Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Samples and Winemaking
2.2. SPME-GC–MS Analyses
2.3. Sensory Analysis
2.4. Statistical Analysis
3. Results and Discussion
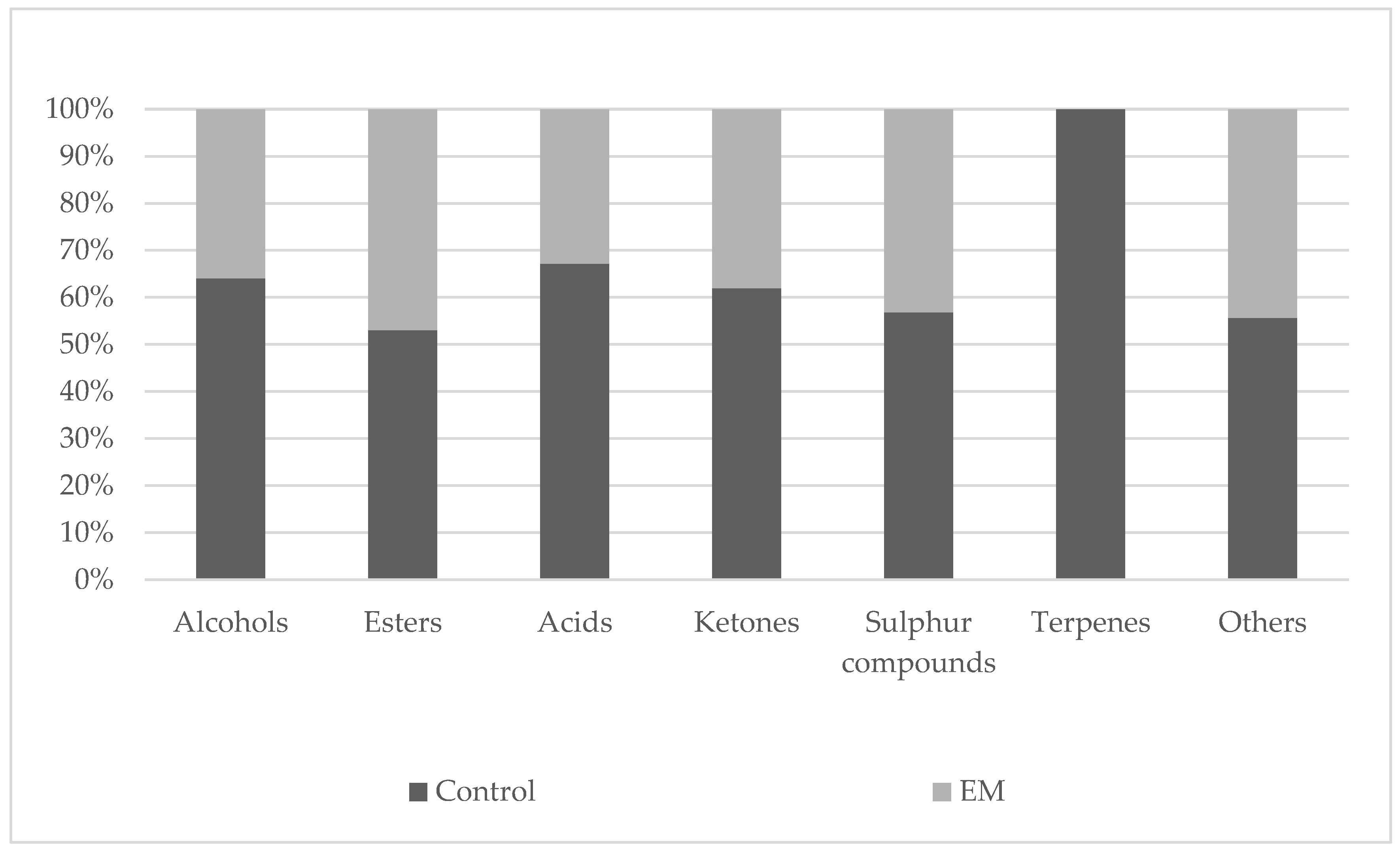
3.1. Characterization of Volatile Signature of Monastrell Wines by Maceration Using SPME/GC–MS
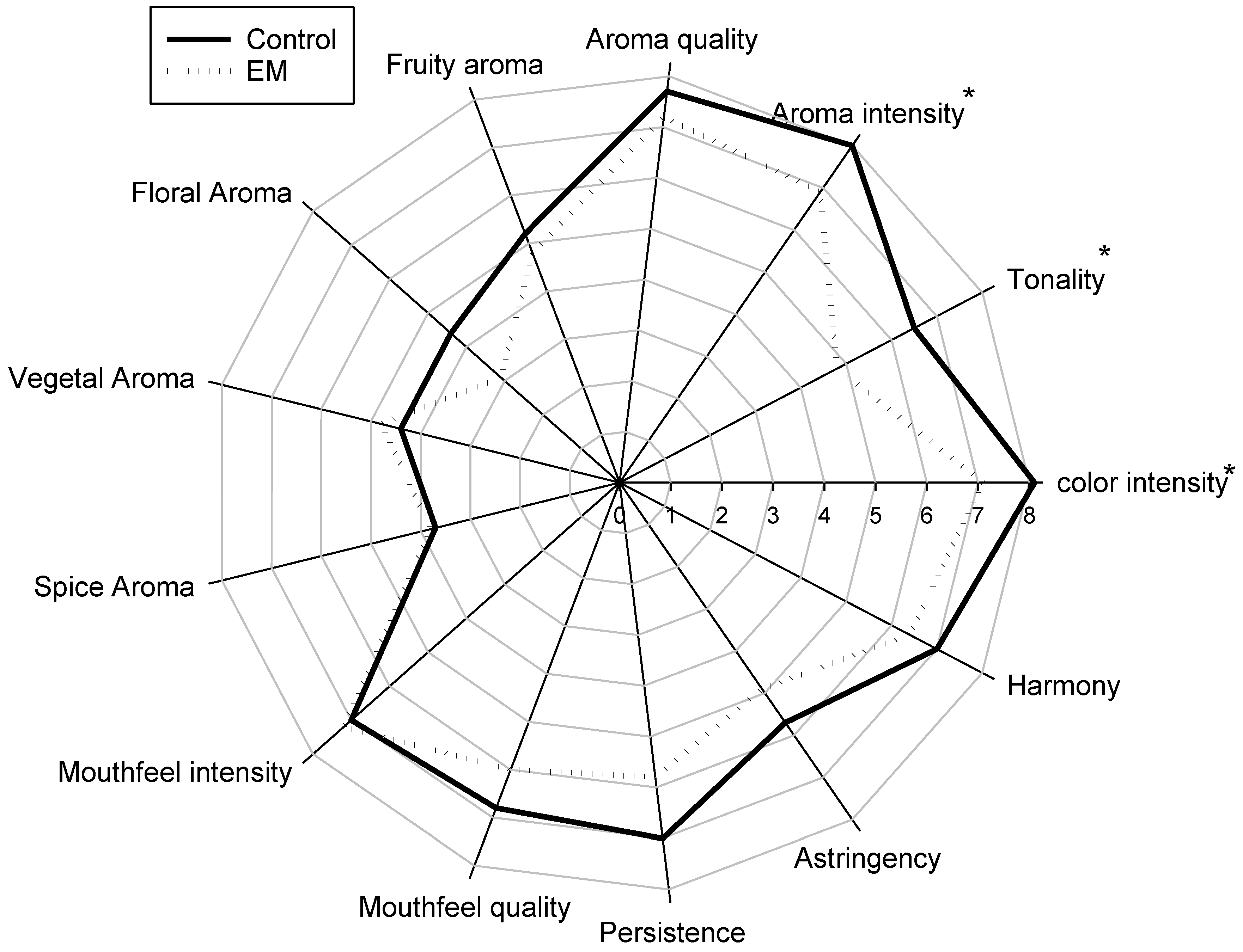
3.2. Sensory Evaluation of Monastrell Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Martínez-Moreno, A.; Pérez-Álvarez, E.P.; López-Urrea, R.; Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Intrigliolo, D.S.; Gil-Muñoz, R. Effects of deficit irrigation with saline water on wine color and polyphenolic composition of Vitis vinifera L. cv. Monastrell. Sci. Hort. 2021, 283, 110085. [Google Scholar] [CrossRef]
- Petropoulos, V.I.; Bogeva, E.; Stafilov, T.; Stefova, M.; Siegmund, B.; Pabi, N.; Lankmayr, E. Study of the influence of maceration time and oenological practices on the aroma profile of Vranec wines. Food Chem. 2014, 165, 506–514. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kan, J.; Tang, J.; Cai, Z.; Ji, L. The profile in polyphenols and volatile compounds in alcoholic beverages from different cultivars of mulberry. J. Food Sci. 2012, 77, C430–C436. [Google Scholar]
- Casassa, L.F.; Huff, R.; Steele, N.B. Chemical consequences of extended maceration and post-fermentation additions of grape pomace in Pinot noir and Zinfandel wines from the Central Coast of California (USA). Food Chem. 2019, 300, 125147. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Romano, R.; Sannino, C.; Le Grottaglie, L.; Settanni, L.; Moschetti, G. Evolution of microbiological and chemical parameters during red wine making with extended post-fermentation maceration. Int. J. Food Microbiol. 2014, 171, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Yilmaztekin, M.; Kocabey, N.; Hayaloglu, A.A. Effect of maceration time on free and bound volatiles of red wines from cv. Karaoğlan (Vitis vinifera L.) grapes grown in Arapgir, Turkey. J. Food Sci. 2015, 80, C556–C563. [Google Scholar] [CrossRef] [PubMed]
- Joscelyne, V.L. Consequences of Extended Maceration of Red Wine Colour and Phenolic. Ph.D. Thesis, University of Adelaide, Adelaide, SA, Australia, 2009; 246p. [Google Scholar]
- Frost, S.C.; Blackman, J.W.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H. Extended maceration and cap management impacts on the phenolic, volatile, and sensory profiles of Merlot wine. Am. J. Enol. Vitic. 2018, 69, 360–370. [Google Scholar] [CrossRef]
- Casassa, F.L.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of extended maceration and ethanol concentration on the extraction and evolution of phenolics, colour components and sensory attributes of Merlot wines. Aust. J. Wine Grape Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Beaver, C.W.; Mireles, M.S.; Keller, M.; Riley, W.R.; Harbertson, J.F. Sensory impact of extended maceration and regulated deficit irrigation on Washington State Cabernet Sauvignon wines. Am. J. Enol. Vitic. 2013, 64, 505–514. [Google Scholar] [CrossRef]
- Kocabey, N.; Yilmaztekin, M.; Hayaloglu, A.A. Effect of maceration duration on physicochemical characteristics, organic acid, phenolic compounds and antioxidant activity of red wine from Vitis vinifera L. Karaoglan. J. Food Sci. Technol. 2016, 53, 3557–3565. [Google Scholar] [CrossRef]
- Bestulic, E.; Rossi, S.; Plavsa, T.; Horvat, I.; Lukic, I.; Bubola, M.; Ilak Persuric, A.S.; Jeromel, A.; Radeka, S. Comparison of different maceration and nonmaceration treatments for enhancement of phenolic composition, colour intensity, and taste attributes of Malvazija istarska (Vitis vinifera L.) white wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- Prezioso, I.; Fioschi, G.; Rustioni, L.; Mascellani, M.; Natrella, G.; Venerito, P.; Paradiso, V.M. Influence of prolonged maceration on phenolic compounds, volatile profile and sensory properties of wines from Minutolo and Verdeca, two Apulian white grape varieties. LWT 2024, 192, 115698. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatile compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Martínez-Pérez, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Use of unripe grape wine as a tool for reducing alcohol content and improving the quality and oenological characteristics of red wines. OENO One 2023, 57, 109–119. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Moreno-Olivares, J.D.; Paladines-Quezada, D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Study of aromatic profile of different crosses of Monastrell white wines. J. Sci. Food Agric. 2020, 100, 38–49. [Google Scholar] [CrossRef]
- Issa-Issa, H.; Guclu, G.; Noguera-Artiaga, L.; López-Lluch, D.; Poveda, R.; Kelebek, H.; Carbonell-Barrachina, Á.A. Aroma-active compounds, sensory profile, and phenolic composition of Fondillón. Food Chem. 2020, 316, 126353. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sa, F.; Barros, F.; Araujo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2000, 563, 300–309. [Google Scholar] [CrossRef]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-analysis of the core aroma components of grape and wine aroma. Front. Plant Sci. 2016, 7, 211530. [Google Scholar] [CrossRef]
- Szikszai, A.R. Characteristic volatile compounds of Monastrell wines. Rev. Dr. UMH 2018, 4, 3. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145. [Google Scholar] [CrossRef]
- Lukic, I.; Jedrejcic, N.; Ganic, K.K.; Staver, M.; Persuric, Đ. Phenolic and aroma composition of white wines produced by prolonged maceration and maturation in wooden barrels. Food Technol. Biotechnol. 2015, 53, 407–418. [Google Scholar]
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Impact of various maceration techniques on the phenolic and volatile composition of Chenin Blanc wines. Int. J. Food Sci. Technol. 2016, 51, 2360–2366. [Google Scholar] [CrossRef]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Gunata, Z. Aroma components of cv. Muscat of Bornova wines and influence of skin contact treatment. Food Chem. 2006, 94, 319–326. [Google Scholar] [CrossRef]
- Palomo, E.S.; Gonzalez-Viñas, M.A.; Dıaz-Maroto, M.C.; Soriano-Perez, A.; Perez-Coello, M.S. Aroma potential of Albillo wines and effect of skin-contact treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Aroma compounds in wine. In Grape and Wine Biotechnology; InTech: London, UK, 2016; pp. 267–322. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Wine Grape Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Henschke, P.A. The ‘buttery’ attribute of wine—Diacetyl—Desirability, spoilage and beyond. Int. J. Food Microbiol. 2004, 96, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Lolkema, J.S.; Konings, W.N.; Santos, H. Enzyme basis for pH regulation of citrate and pyruvate metabolism by Leuconostoc oenos. Appl. Environ. Microbiol. 1995, 61, 1303–1310. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Dagan, L. Potentiel Aromatique des Raisins de Vitis vinifera L. cv. Petit Manseng et Gros Manseng. Contribution à L’arôme des Vins de Pays Côtes de Gascogne. Ph.D. Thesis, Ecole Nationale Supérieure Agronomique de Montpellier, Montpellier, France, 2006. [Google Scholar]
- Lytra, G.; Tempere, S.; Zhang, S.; Marchand, S.; De Revel, G.; Barbe, J.C. Olfactory impact of dimethyl sulfide on red wine fruity esters aroma expression in model solution. Oeno One 2014, 48, 75–85. [Google Scholar] [CrossRef]
- Segurel, M.A.; Razungles, A.J.; Riou, C.; Trigueiro, M.G.L.; Baumes, R.L. Ability of possible DMS precursors to release DMS during wine aging and in the conditions of heat-alkaline treatment. J. Agric. Food Chem. 2005, 53, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Samaniego Solis, J.A.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Dimethyl Sulfide (DMS) in Amarone Wines: Influence of Aging, Withering, Grape Variety, and Geographical Origin. J. Agric. Food Chem. 2023, 72, 1978–1984. [Google Scholar] [CrossRef]
- Anocibar-Beloqui, A.; Kotseridis, Y.; Bertrand, A. Détermination de la teneur en sulfure de diméthyle dans quelques vins rouges. J. Int. Sci. Vigne Vin 1996, 30, 167–170. [Google Scholar]
- Etiévant, P.X. Wine. In Volatile Compounds in Foods and Beverages; Maarse, H., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Sánchez-Palomo, E.; Delgado, J.A.; Ferrer, M.A.; Viñas, M.A.G. The aroma of La Mancha Chelva wines: Chemical and sensory characterization. Food Res. Int. 2019, 119, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Q.; Li, Y.; Liu, S.; Tu, Q.; Yuan, C. Characterization of wine volatile compounds from different regions and varieties by HS-SPME/GC-MS coupled with chemometrics. Curr. Res. Food Sci. 2023, 6, 100418. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bencomo, J.J.; Méndez-Siverio, J.J.; Pérez-Trujillo, J.P.; Cacho, J. Effect of skin contact on bound aroma and free volatiles of Listán blanco wine. Food Chem. 2008, 110, 214–225. [Google Scholar] [CrossRef]
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C. Effect of skin contact on the aroma composition of the musts of Vitis vinifera L. cv. Muscat of Bornova and Narince grown in Turkey. Food Chem. 2003, 81, 341–347. [Google Scholar] [CrossRef]
- Barbará, J.A.; Nicolli, K.P.; Souza-Silva, É.A.; Biasoto, A.C.T.; Welke, J.E.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Rapp, A.; Güntert, M.; Ullemeyer, H. Changes in aroma substances during the storage in bottles of white wines of the Riesling variety. Zeit. Leben. Fors. 1985, 180, 109–116. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Barda, N. Effects of maceration length after prefermentative cold soak: Detailed chromatic, phenolic and sensory composition of cabernet sauvignon, malbec and merlot wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Sato, M.; Ueno, N.; Singleton, V.L. Colour and sensory characteristics of Merlot red wines caused by prolonged pomace contact. J. Wine Res. 2000, 11, 7–18. [Google Scholar] [CrossRef]
- Scudamore-Smith, P.D.; Hooper, R.L.; McLaran, E.D. Color and Phenolic Changes of Cabernet Sauvignon Wine Made by Simultaneous Yeast/Bacterial Fermentation and Extended Pomace Contact. Am. J. Enol. Vitic. 1990, 41, 57–67. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; Cazarin, C.B.B.; Corrêa, L.C.; Maróstica, M.R., Jr.; Biasoto, A.C.T.; Behrens, J.H. Influence of maceration time on phenolic compounds and antioxidant activity of the Syrah must and wine. J. Food Biochem. 2017, 42, e12471. [Google Scholar] [CrossRef]
- Noble, P.A.; Dziuba, M.; Jed Harrison, D.; Albritton, W.L. Factors influencing capacitance-based monitoring of microbial growth. J. Microbiol. Methods 1999, 37, 51–64. [Google Scholar] [CrossRef]
- Gil, M.; Quirós, M.; Fort, F.; Morales, P.; Gonzalez, R.; Canals, J.M.; Zamora, F. Influence of grape maturity and maceration length on polysaccharide composition of Cabernet Sauvignon red wines. Am. J. Enol. Vitic. 2012, 66, 393–397. [Google Scholar] [CrossRef]

| Treatments (mg·L−1) | |||||
|---|---|---|---|---|---|
| Volatile Compounds | RI 1 | RT (min) 2 | Control | Extended Maceration | Score 3 |
| Alcohols | |||||
| 2-Methyl-1-propanol | 1191 | 8.6 | 836.2 ± 53.6 a | 325.5 ± 33.0 b | 88.4 |
| 3-Methyl-1-butanol acetate | 1202 | 9.5 | 1872.7 ± 58.4 a | - | 97.1 |
| 2-Ethyl-1-butanol | 1402 | 16.5 | 13.4 ± 0.6 a | - | 82.8 |
| 4-Methyl-1-pentanol | 1447 | 16.7 | 18.0 ± 2.5 a | - | 83.6 |
| (S)-(+)-3-Methyl-1-pentanol | 1457 | 17.1 | 26.0 ± 6.5 a | 31.6 ± 2.2 a | 87.6 |
| 1-Hexanol | 1463 | 18.1 | 715.9 ± 31.6 a | 603.4 ± 53.4 b | 94.1 |
| 4-Methyl-1-hexanol | 1514 | 21.5 | 16.8 ± 0.9 a | 18.0 ± 1.6 a | 85.8 |
| 1-Heptanol | 1565 | 21.5 | 16.6 ± 0.9 a | - | 85.4 |
| 2-Ethyl-1-hexanol | 1599 | 22.6 | 17.2 ± 1.5 b | 42.8 ± 6.0 a | 83.2 |
| (S)-3-Ethyl-4-methylpentanol | 1609 | 23.1 | 11.6 ± 1.4 a | - | 85.3 |
| 2,3-Butanediol | 1625 | 24.3 | 292.3 ± 45.8 a | 167.2 ± 20.4 b | 95.2 |
| [S-(R*,R*)]-2,3-Butanediol | 1628 | 25.5 | 140.7 ± 32.9 a | 27.4 ± 4.2 b | 91.5 |
| Phenylethyl alcohol | 2112 | 34.5 | 1642.7 ± 87.8 a | 1781.2 ± 258.1 a | 95.9 |
| Total alcohols (mg·L−1) | 5934.3 ± 402.3 a | 3322.6 ± 378.8 b | |||
| Esters | |||||
| Methyl acetate | 964 | 2.6 | 23.6 ± 2.4 a | 17.4 ± 0.2 b | 88.0 |
| Ethyl orthoformate | 1014 | 3.2 | - | 51.0 ± 14.0 a | 87.0 |
| Ethyl propanoate | 1047 | 4.4 | 240.0 ± 83.6 a | 82.6 ± 12.2 b | 92.0 |
| Ethyl isobutyrate | 1071 | 4.6 | 215.0 ± 35.8 a | 263.4 ± 34.6 a | 94.6 |
| Isobutyl acetate | 1107 | 5.8 | 67.6 ± 5.2 a | 32.4 ± 2.0 b | 92.2 |
| Ethyl butanoate | 1128 | 6.5 | 242.0 ± 9.2 a | 196.4 ± 8.0 b | 96.5 |
| Ethyl 2-methylbutanoate | 1173 | 7.0 | 99.1 ± 8.0 b | 128.2 ± 9.6 a | 94.3 |
| Ethyl 3-methylbutanoate | 1175 | 7.6 | 228.4 ± 31.6 a | 242.0 ± 13.0 a | 95.3 |
| Isoamyl acetate | 1215 | 9.5 | - | 647.6 ± 40.0 a | 95.3 |
| Ethyl hexanoate | 1332 | 13.6 | 2466.4 ± 198.0 a | 1914.2 ± 128.0 b | 97.1 |
| Ethyl 2-hydroxypropionate | 1349 | 17.8 | 730.2 ± 98.8 a | 274.0 ± 8.2 b | 92.1 |
| Ethyl octanoate | 1440 | 20.6 | 1872.0 ± 157.0 a | 1278.0 ± 62.0 b | 97.5 |
| Isopentyl hexanoate | 1460 | 21.4 | - | 5.4 ± 0.2 a | 82.8 |
| Ethyl decanoate | 1610 | 26.9 | 169.0 ± 16.0 b | 201.6 ± 8.4 a | 91.1 |
| Diethyl succinate | 1690 | 28.1 | 915.4 ± 38.6 a | 1094.8 ± 174.4 a | 97.7 |
| Total esters (mg·L−1) | 7268.7 ± 684.2 a | 6429.0 ± 514.8 a | |||
| Acids | |||||
| 2- methylpropanoic acid (isobutyric acid) | 1581 | 25.7 | - | 15.4 ± 2.2 a | 85.3 |
| Butanoic acid | 1637 | 27.4 | 12.2 ± 1.4 a | 14.0 ± 9.6 a | 81.9 |
| 3-methylbutanoic acid (isovaleric acid) | 1680 | 28.5 | 46.2 ± 4.6 a | 38.6 ± 6.2 a | 81.5 |
| Hexanoic acid | 1849 | 28.5 | 45.0 ± 3.0 a | - | 86.3 |
| 2-methyl-2-phenylethyl ester—Propanoic acid | 1877 | 31.9 | - | 10.0 ± 0.2 a | 80.1 |
| Pentanoic acid | 1944 | 33.3 | 46.8 ± 6.0 a | 25.2 ± 6.0 b | 82.9 |
| Octanoic acid | 2050 | 38.6 | 44.6 ± 7.0 a | 16.0 ± 3.6 b | 82.7 |
| Total acids (mg·L−1) | 194.8 ± 22.0 a | 119.2 ± 27.8 b | |||
| Aldehydes and Ketones | |||||
| 2,3-Butanedione | 955 | 4.9 | - | 10.8 ± 6.0 a | 82.0 |
| 2-Butanone | 1115 | 8.3 | - | 4.0 ± 2.6 a | 83.7 |
| 2-Octanone | 1287 | 15.4 | 22.8 ± 4.8 a | - | 83.6 |
| Total ketones (mg·L−1) | 22.8 ± 4.8 a | 14.8 ± 8.6 a | |||
| Sulphur compounds | |||||
| Dimethyl sulphide | 857 | 2.2 | 11.2 ± 1.0 a | 8.4 ± 0.4 b | 90.4 |
| Furfural | 1464 | 21.7 | 133.6 ± 26.0 a | 101.8 ± 6.4 b | 95.0 |
| Total sulphur compounds (mg·L−1) | 144.8 ± 27.0 a | 110.2 ± 6.8 b | |||
| Terpenes | |||||
| D-Limonene | 1204 | 12.1 | 34.4 ± 2.0 a | - | 81.5 |
| Eugenol | 2167 | 40.7 | 17.4 ± 4.4 a | - | 83.6 |
| Total terpenes (mg·L−1) | 51.8 ± 6.4 a | - | |||
| Others | |||||
| Butyrolactone | 1626 | 26.7 | 42.8 ± 4.4 a | 34.2 ± 2.6 b | 89.6 |
| Year | Triangular Test | Identifications | Preferences |
|---|---|---|---|
| 2024 | Control vs. EM | 10/18 * | EM (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Moreno, A.; Toledo-Gil, R.; Bautista-Ortin, A.B.; Gómez-Plaza, E.; Yuste, J.E.; Vallejo, F. Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine. Fermentation 2024, 10, 343. https://doi.org/10.3390/fermentation10070343
Martínez-Moreno A, Toledo-Gil R, Bautista-Ortin AB, Gómez-Plaza E, Yuste JE, Vallejo F. Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine. Fermentation. 2024; 10(7):343. https://doi.org/10.3390/fermentation10070343
Chicago/Turabian StyleMartínez-Moreno, Alejandro, Rosa Toledo-Gil, Ana Belén Bautista-Ortin, Encarna Gómez-Plaza, José Enrique Yuste, and Fernando Vallejo. 2024. "Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine" Fermentation 10, no. 7: 343. https://doi.org/10.3390/fermentation10070343
APA StyleMartínez-Moreno, A., Toledo-Gil, R., Bautista-Ortin, A. B., Gómez-Plaza, E., Yuste, J. E., & Vallejo, F. (2024). Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine. Fermentation, 10(7), 343. https://doi.org/10.3390/fermentation10070343






