Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strain Inoculation Quantity and Inoculation Plan
2.3. Preparation of Simulated Juice
2.4. Analytical Method
2.5. Statistical Analysis
3. Results
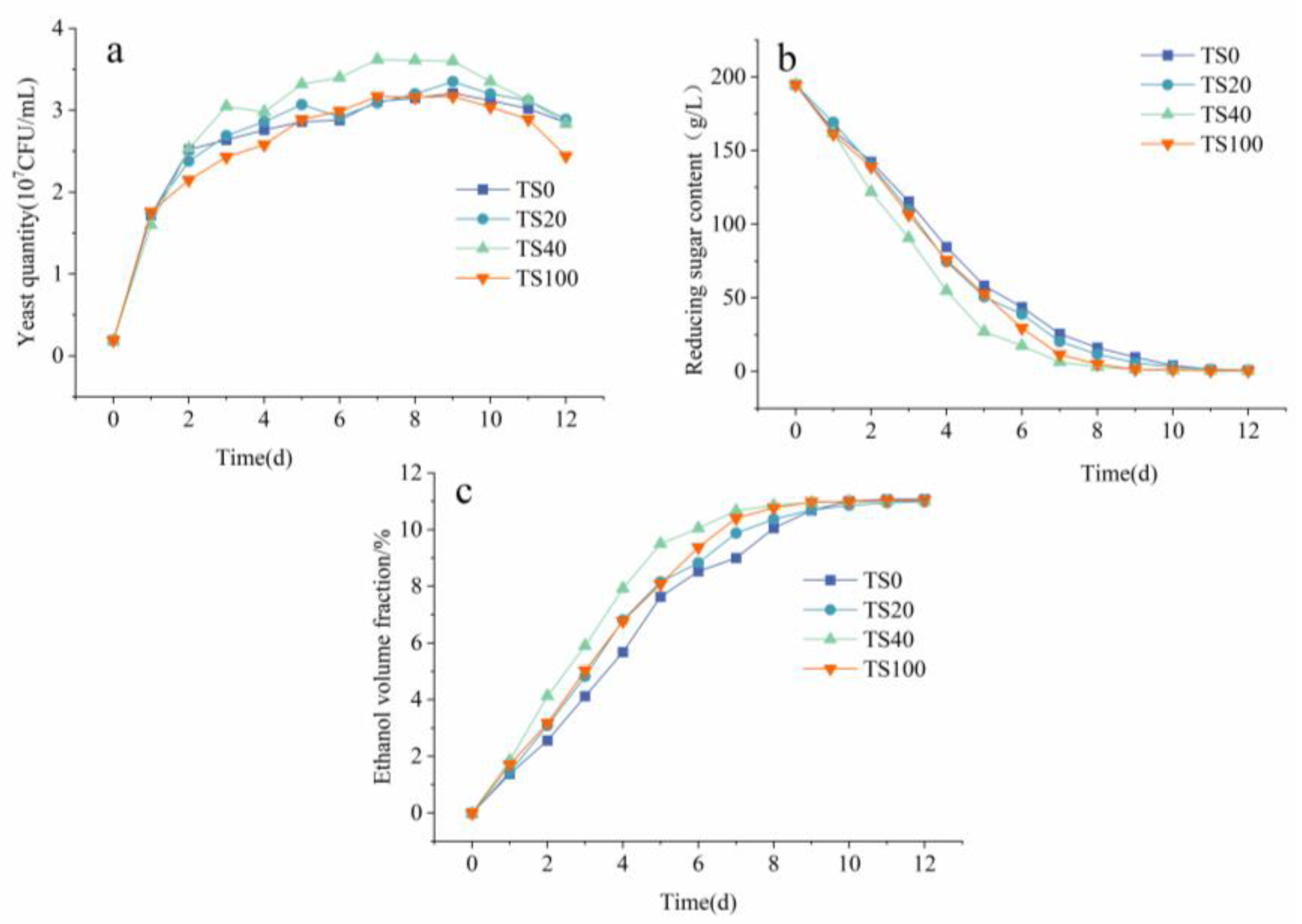
3.1. Changed Trend Chart of Yeast Quantity, Reducing Sugar Content, and Ethanol Volume Fraction during Fermentation
3.2. Kinetic Model of Yeast Growth
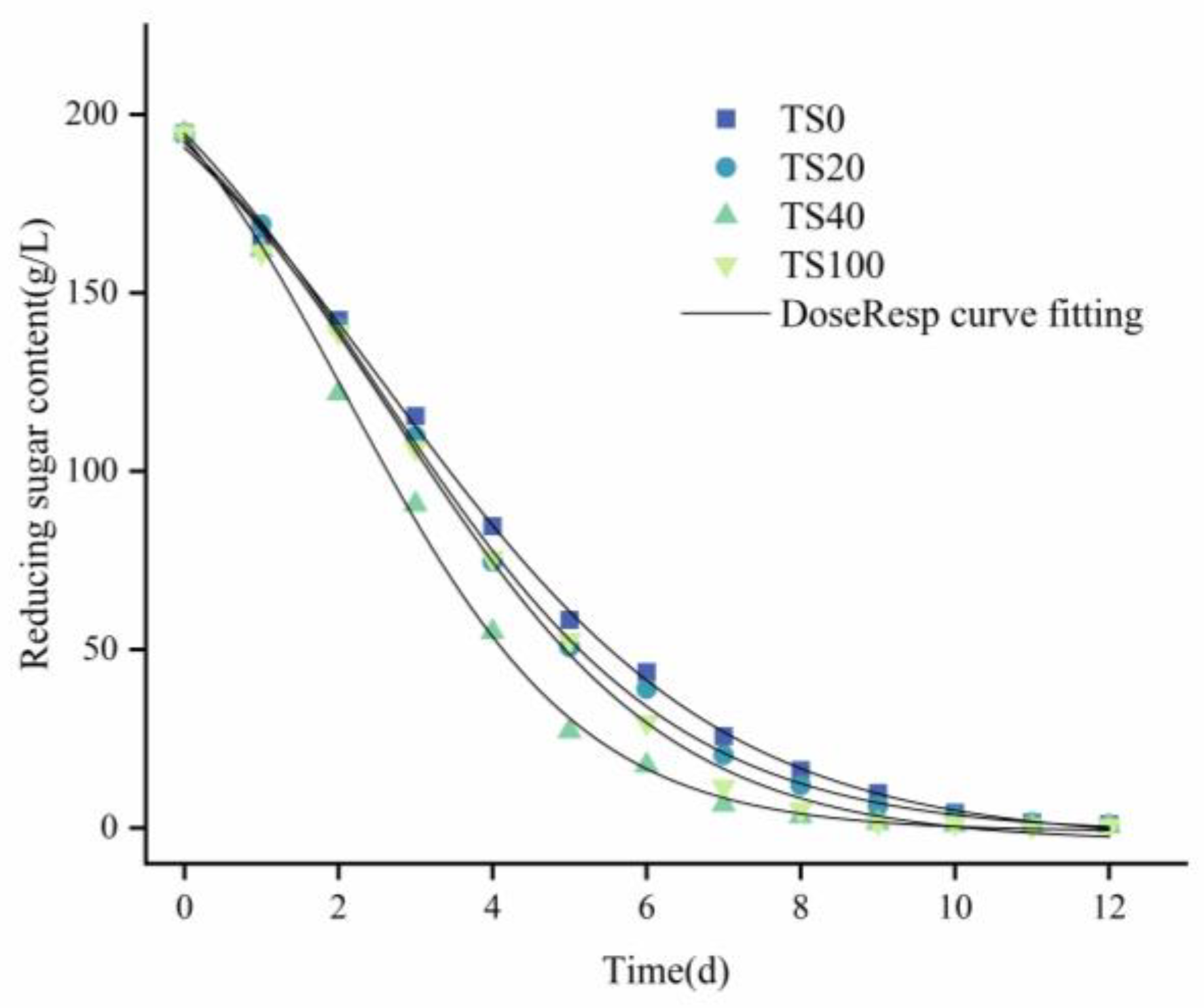
3.3. Kinetic Model of Reducing Sugar Consumption
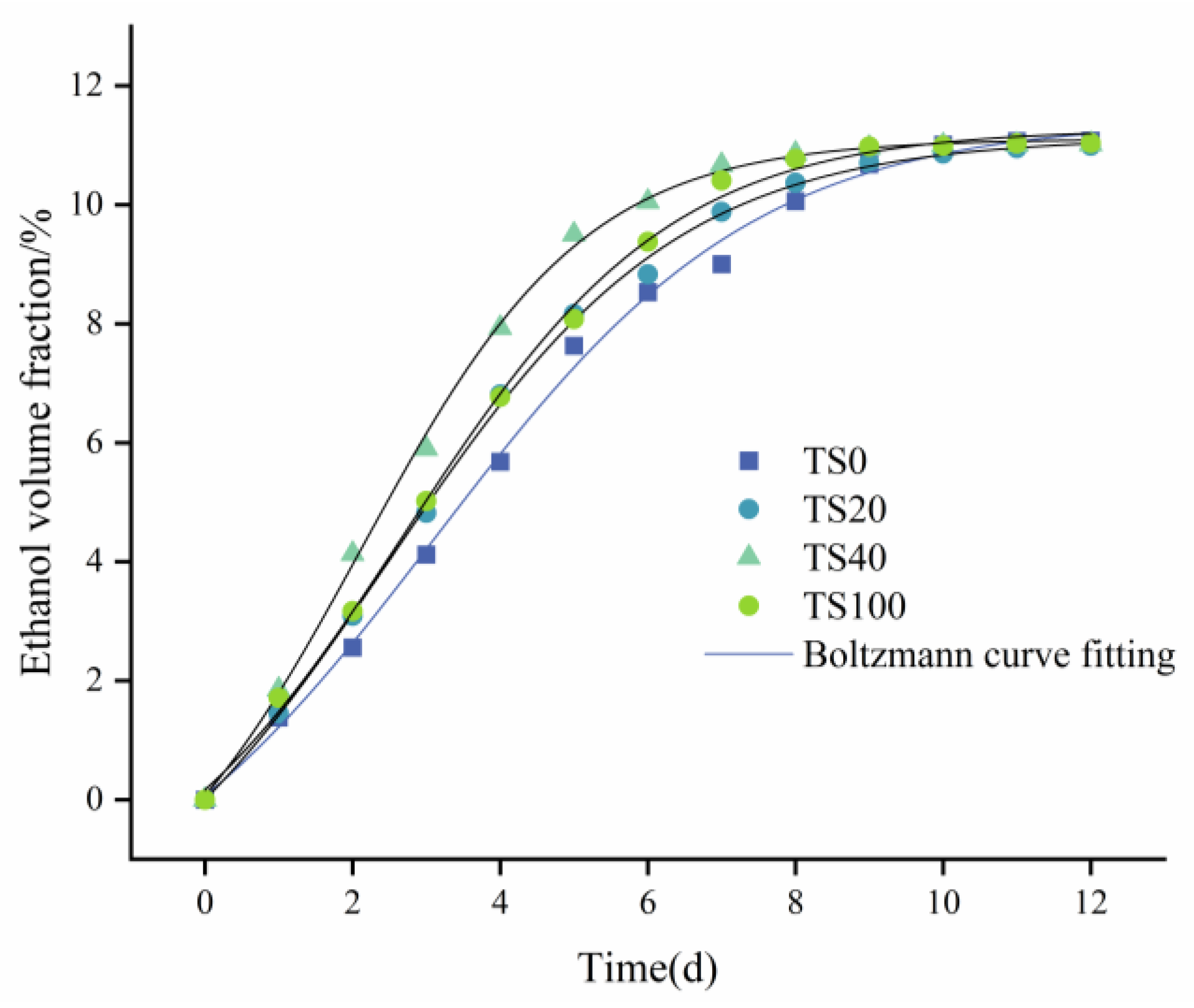
3.4. Kinetic Model of Ethanol Formation
3.5. Volatile Aroma Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological Importance of Torulaspora delbrueckii: From the Obscurity to the Spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef] [PubMed]
- Jianli, Z.; Chuqi, T.; Shuliang, Z.; Liangbo, L.; Yuangen, W.; Wenhua, Y.; Jean Damascene, H.; Jiang, Z.; Wenwen, Z.; Dan, D.; et al. Enhancement of pyranoanthocyanin formation in blueberry wine with non-Saccharomyces yeasts. Food Chem. 2024, 438, 137956. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Zou, S.; Dong, L.; Lin, X.; Chen, Y.; Zhang, S.; Ji, C.; Liang, H. Chemical Composition and Flavor Characteristics of Cider Fermented with Saccharomyces cerevisiae and Non-Saccharomyces cerevisiae. Foods 2023, 12, 3565. [Google Scholar] [CrossRef] [PubMed]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarede, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-inoculations of Lachancea thermotolerans with different Hanseniaspora spp.: Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef]
- Silva-Sousa, F.; Fernandes, T.; Pereira, F.; Rodrigues, D.; Rito, T.; Camarasa, C.; Franco-Duarte, R.; Sousa, M.J. Torulaspora delbrueckii Phenotypic and Metabolic Profiling towards Its Biotechnological Exploitation. J. Fungi 2022, 8, 569. [Google Scholar] [CrossRef]
- Belda, I.; Navascues, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-Sulfanylhexan-1-ol Production in Sequential Mixed Fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae Reveals a Situation of Synergistic Interaction between Two Industrial Strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Luan, Y.; Duan, C.Q.; Yan, G.L. Use of Torulaspora delbrueckii Co-fermentation With Two Saccharomyces cerevisiae Strains With Different Aromatic Characteristic to Improve the Diversity of Red Wine Aroma Profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bencomo, J.J.; Andújar-Ortiz, I.; Moreno-Arribas, M.V.; Simó, C.; González, J.; Chana, A.; Dávalos, J.; Pozo-Bayón, M.Á. Impact of Glutathione-Enriched Inactive Dry Yeast Preparations on the Stability of Terpenes during Model Wine Aging. J. Agric. Food Chem. 2014, 62, 1373–1383. [Google Scholar] [CrossRef]
- Naselli, V.; Prestianni, R.; Badalamenti, N.; Matraxia, M.; Maggio, A.; Alfonzo, A.; Gaglio, R.; Vagnoli, P.; Settanni, L.; Bruno, M.; et al. Improving the Aromatic Profiles of Catarratto Wines: Impact of and Glutathione-Rich Inactivated Yeasts. Antioxidants 2023, 12, 439. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Cao, Y.; Qi, Y.; Yang, K.; Wei, X.; Xu, Y.; Fan, M. Effect of glutathione-enriched inactive dry yeast on color, phenolic compounds, and antioxidant activity of kiwi wine. J. Food Process. Preserv. 2020, 44, e14347. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Andujar-Ortiz, I.; Alcaide-Hidalgo, J.M.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Characterization of Commercial Inactive Dry Yeast Preparations for Enological Use Based on Their Ability To Release Soluble Compounds and Their Behavior toward Aroma Compounds in Model Wines. J. Agric. Food Chem. 2009, 57, 10784–10792. [Google Scholar] [CrossRef] [PubMed]
- Andujar-Ortiz, I.; Pozo-Bayon, M.A.; Garcia-Ruiz, A.; Moreno-Arribas, M.V. Role of specific components from commercial inactive dry yeast winemaking preparations on the growth of wine lactic acid bacteria. J. Agric. Food Chem. 2010, 58, 8392–8399. [Google Scholar] [CrossRef]
- Alfonzo, A.; Prestianni, R.; Gaglio, R.; Matraxia, M.; Maggio, A.; Naselli, V.; Craparo, V.; Badalamenti, N.; Bruno, M.; Vagnoli, P.; et al. Effects of different yeast strains, nutrients and glutathione-rich inactivated yeast addition on the aroma characteristics of Catarratto wines. Int. J. Food Microbiol. 2021, 360, 109325. [Google Scholar] [CrossRef]
- Liu, D.; Qi, Y.M.; Zhao, N.; Cao, Y.F.; Xu, J.N.; Fan, M.T. Multivariate analysis reveals effect of glutathione-enriched inactive dry yeast on amino acids and volatile components of kiwi wine. Food Chem. 2020, 329, 127086. [Google Scholar] [CrossRef]
- Andújar-Ortiz, I.; Chaya, C.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.A. Impact of Using New Commercial Glutathione Enriched Inactive Dry Yeast Oenological Preparations on the Aroma and Sensory Properties of Wines. Int. J. Food Prop. 2014, 17, 987–1001. [Google Scholar] [CrossRef]
- Gabrielli, M.; Aleixandre-Tudo, J.L.; Kilmartin, P.A.; Sieczkowski, N.; du Toit, W.J. Additions of Glutathione or Specific Glutathione-rich Dry inactivated Yeast Preparation (DYP) to Sauvignon blanc Must: Effect on Wine Chemical and Sensory Composition. S. Afr. J. Enol. Vitic. 2017, 38, 18–28. [Google Scholar] [CrossRef]
- Ortiz, I.A.; Bayón, M.Á.P.; Garrido, I.; Álvarez, P.J.M.; Bartolomé, B.; Arribas, M.V.M. Effect of using glutathione-enriched inactive dry yeast preparations on the phenolic composition of rosé Grenache wines during winemaking. OENO One 2012, 46, 241–351. [Google Scholar] [CrossRef]
- Giménez, P.; Just-Borras, A.; Pons, P.; Gombau, J.; Heras, J.M.; Sieczkowski, N.; Canals, J.M.; Zamora, F. Biotechnological tools for reducing the use of sulfur dioxide in white grape must and preventing enzymatic browning: Glutathione; inactivated dry yeasts rich in glutathione; and bioprotection with Metschnikowia Pulcherrima. Eur. Food Res. Technol. 2023, 249, 1491–1501. [Google Scholar] [CrossRef]
- Nioi, C.; Lisanti, M.T.; Meunier, F.; Redon, P.; Massot, A.; Moine, V. Antioxidant activity of yeast derivatives: Evaluation of their application to enhance the oxidative stability of white wine. LWT-Food Sci. Technol. 2022, 171, 114116. [Google Scholar] [CrossRef]
- Xu, W.; Liu, B.; Wang, C.; Kong, X. Organic cultivation of grape affects yeast succession and wine sensory quality during spontaneous fermentation. LWT 2020, 120, 108894. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wu, G.; Xu, F.; Hu, R.; Tan, L. Kinetics model of jackfruit wine in batch fermentation. Food Ferment. Ind. 2021, 47, 74–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, D.; Niu, G.; Wei, W.; Yan, F. Fermentation kinetics and antioxidant activity of sea buckthorn wine. Food Ferment. Ind. 2019, 45, 53–58. [Google Scholar] [CrossRef]
- Chen, X.; Diao, T.; Lai, X.; Wei, X.; Leng, Y.; Ma, Y. Effects of glutathione-enriched inactive dry yeast addition during different brewing stages on the qualities of pear wine. Food Sci. 2022, 44, 132–142. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Hu, K.; Xu, Y.-H.; Mei, W.-C.; Tao, Y.-S. Biomass suppression of Hanseniaspora uvarum by killer Saccharomyces cerevisiae highly increased fruity esters in mixed culture fermentation. LWT 2020, 132, 109839. [Google Scholar] [CrossRef]
- GB 5009.225-2023; National standard for Food Safety-Determination of Ethanol Concentration in Wine and Edible Alcohol. State Health and Wellness Commission, State Administration of Market Supervision: Beijing, China, 2023.
- Xu, J.N.; Qi, Y.M.; Zhang, J.; Liu, M.M.; Wei, X.Y.; Fan, M.T. Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xu, Y.; An, C.; Peng, B.; Wang, J. Impacts of glutathione-enriched inactive dry yeast preparations on the quality of ‘viognier’ dry white wine. Food Ferment. Ind. 2019, 45, 157–164. [Google Scholar] [CrossRef]
- Qin, Z.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2018, 105, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hu, K.; Chen, S.; Xiong, S.; Tao, Y. Increase in Fruity Ester Production during Spine Grape Wine Fermentation by Goal-Directed Amino Acid Supplementation. Fermentation 2021, 7, 231. [Google Scholar] [CrossRef]
- Silva, M.; Pontes, A.; Franco-Duarte, R.; Soares, P.; Sampaio, J.P.; Sousa, M.J.; Brito, P.H. A glimpse at an early stage of microbe domestication revealed in the variable genome of Torulaspora delbrueckii, an emergent industrial yeast. Mol. Ecol. 2023, 32, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Rito, T.; Vieira, D.; Fernandes, T.; Pais, C.; Sousa, M.J.; Soares, P.; Franco-Duarte, R. Improvement of Torulaspora delbrueckii Genome Annotation: Towards the Exploitation of Genomic Features of a Biotechnologically Relevant Yeast. J. Fungi 2021, 7, 287. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Jiang, D.; Zhang, Y.; Zhang, S.; Sun, S. Effect of Saccharomyces cerevisiae, Torulaspora delbrueckii and malolactic fermentation on fermentation kinetics and sensory property of black raspberry wines. Food Microbiol. 2020, 91, 103551. [Google Scholar] [CrossRef]
- Fairbairn, S.; Engelbrecht, L.; Setati, M.E.; du Toit, M.; Bauer, F.F.; Divol, B.; Rossouw, D. Combinatorial analysis of population dynamics, metabolite levels and malolactic fermentation in Saccharomyces cerevisiae/ Lachancea thermotolerans mixed fermentations. Food Microbiol. 2021, 96, 103712. [Google Scholar] [CrossRef]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef]
- Kosel, J.; Čadež, N.; Schuller, D.; Carreto, L.; Franco-Duarte, R.; Raspor, P. The influence of Dekkera bruxellensis on the transcriptome of Saccharomyces cerevisiae and on the aromatic profile of synthetic wine must. FEMS Yeast Res. 2017, 17, fox018. [Google Scholar] [CrossRef] [PubMed]
- Legras, J.L.; Galeote, V.; Bigey, F.; Camarasa, C.; Marsit, S.; Nidelet, T.; Sanchez, I.; Couloux, A.; Guy, J.; Franco-Duarte, R.; et al. Adaptation of S. cerevisiae to Fermented Food Environments Reveals Remarkable Genome Plasticity and the Footprints of Domestication. Mol. Biol. Evol. 2018, 35, 1712–1727. [Google Scholar] [CrossRef] [PubMed]
- Galafassi, S.; Toscano, M.; Vigentini, I.; Piškur, J.; Compagno, C. Osmotic stress response in the wine yeast Dekkera bruxellensis. Food Microbiol. 2013, 36, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Wenwen, Z.; Mengyang, B.; Zufang, W.; Peifang, W.; Yingjie, M. Research Progression in Mixed Culture Fermentation of Fruit Wine with Yeasts. Food Sci. 2018, 39, 252–259. [Google Scholar] [CrossRef]
- Sun, M.; Shi, F.; Wang, X. The Role of Saccharomyces cerevisiae NAD(H) Kinase Pos5p on the Defense of Oxidative Stress. Microbiol. China 2010, 37, 1740–1746. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; Du Toit, W.J. Influence of yeast strain, extended lees contact and nitrogen supplementation on glutathione concentration in wine. Aust. J. Grape Wine Res. 2013, 19, 161–170. [Google Scholar] [CrossRef]
- Lee, J.-C.; Straffon, M.J.; Jang, T.-Y.; Higgins, V.J.; Grant, C.M.; Dawes, I.W. The essential and ancillary role of glutathione in Saccharomyces cerevisiae analysed using a grande gsh1 disruptant strain. FEMS Yeast Res. 2001, 1, 57–65. [Google Scholar] [CrossRef]
- Tello-Padilla, M.F.; Perez-Gonzalez, A.Y.; Canizal-García, M.; González-Hernández, J.C.; Cortes-Rojo, C.; Olivares-Marin, I.K.; Madrigal-Perez, L.A. Glutathione levels influence chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. Yeast 2018, 35, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bencomo, J.J.; Andújar-Ortiz, I.; Sánchez-Patán, F.; Moreno-Arribas, M.V.; Pozo-Bayón, M.A. Fate of the glutathione released from inactive dry yeast preparations during the alcoholic fermentation of white musts. Aust. J. Grape Wine Res. 2016, 22, 46–51. [Google Scholar] [CrossRef]
- Andújar-Ortiz, I.; Pozo-Bayón, M.A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Rodríguez-Bencomo, J.J. Reversed-phase high-performance liquid chromatography-fluorescence detection for the analysis of glutathione and its precursor γ-glutamyl cysteine in wines and model wines supplemented with oenological inactive dry yeast preparations. Food Anal. Methods 2012, 5, 154–161. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Stander, M.A.; Du Toit, W.J. Assessment of glutathione levels in model solution and grape ferments supplemented with glutathione-enriched inactive dry yeast preparations using a novel UPLC-MS/MS method. Food Addit. Contam. Part. A 2013, 30, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Etschmann, M.M.W.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cheng, Z.; Fan, M. Effect of glutathione addition on the aroma components of stored kiwi fruit wine. Sci. Technol. Food Ind. 2017, 08, 183–188. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Marcon, Â.R.; Carnieli, G.J.; Vanderlinde, R. Effect of glutathione addition in sparkling wine. Food Chem. 2014, 159, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Callejón, R.M.; Tesfaye, W.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Volatile compounds in red wine vinegars obtained by submerged and surface acetification in different woods. Food Chem. 2009, 113, 1252–1259. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Zhu, D. Fermentation kinetics of blackcurrant fruit wine. China Brew. 2020, 39, 125–128. [Google Scholar] [CrossRef]
- Moimenta, A.R.; Henriques, D.; Minebois, R.; Querol, A.; Balsa-Canto, E. Modelling the physiological status of yeast during wine fermentation enables the prediction of secondary metabolism. Microb. Biotechnol. 2023, 16, 847–861. [Google Scholar] [CrossRef]

| Treatment Groups | Models | ||
|---|---|---|---|
| Logistic | SGompertz | ||
| Fitting equations | TS0 | ||
| TS20 | |||
| TS40 | |||
| TS100 | |||
| R2 | TS0 | 0.99198 | 0.97062 |
| TS20 | 0.99412 | 0.97062 | |
| TS40 | 0.99353 | 0.97928 | |
| TS100 | 0.99600 | 0.94738 | |
| Treatment Groups | Models | |||
|---|---|---|---|---|
| Logistic | DoseResp | Boltzmann | ||
| Fitting equations | TS0 | |||
| TS20 | ||||
| TS40 | ||||
| TS100 | ||||
| R2 | TS0 | 0.99601 | 0.99901 | 0.99901 |
| TS20 | 0.99738 | 0.99920 | 0.99920 | |
| TS40 | 0.99456 | 0.99900 | 0.99900 | |
| TS100 | 0.99214 | 0.99781 | 0.99781 | |
| Treatment Groups | Models | ||
|---|---|---|---|
| Logistic | Boltzmann | ||
| Fitting equations | TS0 | ||
| TS20 | |||
| TS40 | |||
| TS100 | |||
| R2 | TS0 | 0.99586 | 0.99780 |
| TS20 | 0.99740 | 0.99921 | |
| TS40 | 0.99462 | 0.99902 | |
| TS100 | 0.99347 | 0.99844 | |
| No | RI | Aroma Compounds | Mass Contents/(μg/L) | |||
|---|---|---|---|---|---|---|
| TS0 | TS20 | TS40 | TS100 | |||
| 1 | 714 | Acetaldehyde | nd | 15.18 ± 0.38 b | 18.04 ± 0.27 c | 18.43 ± 0.58 c |
| 2 | 880 | Ethyl acetate | 1661.96 ± 0.12 a | 1980.23 ± 0.21 d | 1756.53 ± 0.08 c | 1719.37 ± 0.09 b |
| 3 | 898 | Acetal | 50.25 ± 1.27 a | 91.15 ± 0.89 b | nd | nd |
| 4 | 1094 | Isobutanol | 165.67 ± 0.19 a | 185.94 ± 0.35 b | 195.88 ± 0.11 c | 198.75 ± 0.24 d |
| 5 | 1126 | Isoamyl acetate | 75.62 ± 0.13 b | 46.81 ± 0.1 a | 93.14 ± 0.16 c | 113.84 ± 0.12 d |
| 6 | 1211 | Isoamyl alcohol | 2222.00 ± 1.56 a | 2449.52 ± 2.54 b | 2491.72 ± 1.35 c | 2578.77 ± 1.69 d |
| 7 | 1227 | Ethyl hexanoate | 68.96 ± 0.05 a | 96.22 ± 0.08 d | 82.62 ± 0.11 b | 84.07 ± 0.09 c |
| 8 | 1283 | 2-Octanone | 22.05 ± 0.21 b | 21.20 ± 0.31 a | 21.44 ± 0.29 a | 22.19 ± 0.19 b |
| 9 | 1359 | N-hexanol | nd | 39.07 ± 1.05 a | 40.18 ± 0.33 b | 41.73 ± 0.38 c |
| 10 | 1382 | Hexyl formate | 36.22 ± 0.63 | nd | nd | nd |
| 11 | 1441 | Ethyl octanoate | 65.37 ± 0.19 a | 65.46 ± 0.15 a | 69.97 ± 0.42 b | 69.77 ± 0.23 b |
| 12 | 1528 | Benzaldehyde | 10.14 ± 0.28 a | 10.18 ± 0.19 a | 20.67 ± 0.24 c | 15.14 ± 0.14 b |
| 13 | 1541 | Ethyl nonanoate | nd | 22.68 ± 0.004 | nd | nd |
| 14 | 1564 | 1-Octanol | nd | 16.11 ± 0.38 b | nd | 14.43 ± 0.65 a |
| 15 | 1580 | 2,3-Butanediol | 32.72 ± 0.64 b | nd | 13.96 ± 0.35 a | 13.37 ± 0.28 a |
| 16 | 1643 | Ethyl decanoate | 27.68 ± 0.19 b | 14.64 ± 0.21 a | 34.42 ± 0.15 c | 35.97 ± 0.31 d |
| 17 | 1702 | 3-methylthiopropyl alcohol | 12.65 ± 0.39 a | 13.12 ± 0.24 b | 17.20 ± 0.15 d | 14.47 ± 0.39 c |
| 18 | 1826 | Phenylethyl acetate | 66.06 ± 0.35 a | 74.51 ± 0.54 c | 71.39 ± 1.02 b | 88.49 ± 0.18 d |
| 19 | 1847 | Ethyl Laurate | 10.20 ± 0.38 b | nd | 13.65 ± 0.26 c | 29.86 ± 0.24 d |
| 20 | 1923 | Phenylethanol | 1705.21 ± 1.25 a | 2398.63 ± 1.89 b | 2466.49 ± 2.18 c | 2682.46 ± 1.64 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Yu, K.; Xiao, X.; Wei, Z.; Xiong, R.; Du, Y.; Li, Y.; Ma, Y. Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast. Fermentation 2024, 10, 329. https://doi.org/10.3390/fermentation10070329
Xie L, Yu K, Xiao X, Wei Z, Xiong R, Du Y, Li Y, Ma Y. Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast. Fermentation. 2024; 10(7):329. https://doi.org/10.3390/fermentation10070329
Chicago/Turabian StyleXie, Liming, Kangjie Yu, Xiongjun Xiao, Ziyun Wei, Rong Xiong, Yong Du, Yajun Li, and Yi Ma. 2024. "Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast" Fermentation 10, no. 7: 329. https://doi.org/10.3390/fermentation10070329
APA StyleXie, L., Yu, K., Xiao, X., Wei, Z., Xiong, R., Du, Y., Li, Y., & Ma, Y. (2024). Study on the Kinetic Model of Mixed Fermentation by Adding Glutathione-Enriched Inactive Dry Yeast. Fermentation, 10(7), 329. https://doi.org/10.3390/fermentation10070329





