the bacteria grow at a rapid rate, which then results in an insufficiency of nutrients and dissolved oxygen, generating excess heat that raises the medium temperature. This, in turn, negatively affects the growth of bacteria and the vitality of enzymes. Moreover, an excessive inoculum dosage can lead to microbial saturation during fermentation, triggering competition for substrates. This competition can negatively impact the survival of some microorganisms, resulting in a rapid decrease in the release rate of active ingredients [34].
3.1.6. Effect of CGF Addition
As shown in Figure 6, the quantity of total polysaccharide in the fermented Astragali Radix broth initially rose with the addition of CGF and peaked at 643.35 mg/g when 3 mL was added. Subsequently, a downward trend occurred with a further increase in the CGF addition. For CGF additions ranging from 0–4 mL, the total flavonoid content increased proportionally and reached its highest peak of 11.30 mg/g at the CGF addition of 4 mL, after which it started to decrease. Similarly, the total saponin content initially increased with increasing CGF addition, reaching a maximum of 8.34 mg/g at 2 mL CGF extract addition, and then showed a decreasing trend. Compared to the group without added CGF, total polysaccharide, total flavonoid, and total saponin were increased by up to 242.98 mg/g, 7.61 mg/g, and 2.29 mg/g, respectively. The total polysaccharide, total flavonoid, and total saponin contents demonstrated a significant increase when the CGF extract was added at a concentration of 2–4 mL.
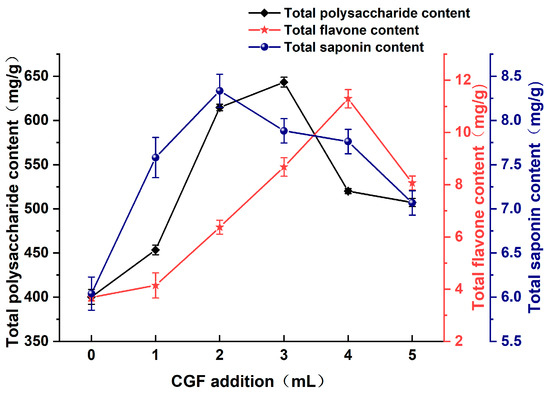
Figure 6.
Effect of CGF addition on the content of active substance in Astragali Radix fermentation broth.
CGF has a powerful growth-promoting function; incorporating an appropriate amount of CGF extract during the lactic acid bacteria fermentation of Astragali Radix can repair damaged microbial cells and stimulate the growth of new and healthy cells to ensure normal growth and metabolism of bacteria flora [35]. During fermentation, the synthetic glycogen in CGF can provide energy for the Lactobacilli to break down macromolecules into smaller organic acids [36], which can provide part of the carbon source necessary for the colony to sustain its normal metabolic activities, enhance enzyme production, and ultimately increase the content of active compounds in the fermentation broth of Astragali Radix. However, excessive addition of CGF extract can elevate the substrate’s water content, which is unfavorable for normal bacteria growth [37].
3.1.7. Optimum Fermentation Conditions and Effects
Based on the consolidated findings from Section 3.1.1, Section 3.1.2, Section 3.1.3, Section 3.1.4, Section 3.1.5 and Section 3.1.6, the optimal fermentation conditions for maximizing the release of active substances in Astragali Radix fermentation broth were identified as follows: fermentation duration of 18–30 h, temperature range of 35–40 °C, substrate concentration of 0.07–0.1 mg/mL, pH value of 5–7, inoculum size of 2–4 mL, and CGF addition of 2–4 mL. To further validate the enhancing effect of lactic acid bacteria fermentation within this optimal range on the content of active substances in Astragali Radix fermentation broth, specific conditions within this range were selected for experimentation: fermentation duration of 24 h, temperature of 37 °C, substrate concentration of 0.07 g/mL, pH of 6, inoculum volume of 2 mL, and CGF addition of 3 mL. The results demonstrated that under these conditions, the total polysaccharide, total flavonoid, and total saponin contents in the Astragali Radix fermentation broth increased by 224.05 mg/g, 4.81 mg/g, and 6.40 mg/g, respectively, representing significant enhancements of 51.42%, 97.76%, and 72.81% compared to pre-fermentation levels. This marked elevation in the concentration of active substances within the fermentation broth is hypothesized to stem from the production of lactic acid and various enzymes during lactic acid bacteria fermentation [38]. Specifically, lactic acid contributes to the acid hydrolysis of cellulose, while enzymes facilitate the enzymatic degradation of cellulose in Astragali Radix, leading to the disruption of cell walls and subsequently enhancing the solubility and release of active substances [39].
3.2. Changes in Microbiological Composition before and after Fermentation
Microbial fermentation is a complex biochemical process, and changes in microbial species and numbers are important factors affecting the fermentation process. Therefore, the analysis of changes in microbiological composition during the fermentation process is of great importance for enhancing the content of specific components of Astragali Radix fermentation broth, as well as for improving the quality of fermented products.
3.2.1. Changes in Colony Abundance and Diversity
The richness and diversity of the microflora before and after fermentation were assessed using α-diversity indices such as Ace, Chao, and Shannon. As shown in Figure 7, the Ace, Chao, and Shannon indices of the LB and LC groups were significantly lower than those of the CK group, indicating that the abundance and diversity of the microflora in the LB and LC groups were significantly reduced compared to the CK group (p < 0.05). This may be related to the stimulatory and inhibitory effects between different strains. The growth of lactic acid bacteria is affected by the acidity of the environment, temperature, and other factors; the different probiotic acid-producing capacities, acid tolerance, optimal growth temperature, and others, constitute the complex ecological relationship and at the same time are also important factors limiting the growth of each probiotic bacteria in the process of mixed culture [24]. In addition, the growth of lactic acid bacteria requires a large amount of nutrients and the existence of the relationship of nutrient competition between bacteria strains, which makes the bacteria population abundant and causes diversity changes in the process of fermentation [40]. The changes in Ace, Chao, and Shannon indices between the LB and LC groups were not significant, indicating that there was no significant change in the abundance and diversity of the microflora in the LB and LC groups (p > 1). This implies that the impact of CGF extract on the structural changes in the lactic acid bacteria was not significant and further indicates that there was no inhibitory effect of CGF extract on the microflora.
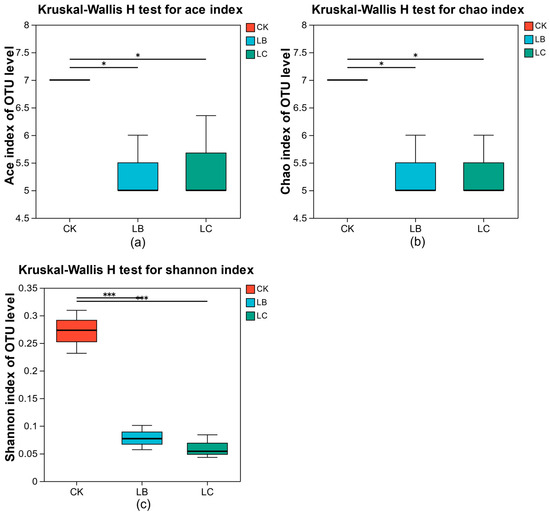
Figure 7.
Alpha diversity of microflora in different groups. (a) ace index (b) chao index (c) shannon index. where "*" and "***" respectively indicate p < 0.05 and p < 0.001.
3.2.2. Changes in the Structural Composition of the Microflora
A number of changes in the microflora structure occurred during the fermentation process. To analyze the composition of the microflora, the OTU values of the bacteria were classified at both the genus and species levels. As shown in Figure 8a, at the genus level, a total of four genera were identified in all samples before and after fermentation, namely Streptococcus sp., Lactobacillus sp., Bifidobacterium sp., and Alcaligenaceae sp. Among these, Streptococcus sp. was the dominant genus, and its relative abundance in each sample was over 95%, while the relative abundance of the other three genera was lower at less than 5%. As shown in Figure 8b, at the species level, a total of seven species were identified in all samples, namely Streptococcus lutetiensis, Lactobacillus plantarum, Streptococcus salivarius subsp. thermophilus, Bifidobacterium animalis subsp. lactis, Lactobacillus rhamnosus GG, Lactobacillus delbrueckii subsp. bulgaricus, and Alcaligenaceae. Among them, Alcaligenaceae is the endophyte of Astragali Radix. Among the added microflora, Streptococcus lutetiensis stood out as the primary dominant strain, with its relative abundance at its lowest in the CK group (94.61%) and increased to 98.61% and 98.98% in the LB and LC groups, respectively. This indicates a marked growth of this strain post-fermentation, suggesting that the fermentation conditions are more conducive to the proliferation of this strain. Lactobacillus plantarum also exhibited a certain degree of increase post-fermentation, while the relative abundance of the remaining five strains all decreased to some extent. In particular, the relative abundance of Lactobacillus delbrueckii subsp. bulgaricus dwindled to 0 after fermentation, indicating its limited role in the fermentation process.
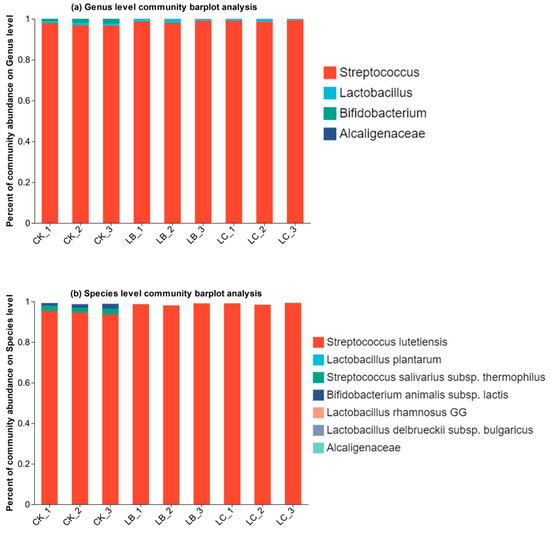
Figure 8.
Relative abundance of microflora composition before and after fermentation.
The composition and structure of the microflora changed dynamically during Astragali Radix fermentation. Microbial diversity and community composition data can furnish insights into the microbial profiles of the fermentation, aiding in comprehending the spatial and temporal shifts within the microbial fermentation process. The dominant strain is Streptococcus lutetiensis. Other strains may indirectly influence the growth and activity of Streptococcus lutetiensis through competition, symbiosis, or synergism by co-regulating key factors such as nutrient conditions, pH, and redox potential in the fermentation environment. This strain may positively influence flavor formation in Astragali Radix fermenters through dynamic succession during the fermentation process, providing valuable guidance for the development of Astragali Radix fermentation technology and the precise selection of flavor-enhancing microorganisms for the fermentation process [26].
3.3. Metabolomic Analysis of Fermentation Products
3.3.1. PCA Analysis of Intergroup Metabolites
Differential metabolites were analyzed using principal component analysis (PCA) for the fermentation after-products of the three treatment groups, CK, LB, and LC. The PCA score plots were obtained with PCA in positive and negative ion modes. In a PCA plot, each sample within a group is represented by a point, reflecting the relationship between samples based on their tendency to cluster or separate in the plot. Points that are closely clustered indicate similarity in observed variables, while dispersed points indicate significant differences in observed variables. As shown in Figure 9, the samples in the three groups of CK, LB, and LC are all within the 95% confidence interval, and the distribution of samples within each group of CK, LB, and LC was more concentrated, indicating better intergroup reproducibility. There were significant differences between the CK and LB groups, as well as between the CK and LC groups. This indicates distinct variations in the metabolite composition of the fermentation broth before and after lactic acid bacteria fermentation. The fermentation process significantly modified the metabolite composition of the fermentation broth [41]. However, the differences between the LB and LC groups were relatively small, implying that the addition of CGF extract had a relatively lower impact on the compositional changes of metabolites in the fermentation broth [42].
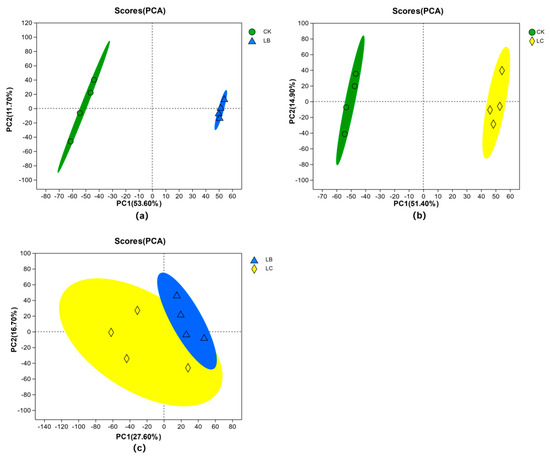
Figure 9.
Intergroup PCA analysis of metabolites in Astragali Radix fermentation broth. (a) PCA scores of CK group vs LB group. (b) PCA scores of CK group vs LC. (c) PCA scores of LB group vs LC groupgroup.
3.3.2. Differential Metabolite Analysis
In order to investigate the differences in metabolites before and after the fermentation of Astragali Radix, as well as between the fermentation treatments with and without the addition of CGF, the metabolites of the samples from the three groups of CK, LB, and LC were analyzed. The results are shown in Figure 10.
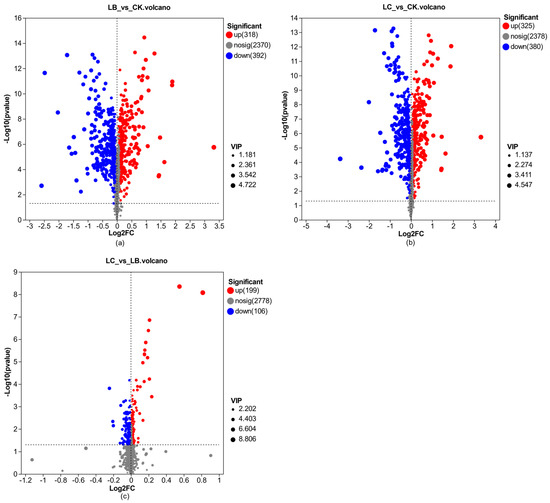
Figure 10.
Volcano plot of metabolite differences in Astragali Radix fermentation broth. (a) LB group vs CK group; (b) LC group vs CK group; (c) LC group vs LB group.
A total of 3083 metabolites were identified in the CK, LB, and LC groups. As shown in Figure 10a, 318 metabolites exhibited an up-regulated pattern, while 392 metabolites showed a down-regulated pattern in the LB group compared to the CK group. The most significantly up-regulated metabolites were (17E,19E,21E,23E,25E)-4,6,8,10,12,14,16,27-Octahydroxy-3-(1-hydroxyhexyl)-17,28-dimethyl-1-oxacyclooctacosa-17,19,21,23,25-pentaen-2-one, which was up-regulated up to 3.72 times. The metabolites Cloversaponin I and Goyasaponin I were also up-regulated significantly, up to 3.08 times and 2.69 times, respectively. On the other hand, the most significantly down-regulated metabolite was L-Aspartyl-L-Phenylalanine with a 0.83 times down-regulation. The metabolites L-Beta-Aspartyl-L-Phenylalanine and Histidinol were relatively significantly down-regulated, with fold changes of 0.69 and 0.66, respectively.
As shown in Figure 10b, 325 metabolites exhibited an up-regulated pattern, while 380 metabolites displayed a down-regulated pattern in the LC group compared to the CK group. The most significantly up-regulated metabolite was (17E,19E,21E,23E,25E)-4,6,8,10,12,14,16,27-Octahydroxy-3-(1-hydroxyhexyl)-17,28-dimethyl-1-oxacyclooctacosa-17,19,21,23,25-pentaen-2-one, which showed a remarkable 3.74-fold up-regulation. The metabolites Cloversaponin I and Goyasaponin I were also up-regulated significantly, up to 3.10 times and 2.70 times, respectively. The metabolite with the most significant down-regulation was L-Aspartyl-L-Phenylalanine, with a 0.09 times down-regulation multiple. The metabolites L-beta-aspartyl-L-phenylalanine, Histidinol, and Asparaginyl-Phenylalanine were also notably down-regulated, with fold changes of 0.70, 0.62, and 0.61, respectively.
As shown in Figure 10c, a total of 199 metabolites were differentially expressed in an up-regulated pattern, while 106 metabolites were differentially expressed in a down-regulated pattern in the LC group compared to the LB group. The most significantly up-regulated metabolite was 6-Aminopenicillanic acid, which was up-regulated up to 1.76 times. The metabolites Flavanone and Adenosine 5’-Monophosphate were also up-regulated significantly, up-regulated up to 1.18 times and 1.16 times, respectively. Conversely, the metabolite with the most significant down-regulation was Dehydroisoandrosterone 3-glucuronide, with a down-regulation multiple of 0.16 times. Furthermore, the metabolites 4-p-Coumaroylquinic acid and D-glycero-L-galacto-Octulose were also down-regulated notably, with down-regulation multiples of 0.13 times and 0.08 times, respectively.
The above findings demonstrate a significant up-regulation of metabolites such as 6-Aminopenicillanic acid, Cloversaponin I, and Goyasaponin I in the fermentation broth before and after the fermentation process of Astragali Radix with lactic acid bacteria. It is possible that lactic acid bacteria reproduction produces extracellular enzymes that break the cell wall of Astragali Radix, resulting in a widened cell gap. This can lead to the precipitation of medicinal components and metabolites of Astragali Radix and cause an increase in certain metabolites [43]. Simultaneously, lactobacilli enzymes catalyze the macromolecular components within Astragali Radix, causing the decomposition or transformation of its local structure and the production of new compounds under specific conditions [44]. The elevated concentration of these metabolites enhances the physiological activities of Astragali Radix, such as its anti-inflammatory, anticancer, and antidiabetic functions [45]. Regarding the presence of amino acid metabolites like L-beta-aspartyl-L-phenylalanine and histidinol before and after fermentation, a significant downregulation in their relative abundance is observed. This can be attributed to the gradual reaction of amino acids with substances like reducing sugars or small molecule peptides during the fermentation process, leading to the formation of new compounds. The difference in metabolites between Astragali Radix fermentation products with added CGF extract (LC group) and without added CGF extract (LB group) is relatively insignificant (p > 1). This could be related to the fact that the CGF extract did not have a very high promoting effect on the lactic acid bacteria in this study, possibly due to the concentration and activity of the CGF extract, which needs to be further investigated.
This study has achieved preliminary results in the field of Astragali Radix fermentation, but it is limited by the finite exploration of microbial diversity, the use of a single optimization method, the lack of in-depth mechanism research, and the absence of functional validation. Future research should strengthen these aspects to enhance the scientific rigor and practical value of Astragali Radix fermentation.
4. Conclusions
In this study, the effect of lactic acid bacteria on Astragali Radix fermentation was studied, the enhancing effect of Chlorella Growth Factor (CGF) on fermentation was investigated, and the best fermentation conditions were optimized. The results showed that the total polysaccharide, total flavonoid, and total saponin contents in the fermentation broth after fermentation were effectively increased by 51.42%, 97.76%, and 72.81%, respectively, under the optimized conditions. High-throughput sequencing revealed that Streptococcus lutetiensis was the absolutely dominant strain during Astragali Radix fermentation, and it had the most significant effect on improving the fermentation process. Non-targeted metabolomic analysis based on UHPLC-MS annotated a total of 3083 differential metabolites, showing that the metabolites in the fermentation broth before and after lactic acid bacteria fermentation differed significantly. The differentials consisted mainly of amino acids and saponin compounds, and the addition of CGF extracts had a certain effect on the fermentation of Astragali Radix. This study provides a basis for the development of new products from Astragali Radix and other medicinal plant resources using lactic acid bacteria fermentation.
Author Contributions
X.L.: conceptualization, methodology, investigation, data curation, writing—original draft; W.L.: conceptualization, funding acquisition, supervision, writing—review and editing; Q.G.: investigation; T.X.: methodology, data curation; X.W.: data curation; R.Z.: validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovation Ability Improvement Project of Small and Medium-sized High-tech Company in Zaozhuang City (2023TSGC15); International Cooperation Project for Pilot Project of Integration of Science, Education, and Industry, Qilu University of Technology (Shandong Academy of Sciences) (2024GH07); Shandong Major Technological Innovation Project (2021CXGC010508); Natural Science Foundation of Shandong Province (ZR2022MC204); Major Science and Technology Innovation Projects in Shandong Province (2019JZZY010723)ter.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Qingyan Ge was employed by the company Shandong Qilu King-Phar Pharmaceutical, Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ren, C.; Zhao, X.; Liu, K.; Wang, L.; Chen, Q.; Jiang, H.; Gao, X.; Lv, X.; Zhi, X.; Wu, X.; et al. Research progress of natural medicine Astragalus mongholicus Bunge in treatment of myocardial fibrosis. J. Ethnopharmacol. 2023, 305, 116128. [Google Scholar] [PubMed]
- Kalaycı, B.; Özek, N.Ş.; Aysin, F.; Özbek, H.; Kazaz, C.; Önal, M.; Güvenalp, Z.J.S.P.J. Evaluation of cytotoxic and apoptotic effects of the extracts and phenolic compounds of Astragalus globosus Vahl and Astragalus breviflorus DC. Saudi Pharm. J. 2023, 31, 101682. [Google Scholar] [PubMed]
- Qiao, Y.; Liu, C.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Wang, Z. Polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota. Poult. Sci. 2022, 101, 101905. [Google Scholar] [PubMed]
- Zhong, R.; Yu, M.; Liu, H.; Sun, H.; Cao, Y.; Zhou, D. Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance, rumen fermentation, immune responses, and antioxidant status of lambs. Anim. Feed. Sci. Technol. 2012, 174, 60–67. [Google Scholar]
- Chen, Y.; Liu, C.; Yang, F.; Chen, H.; Yang, C.; Fan, Z.; Xiao, Y.; Xiao, D. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis, antioxidant and α-glucosidase inhibitory activities of mulberry leaves processed by solid-state fermentation. LWT 2023, 188, 115351. [Google Scholar]
- Wu, H.; Liu, H.-N.; Ma, A.-M.; Zhou, J.-Z.; Xia, X.-D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT 2022, 154, 112687. [Google Scholar]
- Roasa, J.; De Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trends Food Sci. Technol. 2021, 116, 954–974. [Google Scholar]
- Long, M.; Pei, X.; Lu, Z.; Xu, D.; Zheng, N.; Li, Y.; Ge, H.; Cao, W.; Osire, T.; Xia, X. Effective degradation of anthraquinones in Folium Sennae with Monascus fermentation for toxicity reduce and efficacy enhancement. Heliyon 2023, 9, e18735. [Google Scholar] [PubMed]
- Qi, J.; Huang, H.; Wang, J.; Liu, N.; Chen, X.; Jiang, T.; Xu, H.; Lei, H. Insights into the improvement of bioactive phytochemicals, antioxidant activities and flavor profiles in Chinese wolfberry juice by select lactic acid bacteria. Food Biosci. 2021, 43, 101264. [Google Scholar]
- Weon, J.B.; Yun, B.-R.; Lee, J.; Eom, M.R.; Ko, H.-J.; Lee, H.Y.; Park, D.-S.; Chung, H.-C.; Chung, J.Y.; Ma, C.J. Neuroprotective effect of steamed and fermented Codonopsis lanceolata. Biomol. Ther. 2014, 22, 246. [Google Scholar]
- Yim, N.H.; Gu, M.J.; Park, H.R.; Hwang, Y.H.; Ma, J.Y. Enhancement of neuroprotective activity of Sagunja-tang by fermentation with lactobacillus strains. BMC Complement. Altern. Med. 2018, 18, 312. [Google Scholar]
- Ben Abdesslem, S.; Ben Moussa, O.; Boulares, M.; Elbaz, M.; Chouaibi, M.; Ayachi, S.; Hassouna, M. Evaluation of the effect of fennel (Foeniculum vulgare Mill) essential oil addition on the quality parameters and shelf-life prediction of yoghurt. Int. J. Dairy Technol. 2020, 73, 403–410. [Google Scholar]
- Xu, Z.; Li, M.; Wang, Y.; Feng, M.; Gan, Z.; Leng, X.; Li, X. Affecting mechanism of Chlorella sorokiniana meal replacing fish meal on growth and immunity of Litopenaeus vannamei based on transcriptome analysis. Aquac. Rep. 2023, 31, 101645. [Google Scholar]
- Luo, J.-W.; Xiao, S.; Wang, B.; Cai, Y.-X.; Wang, J.-H. In vitro fermentation of pineapple–whey protein fermentation product on human intestinal microbiota derived from fecal microbiota transplant donors. LWT 2024, 191, 115637. [Google Scholar]
- Song, X.; Wang, J.; Wang, Y.; Feng, Y.; Cui, Q.; Lu, Y. Artificial creation of Chlorella pyrenoidosa mutants for economic sustainable food production. Bioresour. Technol. 2018, 268, 340–345. [Google Scholar] [PubMed]
- Barghchi, H.; Dehnavi, Z.; Nattagh-Eshtivani, E.; Alwaily, E.R.; Almulla, A.F.; Kareem, A.K.; Barati, M.; Ranjbar, G.; Mohammadzadeh, A.; Rahimi, P.J.B.; et al. The effects of Chlorella vulgaris on cardiovascular risk factors: A comprehensive review on putative molecular mechanisms. Biomed. Pharmacother. 2023, 162, 114624. [Google Scholar]
- Geada, P.; Francisco, D.; Pereira, F.; Maciel, F.; Madureira, L.; Barros, A.; Silva, J.L.; Vicente, A.A.; Teixeira, J.A. Multivariable optimization process of heterotrophic growth of Chlorella vulgaris. Food Bioprod. Process. 2023, 138, 1–13. [Google Scholar]
- Zhou, J.; Wang, M.; Bäuerl, C.; Cortés-Macías, E.; Calvo-Lerma, J.; Collado, M.C.; Barba, F.J. The impact of liquid-pressurized extracts of Spirulina, Chlorella and Phaedactylum tricornutum on in vitro antioxidant, antiinflammatory and bacterial growth effects and gut microbiota modulation. Food Chem. 2023, 401, 134083. [Google Scholar]
- Solano, R.; Patino-Ruiz, D.; Herrera, A. Preparation of modified paints with nano-structured additives and its potential applications. Nanomater. Nanotechnol. 2020, 10, 1847980420909188. [Google Scholar]
- Peng, K.; Chen, X.; Wei, D.; Zhao, L.; Chen, B.; Mo, W.; Zheng, C.; Sun, Y. Inclusion of Chlorella water extract in Oreochromis niloticus fingerling diets: Effects on growth performance, body composition, digestive enzyme activity, antioxidant and immune capacity, intestine and hepatic histomorphology and sodium nitrite stress resistance. Aquac. Rep. 2020, 18, 100547. [Google Scholar]
- Liu, B.; Lu, H.; Shu, Q.; Chen, Q.; Wang, J. The influence of different pretreatment methods of highland barley by solid-state fermentation with Agaricus sinodeliciosus var. Chaidam ZJU-TP-08 on its nutrient content, functional properties and physicochemical characteristics. J. Fungi 2022, 8, 940. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Song, T.; Xu, X.; Yu, J.; Liu, T.J.H. Optimization of Cordyceps sinensis fermentation Marsdenia tenacissima process and the differences of metabolites before and after fermentation. Heliyon 2022, 8, e12586. [Google Scholar] [PubMed]
- Niazi, A.; Mehrgan, M.S.; Islami, H.R.J.A. Optimizing turmeric and green tea fermentation with Lactobacillus brevis to enhance growth performance, digestive enzymes, and immunity in rainbow trout. Aquaculture 2023, 577, 739962. [Google Scholar]
- Wang, M. Evaluation of Biological Properties of Extracts Obtained from Marine Biomass Assisted by Pulsed Electric Fields (PEF) and Pressurized Liquid Extraction (PLE). Ph.D. Thesis, Universitat de València, Valencia, Spain, 2022. [Google Scholar]
- Chen, Z.; Zhou, P.; Zhao, Z.; Li, B. Enhanced production of L-serine in Escherichia coli by fermentation process optimization and transcriptome sequencing. Biochem. Eng. J. 2023, 200, 109109. [Google Scholar]
- Yang, Y.; Na Wu, Y.; Ce, L.G.E.; Ge, X.G.B.R.; Shuang, Q.; Zhang, F.M. Analysis of microbial community and its correlation with flavor compounds during Congee fermentation. Food Biosci. 2023, 51, 102261. [Google Scholar]
- Guo, Q.; Zabed, H.; Zhang, H.; Wang, X.; Yun, J.; Zhang, G.; Yang, M.; Sun, W.; Qi, X. Optimization of fermentation medium for a newly isolated yeast strain (Zygosaccharomyces rouxii JM-C46) and evaluation of factors affecting biosynthesis of D-arabitol. LWT 2019, 99, 319–327. [Google Scholar]
- Zhou, S.; Ding, N.; Han, R.; Deng, Y. Metabolic engineering and fermentation optimization strategies for producing organic acids of the tricarboxylic acid cycle by microbial cell factories. Bioresour. Technol. 2023, 379, 128986. [Google Scholar]
- Bitew, D.; Alemu, M.; Tesfaye, A.; Andualem, B.J.R.; Reviews, S.E. Ethanologenic yeasts from Ethiopian fermented beverages and optimization of fermentation conditions. Renew. Sustain. Energy Rev. 2024, 190, 114090. [Google Scholar]
- Ni, K.; Wang, X.; Lu, Y.; Guo, L.; Li, X.; Yang, F. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresour. Technol. 2020, 297, 122249. [Google Scholar]
- Zheng, Z.; Wei, L.; Zhu, M.; Qian, Z.; Liu, J.; Zhang, L.; Xu, Y. Effect of lactic acid bacteria co-fermentation on antioxidant activity and metabolomic profiles of a juice made from wolfberry and longan. Food Res. Int. 2023, 174, 113547. [Google Scholar]
- Guo, M.; Kong, Q.; Wang, W.; Yu, H. Biotransformation of amygdalin by lactic acid bacteria fermentation. Process Biochem. 2023, 132, 221–227. [Google Scholar]
- Shi, H.; Zhao, Y.; Wang, W.; Zhou, Y.; Liang, Y.; Wu, R.; Wu, J. The potential of lactic acid bacteria in fermented herbs-derived food products. Food Biosci. 2024, 61, 104714. [Google Scholar]
- Akpabli-Tsigbe, N.D.K.; Ma, Y.; Ekumah, J.-N.; Osabutey, J.; Hu, J.; Xu, M.; Johnson, N.A.N.J.L. Novel solid-state fermentation extraction of 5-O-caffeoylquinic acid from heilong48 soybean using Lactobacillus helviticus: Parametric screening and optimization. LWT 2021, 149, 111809. [Google Scholar]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B.J.L. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT 2021, 135, 110029. [Google Scholar]
- Zhang, R.; Song, X.; Liu, W.; Gao, X. Mixed fermentation of Chlorella pyrenoidosa and Bacillus velezensis SW-37 by optimization. LWT 2023, 175, 114448. [Google Scholar]
- Wu, T.; Deng, C.; Luo, S.; Liu, C.; Hu, X. Effect of rice bran on properties of yogurt: Comparison between addition of bran before fermentation and after fermentation. Food Hydrocoll. 2023, 135, 108122. [Google Scholar]
- Jiang, Y.; Wu, J.; Tian, L.; Liu, Y.; Zhao, F.; He, Z.; Mao, Y.; Jia, J.; Guan, T. The therapeutic effects of fermented milk with lactic acid bacteria from traditional Daqu on hypertensive mice. J. Dairy Sci. 2024, 107, 742–758. [Google Scholar]
- Charlier, C.; Cretenet, M.; Even, S.; Le Loir, Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009, 131, 30–39. [Google Scholar]
- Pau, S.; Tan, L.C.; Arriaga, S.; Lens, P.N. Lactic acid fermentation of food waste in a semicontinuous SBR system: Influence of the influent composition and hydraulic retention time. Environ. Technol. 2024, 45, 2993–3003. [Google Scholar]
- Zhao, W.; Zhang, Z.; Gao, Y.; Liu, X.; Du, C.; Ma, F.; Wang, S.; Shi, W.; Yang, Y.; Deng, R.; et al. Fungal dynamic changes in naturally fermented ‘Kyoho’grape juice. Arch. Microbiol. 2022, 204, 556. [Google Scholar]
- Zhao, X.; Tang, F.; Cai, W.; Peng, B.; Zhang, P.; Shan, C. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube–wolfberry composite juice. LWT 2023, 184, 114884. [Google Scholar]
- Huang, W.; Zhang, C.; Gu, Z.; Li, C.; Fang, Z.; Zeng, Z.; Zhang, Z.; Hu, B.; Chen, H.; Wu, W.; et al. Effect of microbial fermentation on the sensory characteristics and chemical compositions of Chinese sweet tea (Lithocarpus litseifolius (Hance) Chun). Food Biosci. 2022, 46, 101567. [Google Scholar]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P.J.P. Flavonoids and saponins: What have we got or missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).