Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sugar Recovery from Agro-Industrial Wastes
2.1.1. Agro-Industrial Wastes
2.1.2. Chemical Characterization
2.1.3. Agro-Industrial Waste Pretreatment
2.1.4. Hydrolysate Preparation
2.2. Fermentation
2.2.1. Microbial Consortium and Inoculum Production
2.2.2. Culture Medium from Agro-Industrial Wastes and Fermentation Conditions
2.3. FTIR Characterization
2.4. BC Production in a Stirred Tank Bioreactor (STB)
2.4.1. Operating Conditions Setup
2.4.2. Oxygen Uptake Rate (OUR)
2.4.3. Fermentation in STB
2.5. Reducing Sugars Determination
2.6. BC Recovery
3. Results and Discussion
3.1. BC Production from Agro-Industrial Wastes
3.1.1. Characterization and Hydrolysis of Agro-Industrial Wastes
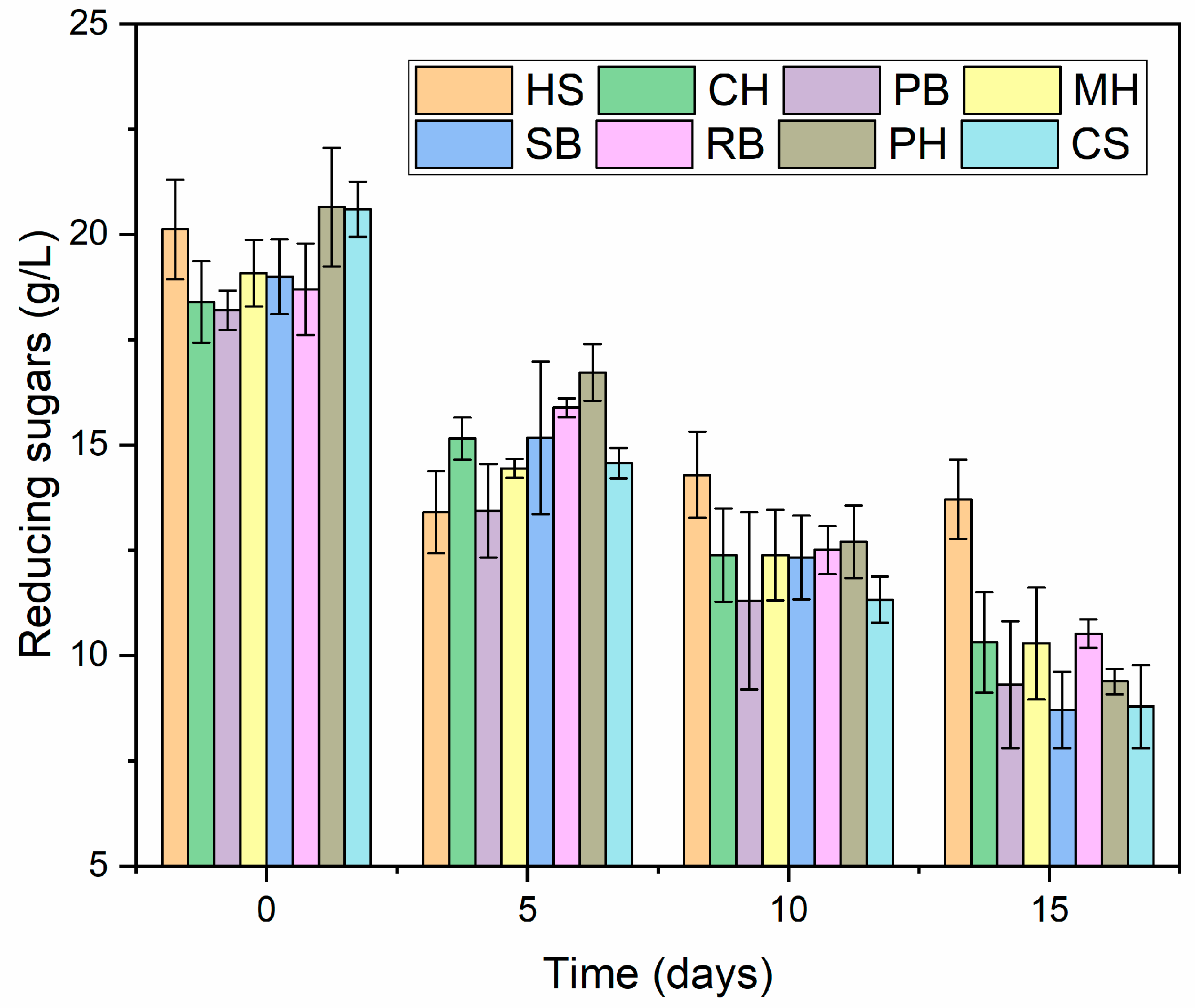
3.1.2. Fermentation
3.1.3. FTIR Characterization
3.2. BC Production in STB
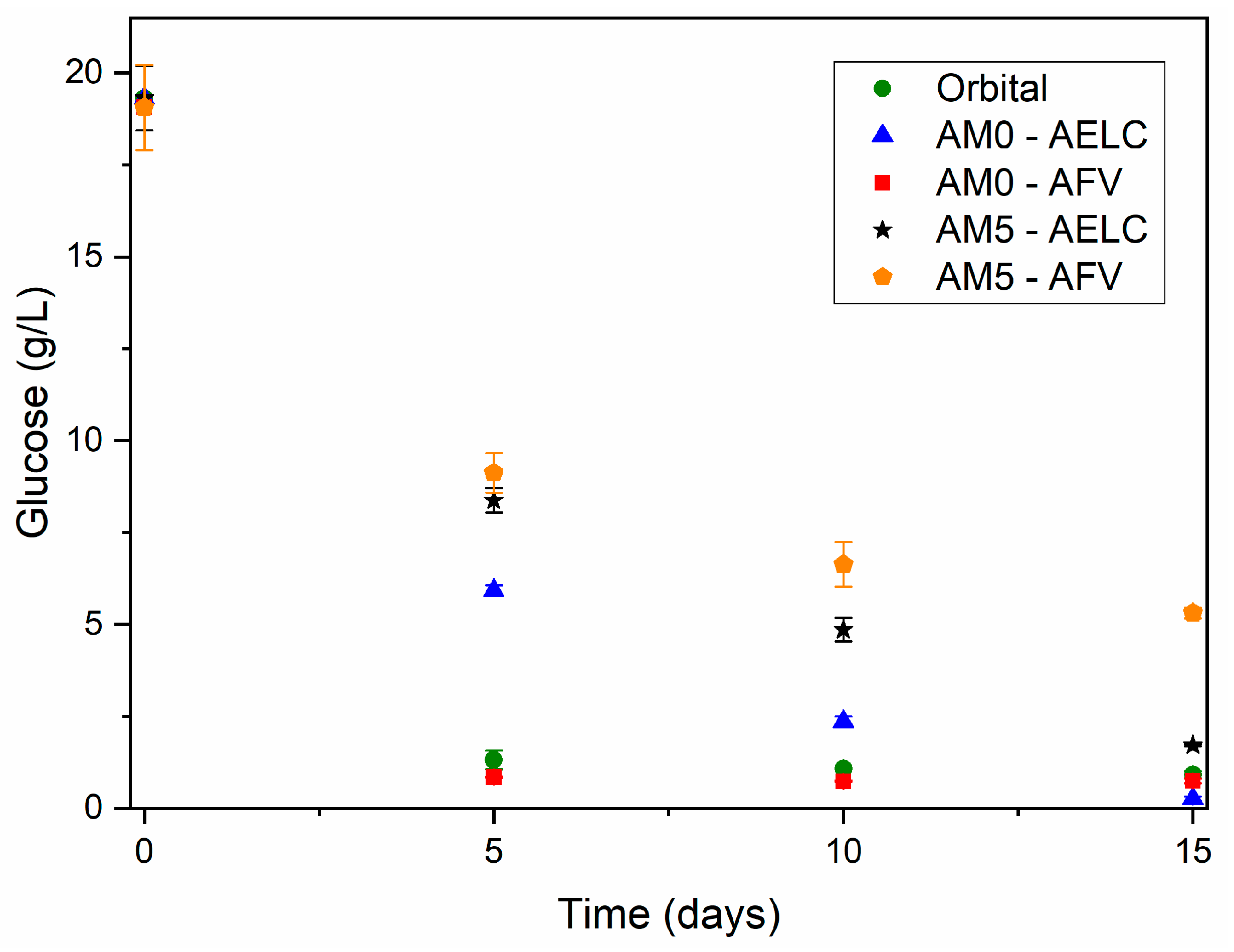
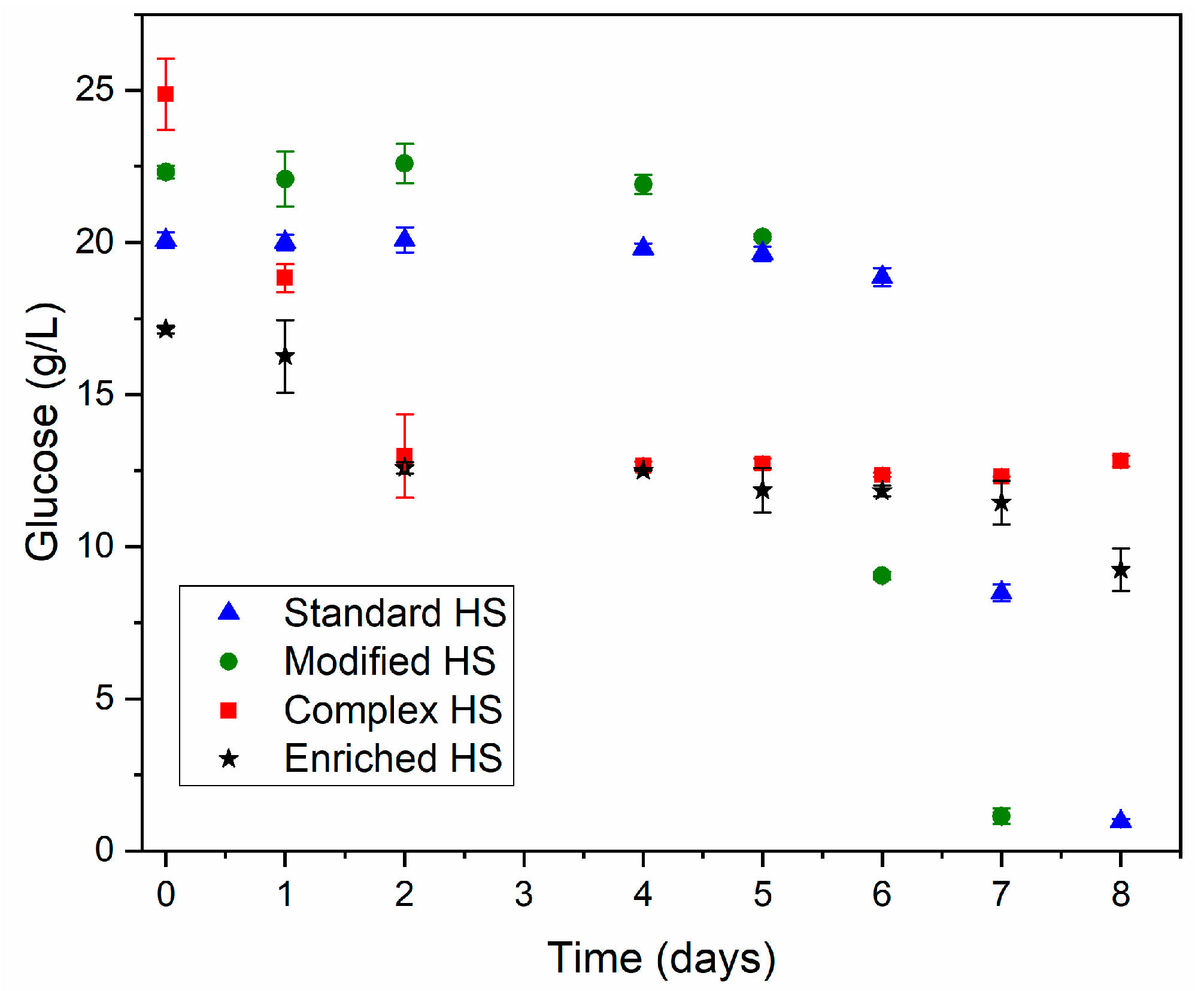
3.2.1. Effect of Orbital Stirring and Increase of the Nitrogen Source
3.2.2. Effect of Magnetic Stirring and Different Oxygen Supply
3.2.3. Fermentation Performance in STB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Sani, A.; Dahman, Y. Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J. Chem. Technol. Biotechnol. 2010, 85, 151–164. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial cellulose production from agricultural residues by two Komagataeibacter sp. strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Son, J.; Lee, J.; Yoo, H.Y.; Lee, T.; Jang, M.; Oh, J.; Park, C. Improved production of bacterial cellulose through investigation of effects of inhibitory compounds from lignocellulosic hydrolysates. GCB Bioenergy 2021, 13, 436–444. [Google Scholar] [CrossRef]
- Wang, Q.; Nnanna, P.C.; Shen, F.; Huang, M.; Tian, D.; Hu, J.; Zeng, Y.; Yang, G.; Deng, S. Full utilization of sweet sorghum for bacterial cellulose production: A concept of material crop. Ind. Crop. Prod. 2021, 162, 113256. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Tran, T.; Verdier, F.; Martin, A.; Alexandre, H.; Grandvalet, C.; Tourdot-Maréchal, R. Oxygen management during kombucha production: Roles of the matrix, microbial activity, and process parameters. Food Microbiol. 2022, 105, 104024. [Google Scholar] [CrossRef]
- Tapias, Y.A.R.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial cellulose films production by Kombucha symbiotic community cultured on different herbal infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef]
- Betlej, I.; Salerno-Kochan, R.; Krajewski, K.J.; Zawadzki, J.; Boruszewski, P. The influence of culture medium components on the physical and mechanical properties of cellulose synthesized by kombucha microorganisms. BioResources 2020, 15, 3125–3135. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Korchagina, A.A.; Gismatulina, Y.A.; Gladysheva, E.K.; Budaeva, V.V.; Sakovich, G.V. Simultaneous Production of Cellulose Nitrates and Bacterial Cellulose from Lignocellulose of Energy Crop. Polymers 2023, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Lončar, E.; Djurić, M.; Malbaša, R.; Kolarov, L.; Klašnja, M. Influence of working conditions upon kombucha conducted fermentation of black tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Caicedo, L.A.; Da França, F.; Lopez, L. Factores para el escalado del proceso de producción de celulosa por fermentación estática. Rev. Colomb. Química 2001, 30, 155–162. [Google Scholar]
- Shavyrkina, N.A.; Budaeva, V.V.; Skiba, E.A.; Mironova, G.F.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Sitnikova, A.E.; Shilov, A.I.; Kuznetsov, P.S.; et al. Scale-up of biosynthesis process of bacterial nanocellulose. Polymers 2021, 13, 1920. [Google Scholar] [CrossRef] [PubMed]
- Shavyrkina, N.A.; Skiba, E.A.; Kazantseva, A.E.; Gladysheva, E.K.; Budaeva, V.V.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Mironova, G.F.; Korchagina, A.A.; et al. Static culture combined with aeration in biosynthesis of bacterial cellulose. Polymers 2021, 13, 4241. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M. Formation and characterization of spherelike bacterial cellulose particles produced by Acetobacter xylinum JCM 9730 strain. Biomacromolecules 2010, 11, 1727–1734. [Google Scholar] [CrossRef]
- Jung, J.Y.; Khan, T.; Park, J.K.; Chang, H.N. Production of bacterial cellulose by Gluconacetobacter hansenii using a novel bioreactor equipped with a spin filter. Korean J. Chem. Eng. 2007, 24, 265–271. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial cellulose production by Acetobacter xylinum in a 50-L internal-loop airlift reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Cheng, H.P.; Wang, P.M.; Chen, J.W.; Wu, W.T. Cultivation of Acetobacter xylinum for bacterial cellulose production in a modified airlift reactor. Biotechnol. Appl. Biochem. 2002, 35, 125–132. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, J.-N.; Wee, Y.-J.; Park, D.-H.; Ryu, H.-W. Bacterial cellulose production by Gluconacetobacter sp. PKY5 in a rotary biofilm contactor. Appl. Biochem. Biotechnol. 2007, 137, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell as Promising Feedstock for the Production of Poly (3-Hydroxybutyrate)(PHB). Appl. Sci. 2023, 13, 975. [Google Scholar] [CrossRef]
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrogen Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Santoso, S.P.; Lin, S.-P.; Wang, T.-Y.; Ting, Y.; Hsieh, C.-W.; Yu, R.-C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.-Y.; Cheng, K.-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef]
- Najafpour, G. Comparative studies on effect of pretreatment of rice husk for enzymatic digestibility and bioethanol production. Int. J. Eng. 2013, 26, 455–464. [Google Scholar]
- Siacor, F.D.C.; Lobarbio, C.F.Y.; Taboada, E.B. Pretreatment of mango (Mangifera indica L. Anacardiaceae) seed husk for bioethanol production by dilute acid treatment and enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2021, 193, 1338–1350. [Google Scholar]
- Chen, L.; Li, J.; Lu, M.; Guo, X.; Zhang, H.; Han, L. Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aswini, K.; Gopal, N.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Dávalos, P. Aislamiento y Caracterización de Cepas Nativas de Komagataeibacter xylinus y c Omparación de su Crecimiento en Diferentes Sustratos; Ingeniera Bioquímica, Facultad de Ciencia e Ingeniería en Alimentos y Biotecnología, Universidad Técnica de Ambato: Ambato, Ecuador, 2022. [Google Scholar]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wu, J.-Y.; Ho, K.-P. Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem. Eng. J. 2006, 27, 331–335. [Google Scholar] [CrossRef]
- Ascanio, G.; Castro, B.; Galindo, E. Measurement of power consumption in stirred vessels—A review. Chem. Eng. Res. Des. 2004, 82, 1282–1290. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of cell growth and crystalline nanocellulose production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Silva, A.S.; Correa, L.G.; Kanai, R.S.; Shirai, M.A. Effect of sugarcane bagasse addition on physical, chemical, and sensory properties of oat flour and banana cake. J. Texture Stud. 2020, 51, 902–908. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Sánchez-Camargo, A.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Huang, C.; Lin, I.; Liu, Y.; Mau, J. Composition, enzyme and antioxidant activities of pineapple. Int. J. Food Prop. 2021, 24, 1244–1251. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Kalschne, D.L.; da Silva-Buzanello, R.A.; Byler, A.P.I.; Scremin, F.R.; de Magalhães Junior, A.M.; Canan, C. Rice and rice bran from different cultivars: Physicochemical, spectroscopic, and thermal analysis characterization. Semin. Agrar. 2020, 41, 3081–3092. [Google Scholar] [CrossRef]
- Hall, M.B. Methodological challenges in carbohydrate analyses. Rev. Bras. Zootec. 2007, 36, 359–367. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R.; et al. Fruit characteristics, peel nutritional compositions, and their relationships with mango peel pectin quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Rosniyana, A.; Hashifah, M.; Norin, S.S. The physico-chemical properties and nutritional composition of rice bran produced at different milling degrees of rice. J. Trop. Agric. Food Sci. 2007, 35, 99. [Google Scholar]
- Shi, R.; Pang, C.; Wu, X.; Zhao, X.; Chen, F.; Zhang, W.; Sun, C.; Fu, S.; Hu, M.; Zhang, J.; et al. Genetic dissection and germplasm selection of the low crude fiber component in Brassica napus L. shoots. Foods 2023, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Value-added products from fruit and vegetable wastes: A review. CLEAN–Soil Air Water 2021, 49, 2000376. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.; Rooney, D.; Walker, G. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, D.; Zhao, X. Conversion of lignocellulose to biofuels and chemicals via sugar platform: An updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew. Sustain. Energy Rev. 2021, 146, 111169. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Widyaya, V.T.; Ha, J.-M.; Suh, D.J.; Kim, C.S. Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Sjulander, N.; Kikas, T. Origin, impact and control of lignocellulosic inhibitors in bioethanol production—A review. Energies 2020, 13, 4751. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Cheng, K.-K.; Cai, B.-Y.; Zhang, J.-A.; Ling, H.-Z.; Zhou, Y.-J.; Ge, J.-P.; Xu, J.-M. Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem. Eng. J. 2008, 38, 105–109. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.-Y.; Kasim, N.S.; Diem, Q.-D.; Huynh, L.-H.; Ho, Q.-P.; Truong, C.-T.; Ju, Y.-H. Oil production from Yarrowia lipolytica Po1g using rice bran hydrolysate. BioMed Res. Int. 2012, 2012, 378384. [Google Scholar]
- He, J.; Huang, C.; Lai, C.; Huang, C.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yong, Q. The effect of lignin degradation products on the generation of pseudo-lignin during dilute acid pretreatment. Ind. Crop. Prod. 2020, 146, 112205. [Google Scholar] [CrossRef]
- Rogalinski, T.; Ingram, T.; Brunner, G. Hydrolysis of lignocellulosic biomass in water under elevated temperatures and pressures. J. Supercrit. Fluids 2008, 47, 54–63. [Google Scholar] [CrossRef]
- Saavedra-Sanabria, O.L.; Durán, D.; Cabezas, J.; Hernández, I.; Blanco-Tirado, C.; Combariza, M.Y. Cellulose biosynthesis using simple sugars available in residual cacao mucilage exudate. Carbohydr. Polym. 2021, 274, 118645. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.; Sameshima, K. Evaluation of different carbon sources for bacterial cellulose production. Afr. J. Biotechnol. 2005, 4, 478–482. [Google Scholar]
- Yang, X.-Y.; Huang, C.; Guo, H.-J.; Xiong, L.; Luo, J.; Wang, B.; Chen, X.-F.; Lin, X.-Q.; Chen, X.-D. Beneficial effect of acetic acid on the xylose utilization and bacterial cellulose production by Gluconacetobacter xylinus. Indian J. Microbiol. 2014, 54, 268–273. [Google Scholar] [CrossRef] [PubMed]
- van der Maas, L.; Driessen, J.L.; Mussatto, S.I. Effects of inhibitory compounds present in lignocellulosic biomass hydrolysates on the growth of Bacillus subtilis. Energies 2021, 14, 8419. [Google Scholar] [CrossRef]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa bean shell: A by-product with high potential for nutritional and biotechnological applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial cellulose production from industrial waste and by-product streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Hungund, B.S.; Gupta, S. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol 2010, 2, 127–133. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Zhu, C.; Sun, D. Production of bacterial cellulose by Acetobacter xylinum: Effects of carbon/nitrogen-ratio on cell growth and metabolite production. Cellul. Chem. Technol 2016, 50, 997–1003. [Google Scholar]
- Matsuoka, M.; Tsuchida, T.; Matsushita, K.; Adachi, O.; Yoshinaga, F. A synthetic medium for bacterial cellulose production by Acetobacter xylinum subsp. sucrofermentans. Biosci. Biotechnol. Biochem. 1996, 60, 575–579. [Google Scholar] [CrossRef]
- Narh, C.; Frimpong, C.; Mensah, A.; Wei, Q. Rice Bran, an Alternative Nitrogen Source for Acetobacter xylinum Bacterial Cellulose Synthesis. Bioresources 2018, 13, 4346–4363. [Google Scholar] [CrossRef]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of substrate mass transfer influences on the bioreaction (Part 1). Eng. Life Sci. 2006, 6, 537–545. [Google Scholar] [CrossRef]
- Ryngajłło, M.; Jacek, P.; Cielecka, I.; Kalinowska, H.; Bielecki, S. Effect of ethanol supplementation on the transcriptional landscape of bionanocellulose producer Komagataeibacter xylinus E25. Appl. Microbiol. Biotechnol. 2019, 103, 6673–6688. [Google Scholar] [CrossRef]
- Lee, J.-E.; Seo, E.-J.; Kweon, D.-H.; Park, K.-M.; Jin, Y.-S. Fermentation of rice bran and defatted rice bran for butanol production using Clostridium beijerinckii NCIMB 8052. J. Microbiol. Biotechnol. 2009, 19, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Cielecka, I.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Response surface methodology-based improvement of the yield and differentiation of properties of bacterial cellulose by metabolic enhancers. Int. J. Biol. Macromol. 2021, 187, 584–593. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Voon, W.; Muhialdin, B.; Yusof, N.; Rukayadi, Y.; Hussin, A.M. Bio-cellulose Production by Beijerinckia fluminensis WAUPM53 and Gluconacetobacter xylinus 0416 in Sago By-product Medium. Appl. Biochem. Biotechnol. 2019, 187, 211–220. [Google Scholar] [CrossRef]
- Pacheco, G.; Nogueira, C.R.; Meneguin, A.B.; Trovatti, E.; Silva, M.C.C.; Machado, R.T.A.; Ribeiro, S.J.L.; da Silva Filho, E.C.; Barud, H.d.S. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crop. Prod. 2017, 107, 13–19. [Google Scholar] [CrossRef]
- Lotfiman, S.; Biak, D.R.A.; Ti, T.B.; Kamarudin, S.; Nikbin, S. Influence of date syrup as a carbon source on bacterial cellulose production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018, 37, 1085–1091. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Popescu, C.-M.; Lisa, G.; Sakata, Y. Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Samadi, Z.; Allaf, M.M.; Saifi, R.; De Groot, C.T.; Peerhossaini, H. Effects of turbulent mixing and orbitally shaking on cell growth and biomass production in active fluids. AJBSR 2022, 15, 396–404. [Google Scholar]
- Reynoso-Cereceda, G.I.; Garcia-Cabrera, R.I.; Valdez-Cruz, N.A.; Trujillo-Roldán, M.A. Shaken flasks by resonant acoustic mixing versus orbital mixing: Mass transfer coefficient kLa characterization and Escherichia coli cultures comparison. Biochem. Eng. J. 2016, 105, 379–390. [Google Scholar] [CrossRef]
- Hegde, S.; Bhadri, G.; Narsapur, K.; Koppal, S.; Oswal, P.; Turmuri, N.; Jumnal, V.; Hungund, B. Statistical optimization of medium components by response surface methodology for enhanced production of bacterial cellulose by Gluconacetobacter persimmonis. J. Bioprocess Biotech. 2013, 4, 1–5. [Google Scholar]
- Tran, T.; Grandvalet, C.; Winckler, P.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Shedding light on the formation and structure of kombucha biofilm using two-photon fluorescence microscopy. Front. Microbiol. 2021, 12, 725379. [Google Scholar] [CrossRef]
- Mooiman, C.; Bouwknegt, J.; Dekker, W.J.C.; Wiersma, S.J.; A Ortiz-Merino, R.; de Hulster, E.; Pronk, J.T. Critical parameters and procedures for anaerobic cultivation of yeasts in bioreactors and anaerobic chambers. FEMS Yeast Res. 2021, 21, foab035. [Google Scholar] [CrossRef] [PubMed]
- Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of bacterial cellulose by extended cultivation with multiple removal of BC pellicles. Polymers 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
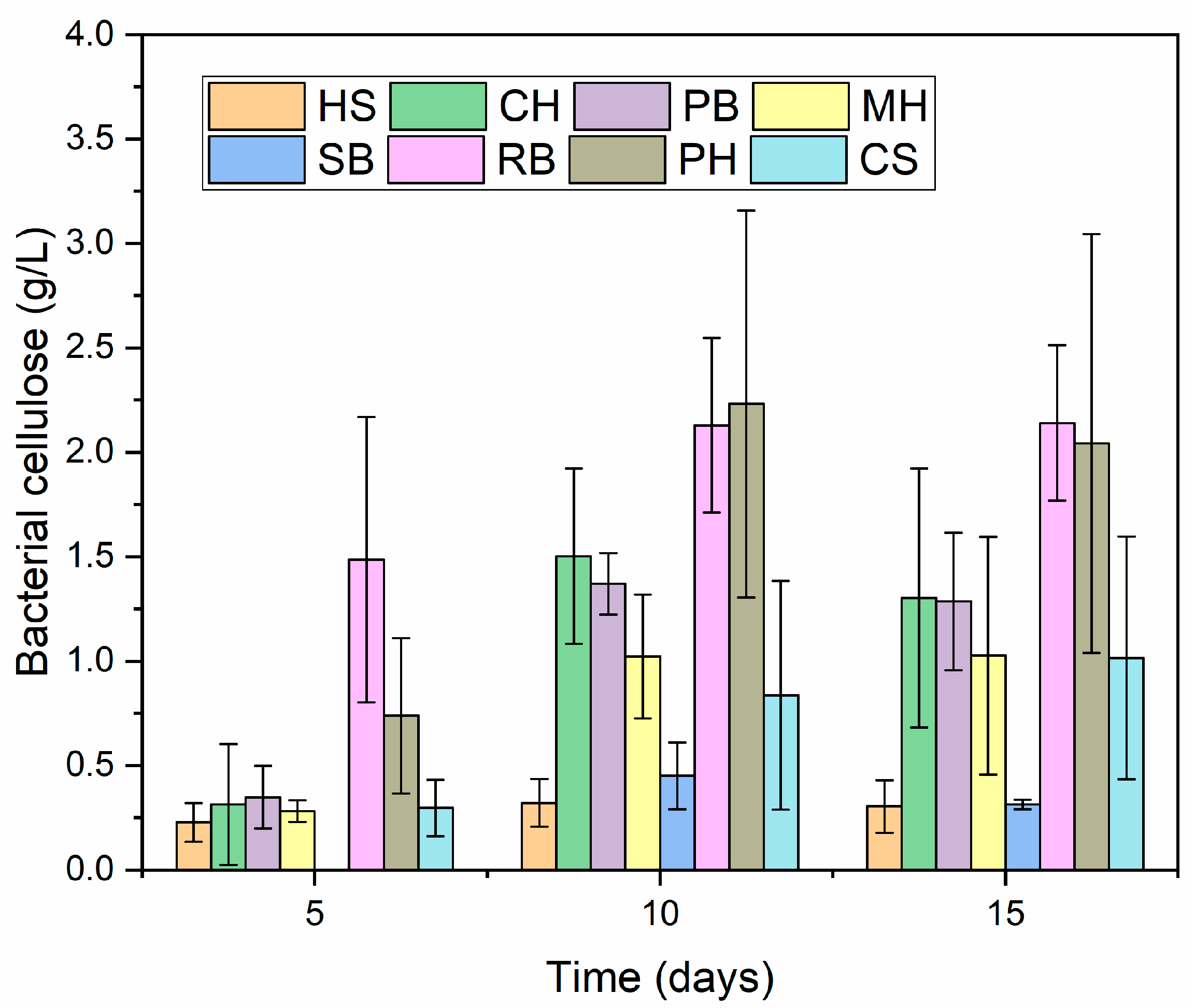



| Parameters | Agro-Industrial Wastes | ||||||
|---|---|---|---|---|---|---|---|
| CH | SB | PB | RB | MH | PH | CS | |
| Phase | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Solid/liquid ratio | 1:05 | 1:15 | 1:10 | 1:10 | 1:10 | 1:10 | 1:10 |
| Temperature (°C) | 121 | 121 | 121 | 121 | 121 | 121 | 121 |
| Sulphuric acid concentration (% v/v) | 3 | 1 | 2.5 | 1 | 4.0 | 2.5 | 1.5 |
| Incubation time (min) | 15 | 60 | 60 | 30 | 60 | 60 | 60 |
| Particle size (µm) | <425 | 425–600 | <250 | 300–425 | <250 | <250 | 425–600 |
| Reference | [22] | [23] | [24] | [25] | [26] | [24] | [27] |
| Materials | With Acid | Without Acid | Acid Contribution | With Acid Ysm (g/g) | Without Acid Ysm (g/g) |
|---|---|---|---|---|---|
| CH | 28.57 | 6.19 | 22.38 | 0.14 | 0.03 |
| SB | 23.20 | 6.30 | 16.90 | 0.35 | 0.09 |
| PB | 54.74 | 51.46 | 3.28 | 0.55 | 0.51 |
| RB | 27.01 | 1.71 | 25.30 | 0.27 | 0.02 |
| MH | 41.54 | 51.96 | NA | 0.42 | 0.52 |
| PH | 45.75 | 44.86 | 0.89 | 0.46 | 0.45 |
| CS | 41.94 | 18.74 | 23.20 | 0.42 | 0.19 |
| Materials | Day 10 | Day 15 | ||||
|---|---|---|---|---|---|---|
| Reducing Sugars Consumed (g/L) | BC (g/L) | Yps (g/g) | Reducing Sugars Consumed (g/L) | BC (g/L) | Yps (g/g) | |
| HS | 5.83 ± 1.54 | 0.32 ± 0.11 | 0.061 ± 0.031 a | 6.41 ± 1.20 | 0.30 ± 0.13 | 0.049 ± 0.022 a,b |
| CH | 6.01 ± 0.44 | 1.50 ± 0.42 | 0.251 ± 0.069 c,d | 8.08 ± 0.94 | 1.30 ± 0.62 | 0.164 ± 0.079 c,d |
| PB | 6.90 ± 2.39 | 1.37 ± 0.15 | 0.213 ± 0.063 b,c | 8.89 ± 1.71 | 1.29 ± 0.33 | 0.143 ± 0.015 c,d |
| MH | 6.69 ± 0.49 | 1.02 ± 0.30 | 0.151 ± 0.036 a,b,c | 8.79 ± 1.14 | 1.03 ± 0.57 | 0.113 ± 0.056 b,c,d |
| SB | 6.67 ± 0.77 | 0.45 ± 0.16 | 0.067 ± 0.020 a | 10.29 ± 0.59 | 0.31 ± 0.02 | 0.031 ± 0.004 a |
| RB | 6.19 ± 1.30 | 2.13 ± 0.42 | 0.362 ± 0.139 d | 8.18 ± 0.88 | 2.14 ± 0.37 | 0.261 ± 0.029 e |
| PH | 7.95 ± 0.97 | 2.23 ± 0.93 | 0.277 ± 0.097 c,d | 11.27 ± 1.54 | 2.04 ± 1.00 | 0.175 ± 0.067 d |
| CS | 9.27 ± 0.21 | 0.84 ± 0.55 | 0.090 ± 0.059 a,b | 11.81 ± 0.40 | 1.02 ± 0.58 | 0.087 ± 0.053 a,b,c |
| Parameter | Standard HS | Modified HS |
|---|---|---|
| Glucose consumption (%) | 96.45 ± 0.57 | 94.90 ± 1.32 |
| BC concentration (g/L) | 0.126 ± 0.015 | 0.306 ± 0.071 |
| Yps (mg/g) | 6.53 ± 0.91 | 16.12 ± 0.22 |
| Fermentation Days | Magnetic Stirring at Beginning AELC | Magnetic Stirring at 5th Day AFV | ||
|---|---|---|---|---|
| CL0 | OUR | CL0 | OUR | |
| 5 | 7.23 ± 0.37 | 0.028 ± 0.003 | 8.26 ± 0.30 | 0.027 ± 0.002 |
| 10 | 6.69 ± 0.19 | 0.033 ± 0.001 | 7.25 ± 0.46 | 0.029 ± 0.001 |
| 15 | 6.04 ± 0.54 | 0.038 ± 0.003 | 7.10 ± 0.34 | 0.030 ± 0.001 |
| Parameter | Standard HS | Modified HS A | Complex HS B | Enriched HS C |
|---|---|---|---|---|
| BC concentration (g/L) | 0.14 | 0.38 | 0.16 | 0.28 |
| Yps (mg/g) | 6.91 | 17.58 | 7.97 | 13.86 |
| Reducing sugars consumption (%) | 94.00 | 95.73 | 48.47 | 46.09 |
| Parameter | Standard HS | Modified HS A | Complex HS B | Enriched HS C |
|---|---|---|---|---|
| Viscosity at 20 °C × (Pa·s) | 1.087 | 1.213 | 0.939 | 1.002 |
| Density (kg/m3) | 1006.45 | 1011.02 | 1001.80 | 1007.06 |
| Agitation power × (W) | 1.908 | 1.917 | 1.899 | 1.909 |
| Reynolds number | 2469.14 | 2221.23 | 2864.11 | 2671.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páez, M.A.; Casa-Villegas, M.; Aldas, M.; Luna, M.; Cabrera-Valle, D.; López, O.; Fernández, D.; Cruz, M.A.; Flor-Unda, O.; García, M.D.; et al. Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes. Fermentation 2024, 10, 425. https://doi.org/10.3390/fermentation10080425
Páez MA, Casa-Villegas M, Aldas M, Luna M, Cabrera-Valle D, López O, Fernández D, Cruz MA, Flor-Unda O, García MD, et al. Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes. Fermentation. 2024; 10(8):425. https://doi.org/10.3390/fermentation10080425
Chicago/Turabian StylePáez, María Augusta, Mary Casa-Villegas, Miguel Aldas, Maribel Luna, Daniel Cabrera-Valle, Orestes López, Danae Fernández, María Alejandra Cruz, Omar Flor-Unda, Mario D. García, and et al. 2024. "Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes" Fermentation 10, no. 8: 425. https://doi.org/10.3390/fermentation10080425
APA StylePáez, M. A., Casa-Villegas, M., Aldas, M., Luna, M., Cabrera-Valle, D., López, O., Fernández, D., Cruz, M. A., Flor-Unda, O., García, M. D., & Cerda-Mejía, L. (2024). Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes. Fermentation, 10(8), 425. https://doi.org/10.3390/fermentation10080425








