1. Introduction
In living organisms, oxidation–reduction systems are playing so intimate and so essential a part that life itself might be defined as a continuous oxidation–reduction reaction. It is not surprising, therefore, that theoretical speculations and experimental studies on oxidation and reduction processes in animals and plants have been actively pursued since the isolation of oxygen over 150 years ago by Hewitt (1950) [
1].
Oxidation–reduction potential began to be used in winemaking in 1932 and was used to explain many phenomena and interventions in the ripening and aging of wine. Oxidizing and reducing substances are found in a certain balance in wine. The optimal Eh value of the wine is the value at which the taste, aroma, and quality of the wine are optimally formed in the wine. The Eh value of wine is specific for each variety [
2].
Knowing the value of the redox potential of the measured sample gives us general information about the redox reactions that are currently taking place in the wine and affect the quality and stability of the wine. In general, wine has a reductive character. A variety of chemical, enzymatic, and biological processes in wine are related to the reduction–oxidation state of the wine. In wine technology, on the one hand, redox potential can be used as a criterion for the addition of sulfuric (IV) acid to must or wine after fermentation, and on the other hand, it can be used as a physiological factor important for the control and regulation of condensation and polymerization of phenolic components [
2,
3].
Wine is a complex of oxidation and reduction reactions between individual compounds. Various organic and inorganic redox systems can be distinguished in wine. A redox system of an inorganic nature represents a transition from a reduced to an oxidized form with the release of electrons, and by accepting electrons, it returns to the reduced form. The most common are compounds of heavy metals—iron, copper, zinc, and their salts, which under certain conditions pass into the oxidized state and vice versa back into the reduced state. Ethanol, organic acids, and polyphenols represent redox systems of an organic nature. These are reduced forms and their oxidized forms are acetaldehyde and acetic acid, keto acids, dehydroascorbic acid, and quinones. Redox systems of an organic nature pass from one form to another. These oxidation–reduction reactions take place under the influence of enzymes and depending on the chemistry of the medium [
2].
The value and change of the redox potential give us information about the following [
3]:
Course of the reaction: whether oxidation or reduction takes place;
An insight into the current state of the wine, which we can compare with other wines;
The value of the redox potential in must and wine is important for the development or inhibition of yeast growth;
Deposits are formed at a certain redox potential, especially if metal ions are present.
In grape must, the value of the measured redox potential represents information about the oxidative state of the fermentation substrate which is an extremely important factor for the further course of yeast metabolism and thus for a series of chemical, enzymatic, and biological processes that take place. The information on the oxidative–reductive state of the must gives us the possibility of regulating the process in the direction of ensuring a favorable environment for the growth and reproduction of yeasts, which leads to the quality of the wine.
2. Theory of Redox Potential
Oxidation is a process in which a substance, molecule, or ion loses or gives up electrons. Reduction, on the other hand, is a process in which a substance, molecule, or ion is involved in taking up a number of electrons. Whenever one substance is oxidized in a system, another substance must be reduced. However, since free electrons never exist in any noteworthy concentration, reduction and oxidation reactions are always coupled together, complementary to each other, so that one reaction releases just as many electrons as the other one consumes. Thus, a pair of reactions always take part in such a process. These simultaneous and complementary reduction and oxidation processes are generally known as redox reactions [
4].
The oxidation potential is related to the electron activity in solution
E° is the standard redox potential of a 50% reduced substance, based on a standard hydrogen electrode. Equation (1) is well known as the Nernst equation. This equation expresses the information on the redox potential of the oxido-reduction reaction. E° is the potential when all of the activities of the products and the reactants would be 1.
Oxidation and reduction reactions in homogenous systems are always complementary in reaction pairs, where free electrons are not existing. In each reaction, the same number of electrons is released and consumed [
4].
For more complex redox systems with a large number of oxidants and reductants that could interact between themselves, the following scheme is used:
The redox potential of a such system could be defined as the following:
where
(Equation (4)) is a product of all the oxidant and reductant activities present in the system [
5].
The potential values measured are dependent on pH, so that in each case, measurements of redox potential should be accompanied by a statement of the pH value at which they were taken. In general, a pH variation of one unit (e.g., from pH 7.0 to pH 6.0) causes the potential to become more positive by 57.7 mV. Clark and Cohen (1923) introduced the concept of rH in order to eliminate this pH dependence of the potential by calculation. The rH value is the negative logarithm of the partial pressure of gaseous hydrogen pH
2: an rH of 0 corresponds to a pH
2 of 1 atm, and an rH of 10 corresponds to a pH
2 of 10
−10 atm, at T = 25 °C [
6].
Later, it was seen that the assumptions made were not correct in every case. Firstly, the value taken as a basis by Clark varied if the reduced phase dissociated to yield hydrogen ions in the alteration of pH. An example of this is methylene blue. The variation of pH from 5 to 6 causes the potential to become more negative by 54 mV but from 6 to 7 only by 36 mV. Secondly, the system being measured contains redox systems for which the relation between E
h and pH is not known, so that the calculation with 57.7 mV/pH leads to false results. According to Michaelis (1933), the E
h variation for each pH unit can amount to 120 mV in certain cases [
6,
7,
8].
Therefore, Clark (1960) himself has demanded that the term rH should no longer be used [
9]. Hewitt (1950), on the other hand, also considered it confusing and suggested that it should not be used [
1,
8,
10].
2.1. Measurement of Redox Potential
In biochemical engineering, combined sterilizable platinum as indicator and calomel or silver/silver chloride electrodes as reference electrodes are in use. As the electrolyte, 3 M KCl solution or, less often, KCl gel are used. However, other authors calculated the decrease in E
h for a tenfold decrease in concentration of dissolved oxygen to be 14.8 mV [
9].
Measuring a sample’s
off-line redox potential, it is necessary to take into account that the dissolution of ambient atmospheric oxygen causes erroneous results and a higher measured potential in the liquid phase of the sample [
8,
10]. In addition, the electrode also has a certain reaction time to reach the value of the redox potential in the sample. Therefore,
on-line is the preferred method of measurement. Electrodes are usually sterilized by chemical sterilization immersed for 5 min in a 35% hydrogen peroxide solution or in ethylene oxide [
8,
10].
2.2. Calibration of Redox Electrodes
For the calibration of the redox electrodes, various redox buffers are in use. In this case, two saturated solutions of qinhydrone at two different pH values at 25 °C are suggested [
10].
A relatively easy method is also the use of ascorbic acid (Vitamin C) at various pH values [
1].
Redox potential measurements should be applied as
on-line measurements, because at
off-line measurements, there exists a danger that the sampling oxygen may enter from the side into the liquid of the testing sample [
9,
11].
3. Redox Potential in Wine Technology
In wine, various organic and inorganic redox couples can be distinguished. Redox systems of an organic nature are represented by ethanol, organic acids, and polyphenols. These are reduced forms and their oxidized forms are acetaldehyde and acetic acid, keto acids, dihydroascorbic acid, and quinones. These oxidation–reduction reactions take place under the influence of enzymes and depending on the chemistry of the medium [
2]. The redox system of an inorganic nature represents a transition from a reduced to an oxidized form with the release of electrons, and by accepting electrons, it returns to the reduced form. The most common are compounds of heavy metals—iron, copper, zinc, and their salts, which under certain conditions pass into the oxidized state and vice versa back into the reduced state. The main anorganic components of the redox buffer in wine are considered to be the Fe(II)-tartrate/Fe(III)-tartrate couple and other iron complexes, the Cu(I) and Cu(II) complexes, and the reduced glutathione (GSH)/oxidized glutathione (GSSH) couple [
9,
11].
At constant temperature and with the presence of several constants in the Nernst equation, the main variable or dominant measurement of the redox potential is the ratio Σa
ox/Σa
red, which represents the microbial activity of
Saccharomyces cerevisiae during wine fermentation. This measurement is for several orders of magnitude higher than the changes in the values of all other redox complexes, which, compared to the metabolic activities of yeasts in the fermentation process, thus only represent the background of the measurements [
11].
The measurement of redox potential expresses the ability of a microorganism to live, grow, and perform physiological activity in a certain environment. The practical importance of redox potential and the influence of oxygen content at different stages of winemaking were studied by Mazzoleni, 1979 [
12].
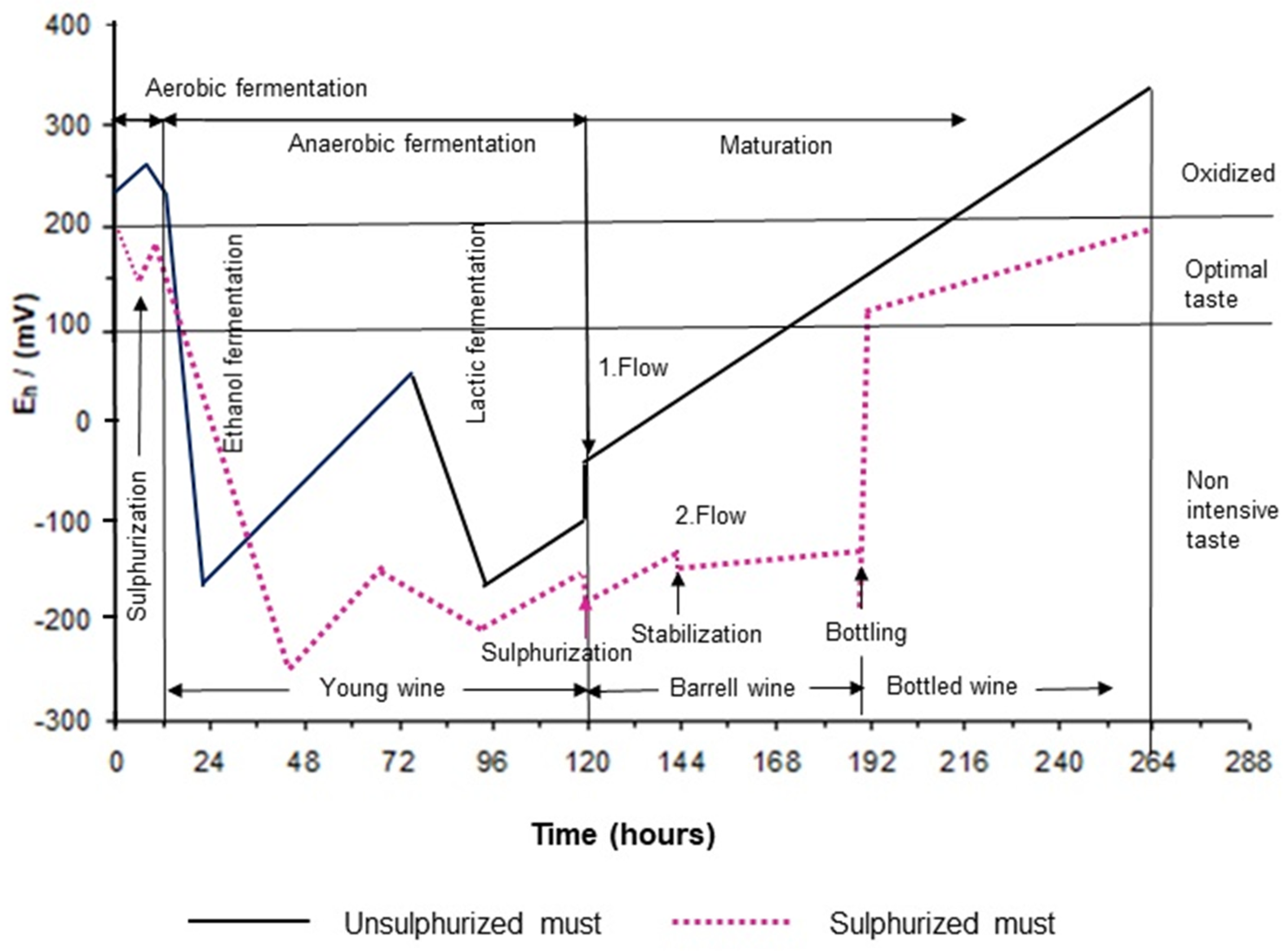
The alcoholic fermentation of grape must takes place in several phases. The redox potential of juice is usually between Eh +300 and +350 mV before the onset of yeast growth [
13]. According to wine fermentation, the first part of the redox potential measurements represents a pseudo-aerobic phase where all of the oxygen in the fermentation substrate is consumed. In this phase, the final biomass concentration is achieved [
12,
14]. In this phase, most of the acetaldehyde is synthesized [
15]. Enzyme alcohol dehydrogenase converted acetaldehyde into reductive ethanol, and therefore reductive conditions in the liquid phase were achieved [
16]. High levels of acetaldehyde may cause yeast growth inhibition and could significantly diminish the glucose consumption rate [
17]. After that, the process turns into the production of ethanol and alcohol formation, where the value of the redox potential decreases from the initial value of Eh +200 to at least −200 mV [
14,
18]. A change in the redox potential to a more negative value indicates the start of ethanol production. The speed and extent to which the potential becomes more negative depends on the specific growth rate, the physiological type of the wine yeast, and the applied fermentation temperature [
18].
At the minimum measured value of the redox potential, the concentration of ethanol reached its maximal value that became constant until the end of fermentation [
19]. Soon after, accumulated ethanol finally blocked microbial growth and prevented the yeast metabolism activities, and the redox potential slowly rose until the end of fermentation [
16]. In the further steps, esterification raised redox values while malolactic fermentation shortly decreased redox values. This period is the most suitable for the first flow and further maturation (
Figure 1) [
20].
With the addition of sulfur dioxide into the grape juice, the activity of the yeast would be reduced and the fermentation processes will be slow. Finally, in both cases of unsulfurized and sulfurized must, ethanol production would stop at the most negative redox potential values.
Generally speaking, high-quality wines show an oxidative-protected, reducing character. During the ripening, storing, and aging of wine, the oxidation and reduction processes which take place affect the character and taste of wine to a considerable extent. The intensity of the oxidation and reduction can be measured in redox potential units [
21]. The treatment of wines with bentonite clay absorbents reduces the redox potential and leads to an increase in wine oxidation. The sphere of yeast activity is within the limits of Eh 350 mV and –240 mV. Above this value, oxygen acts toxic and inhibitory, and below the value Eh –240 mV, the concentration of dissolved oxygen is too low for normal life conditions of yeast (
Figure 1) [
22]. The addition of sulfur to grape must also has a significant effect on the metabolism of
Saccharomyces cerevisiae and thus on the prolongation of the lag phase, which is also reflected in the course of the redox potential (
Figure 1).
Dikanović-Lućan and Palić (1995) pointed out that it is necessary to take into account a reducing action when bottling wines, so the measurement of redox potential could serve as an accurate control of the maturation state [
23]. Recommended values for white bottled wines are Eh 120 to 180 mV and for red wines they are Eh 100 to 240 mV [
14,
15,
18]. Schanderl, 1950, emphasizes that redox potential is of great importance for the organoleptic characteristics of wines. The assumed quality of wine could be established by measuring the oxidation–reduction potential [
13,
20]. There is a relationship between redox potential and wine quality [
24]. Various authors are of the opinion that wines having a lower redox potential are of better quality [
22,
23].
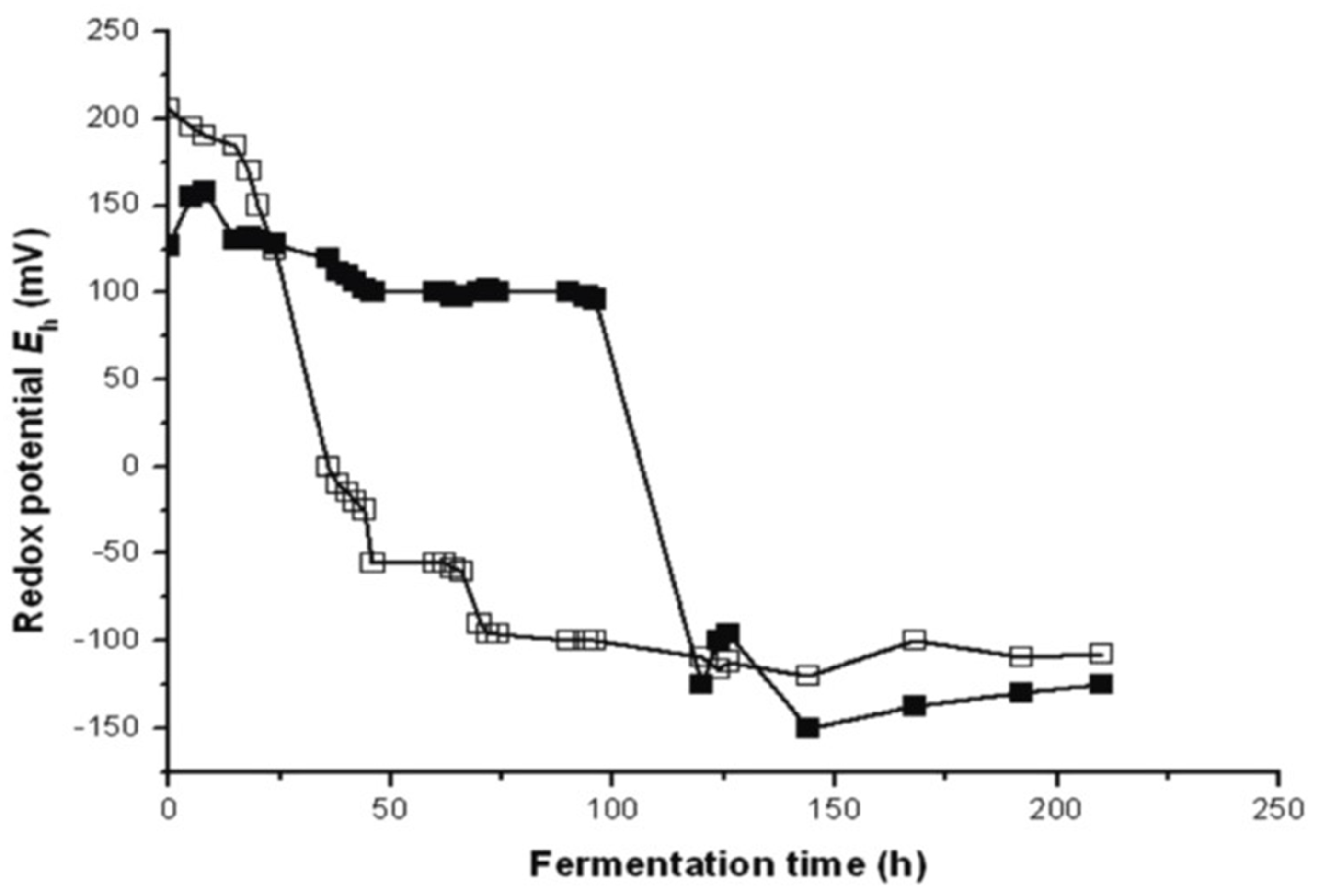
As an example of redox potential’s course in the fermentation of unsulfurized and sulfurized grape must of Blau Fränkish,
on-line measurements of the redox potential are presented in
Figure 2.
Fermentation of grape must proceeded in 3000 L tanks at 18 °C. The first tank contained sulfurized grape must with 50 mg/L 6% sulfuric (IV) acid and the second one was without any addition. In the unsulfurized run, the fermentation started at Eh 200 mV. The initial aerobic phase proceeded for 15 h. Alcohol production started at 17 h at Eh 168 mV and ended at 60 h at Eh −100 mV. Fermentation was finished after 216 h at Eh −108 mV. In the sulfurized substrate, fermentation started at a lower redox potential of Eh 125 mV and the yeast adoption phase proceeded from Eh 166 to 130 mV for 90 h. Alcohol production in the sulfurized grape must started after a 90 h delay at Eh +105 mV and ended after 124 h at a lower redox potential of Eh −125 mV. Both fermentations ended after 216 h [
25].
3.1. Influence of Mixing
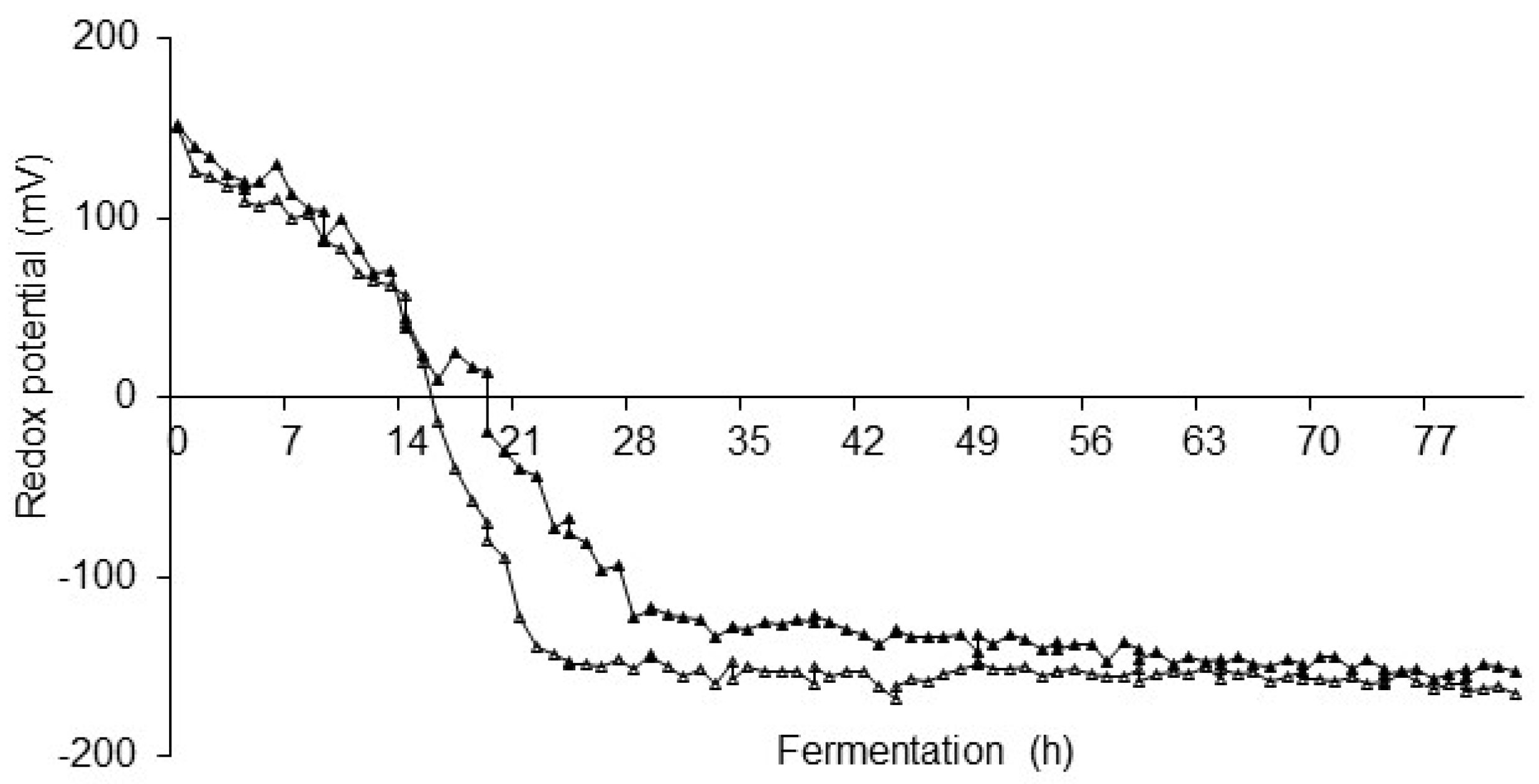
The redox potential curves in
Figure 3 show the values of
on-line measurements during fermentation at 18 °C in a 100 L Stirred Tank Reactor with stirring at 100 rpm and the one in the same reactor without stirring during fermentation.
The initial redox potential in the case of fermentation without stirring was 240 mV, which is 90 mV more than the redox potential of fermentation with stirring. This difference in the initial redox potentials occurs due to the surface contact of oxygen in the case of fermentation, where the surface above the substrate was not degassed with gaseous nitrogen as it was in the experiment with mixing.
A few hours after inoculation, the fermentation started. The redox potential started to drop already after 17 h and reached its minimum at Eh −140 mV after 16 h. In the next part, redox slowly rose to Eh −120 mV after 100 h of fermentation. A further increase to Eh 150 mV was caused by the entrance of the outlet air and the oxidation. The redox potential curve of the stirred fermentation in the first phase of the process lasted 30 h. After this lag phase, the value of the redox potential slowly dropped from the initial value of Eh 150 mV to −110 mV after 120 h at the end of fermentation [
20].
3.2. Influence of Temperature Changes
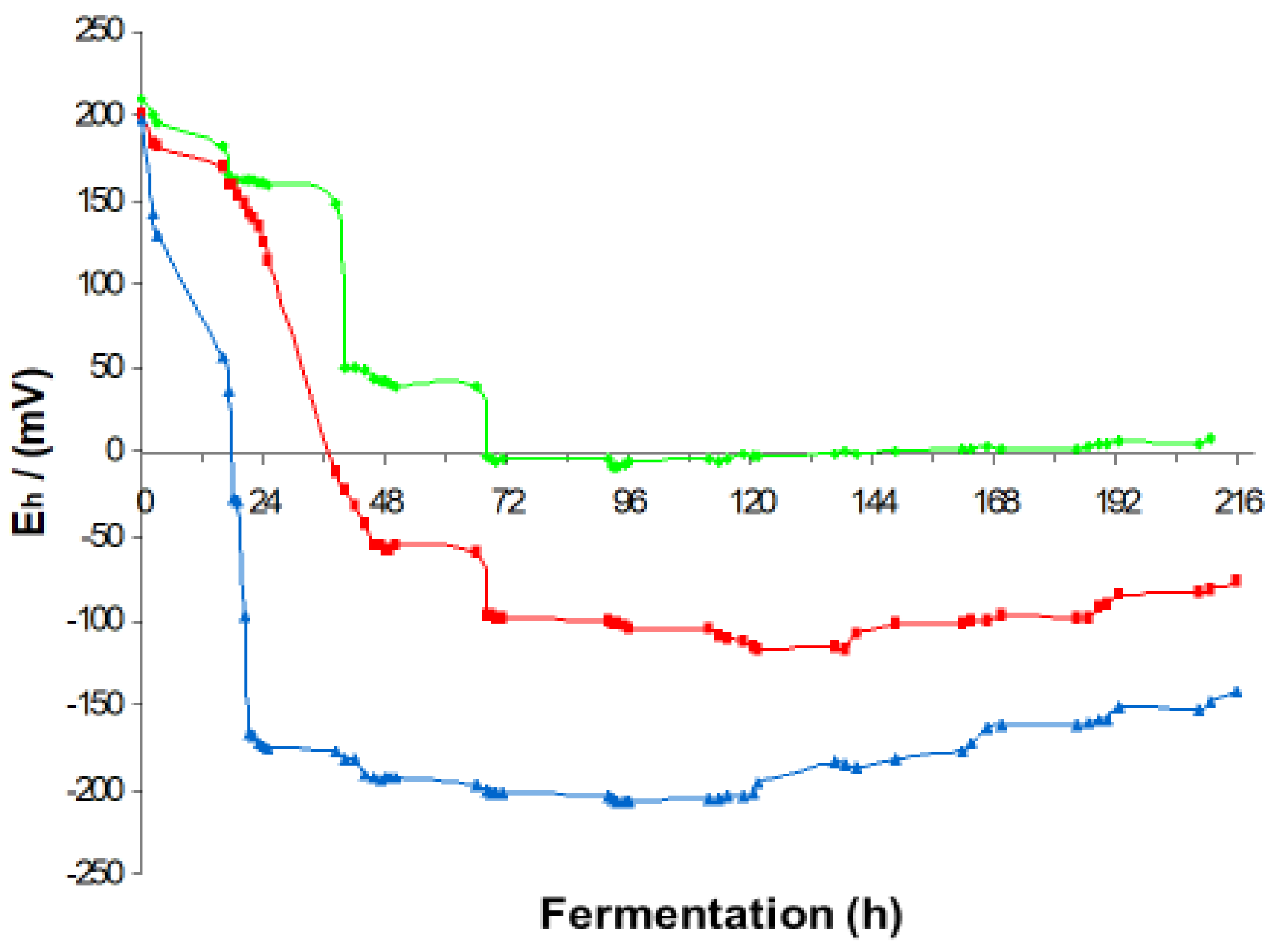
The effect of temperature on fermentation kinetics in Sauvignon Blanc fermentation is presented in
Figure 4. Changing temperature significantly influences the rate of fermentation. The duration of the pseudo-aerobic lag phase is remarkably shortened by increasing the fermentation temperature. It was detected that at 15 °C it lasted 36 h, 18 h at 18 °C, and at 24 °C it is the shortest, as it lasted 4 h only. In the next phase that follows, the exponential growth phase, the highest amounts of glycerol and ethanol are produced. In this phase, the highest drop in the redox potential is detected. The decrease in the redox potential is also faster and most expressed at 24 °C with a more extended change in redox potential up to Eh −170 mV after 22 h; at 18 °C to Eh −100 mV at 72 h; and at 15 °C to Eh −10 mV at 72 h [
26]. In the stationary phase that follows, the final ethanol concentration blocks the metabolic activity of yeast, so the redox potential in the following step rises only slightly, influenced by some background redox reactions (
Figure 4) [
27].
During the winemaking process, yeast metabolism and the associated ethanol production are reflected in redox potential measurements and changes. The maximum concentration of ethanol is reflected in the minimum value of the measured redox potential. In the stationary phase, this concentration remains constant until the end of fermentation. The redox minimum is a function of fermentation temperature. At 24 °C, it reached Eh −200 mV; at 18 °C is was Eh −120 mV; and at 15 °C, the redox minimum was Eh −7 mV [
27].
Raising the fermentation temperature also influences biomass production. At a higher fermentation temperature, more biomass is produced and, as a consequence, there is a higher production of ethanol. In the phase of exponential growth, the redox potential of the process is balanced by the production of glycerol, that acts as a process osmoregulator. The process of glycerol formation and the amount of glycerol produced are also related to the fermentation temperature, the partial pressure of the generated carbon dioxide, and the length and intensity of the lag phase [
14,
18]. At 15 °C at the end of fermentation, glycerol production was 6.5 g/L, while at higher temperatures at 18 °C, it was 7.9 g/L, and at 24 °C, it was 8.6 g/L. Elevated fermentation temperature also increases the biosynthesis of 1-propanol, 2-butanol, and isoamyl alcohol. In the case of 1-propanol, a maximum of 23 mg/L was detected at 24 °C after 93 h, while 17 mg/L was detected at 18 °C, after 142 h of fermentation. In the case of 15 °C, 14 mg/L of 1-propanol was detected after 142 h. The amount of 2-butanol was 25 mg/L after 119 h at 24 °C, 17 mg/L at 18 °C after 164 h, and 15 mg/L at 15 °C after 140 h of fermentation. Similar results were detected in the production of isoamyl alcohol. A maximum of 35 mg/L was detected at 24 °C in 90 h, 33 mg/L at 18 °C was detected after 122 h of fermentation, and in fermentation at 18 °C, 28 mg/L of isoamyl alcohol was detected after 140 h of fermentation [
20].
The activation of enzymes involved in the anabolism of ammonia, which occurs during biomass growth, also affects the higher production of alcohol. A higher fermentation temperature also causes an increase in enzyme activity. A lower fermentation temperature of 18 °C, on the other hand, has the opposite effect on reducing the metabolic rate of Saccharomyces cerevisiae and thus the production of higher alcohols and ethanol. At a temperature of 24 °C, glucose was completely consumed after 120 h, at 18 °C after 141 h, and at 15 °C after 164 h. Consumption of fructose at different temperatures increased glucose consumption in parallel. During fermentation at 18 ºC, 20 g/L of residual fructose was detected after 168 h. The fastest yeast metabolism detected at the highest fermentation temperature is associated with a faster metabolism of glucose and fructose. This was also manifested in a faster reduction of the redox potential.
At 15 °C, the lag phase was extended to 27 h followed by an exponential phase from 23 to 36 h and a stationary phase from 36 to 165 h of fermentation. In the second case, at 22 °C, the lag phase was eight hours shorter and a slightly prolonged exponential growth phase of 17 to 28 h proceeded from 28 h to 166 h into the stationary phase. In the last run at 24 °C, the lag phase lasted only 5 h. From 5 to 26 h, it went into a longer phase of exponential growth, which after 24 h to 164 h went into a stationary phase.
3.3. Influence of Additional Carbon Dioxide Fluxes
Increasing the partial pressure of CO
2 with additional inlet flows, it is possible, similarly to lowering the temperature, to reduce the metabolic activity of wine yeasts and thus also to reduce the ethanol production and increase the biosynthesis of higher alcohols [
18,
20]. By introducing additional carbon dioxide flows, during fermentation, it is also possible to balance the transport of metabolites from yeast cells to the liquid phase. A higher concentration of CO
2 in the cell regulates cellular metabolism, which is also reflected in the further synthesis of products [
27]. By introducing additional flows of carbon dioxide into the fermentation process, it is also possible to regulate the balance between the carboxylation and decarboxylation reactions and thereby to regulate additional resistance against the decarboxylation of pyruvate to acetaldehyde [
17,
19,
28]. The major inhibitory effects of CO
2 buildup seem to result from changes in yeast membrane composition and permeability [
29].
A method for steering the fermentation process, using additional fluxes of carbon dioxide to manage the fermentation process’s redox potential in the direction of the previously optimized redox of the optimal product fermentation, was successfully applied in the fermentation of Cabernet Sauvignon grape must. Compared to the control fermentation, in fermentations using additional carbon dioxide streams, the fermentation process proceeded with lower ethanol and biomass production, while there were no significant differences in the other compounds (
Figure 5).
Table 1 presents the results of the analysis [
22].
3.4. Regulation of Redox—Case Study
In wine technology, possibilities and sources for the regulation of redox potential in the fermentation process were studied. As wine is one of the most sensible food articles, the most suitable tools for redox potential regulation, as well as for the regulation of the rate of the fermentation process and the control of the wine yeast metabolism cycle, are the introduction of the additional carbon dioxide fluxes and the implementation of temperature changes. Varying the process temperature is a tool for the regulation of the rate of Sauvignon Blanc grape must fermentation, and additional inlet carbon dioxide fluxes were applied. The effectivity of this process was monitored by
on-line redox potential measurement. The results are presented in the text below (
Figure 6) [
20].
Measuring the on-line redox profiles, of the same grape variety, of every harvest could statistically establish a fingerprint of great fermentation. A great, vintage-optimized redox profile should be selected. Furthermore, this optimized profile should be applied as a guide or a steering trajectory for each next run’s guidance.
The wine fermentation process could be also regulated and steered using a combination of temperature variation and the introduction of the additional carbon dioxide fluxes [
14].
Using this method, the best results from a humble or average harvest could be improved. By employing this approach, one could enhance the outcomes of the modest or ordinary harvests. While the suggested method could significantly enhance average fermentation, it cannot elevate it to greatness from a poor harvest. The same principle could be used also as a
scaling-up criterion in a production plant [
11].
For steering the fermentation process to the requested redox potential levels, a combination of temperature variation and the introduction of additional carbon dioxide fluxes was used. By this regulation, the redox potential was well balanced and finally the results of the analysis showed that the consumption of reductive sugars as well as the production of ethanol were reproducible. Special attention has to be brought to redox balancing in the exponential growth phase, where without supervision (mostly in the night time), the course of steering fermentation could easily jump out of the planned direction. See the steering redox fermentation line at 36 h (
Figure 6). Using this method, a similar agreement is also shown in the analysis of several other metabolites. Results of the analysis are presented in
Table 2. The sensorial analyses of both fermentation wines were also in good agreement [
20].
4. Redox Potential in Oak Barrel Wine Maturation
Redox potential measurement was efficiently used for monitoring the three-year Blau Fränkisch wine maturation in 225 L Slovenian oak barrique barrels (Kranjc d.o.o., Slovenia). The metabolic activity of the rest of the yeast biomass was measured by immersing the redox electrode throughout the depth profile of the horizontally positioned barrels. In the first and second year of maturation, the biomass was still in a slightly active state in both cases.
According to the measurements, six levels of redox potential, two on both sides of the barrel and four from the top to the bottom of the barrel, were recognized. As it was indicated during the process of wine maturation, the formation and heterogeneity of redox presents the
turn-off layer activity of wine yeast autolysis in the direction of the bottom, as well as the formation of various oxido-reductive zones on both sides of the barrel. The highest redox potential and therefore the most oxidative area were measured at the top entrance and on both sides of the barrel. Measured potential strongly decreased in the direction of the bottom of the barrel, where the rest of the yeast biomass was sediment. These various oxidation–reduction zone measurements are indicated as a clear consequence that indicates a course of the wine maturation process (
Figure 7). The first and second years of wine maturation were characterized by the heterogeneity of various redox layers, while the third year indicated the homogeneity of redox potential measurements, 186 mV in unsulfurized and 168 mV in sulfurized wine, in the layers with a deviation of ±4 mV [
16].
Measurements of the redox potential in barrels showed, in spite of the micro-oxidation area in both cases, that the addition of sulfur caused a faster final deactivation of the yeast biomass than in the case of unsulfurized wine (
Table 3).
In a sulfurous wine, in a more reductive environment, the
turn-off of the rest of biomass was faster and the ripening time was shorter. At the end of ripening, in the sulfurous wine, after three years, the total amount of SO
2 was 46.87 mg/L, and the residual biomass level was 0.87 g/L. In the unsulfurized wine maturation process, much higher redox potential values were shown, indicating more expressed metabolic activity of the residual wine yeast in the barrel [
16].
In the case of unsulfurized wine, after three years of maturation, the amount of free SO
2 was 19.13 mg/L and the total sulfur amount was 33.57 mg/L. That means that the value was higher by 1.52 mg/L or 8.6% in comparison to the 17.61 mg/L of the final free SO
2 amount in the sulfurized wine. In the unsulfurized wine, even after 30 months of maturation, the presence of various redox layers still indicated wine yeast metabolic activity. The detection of unchanged redox potential values in the entire barrel profile after 36 months showed the end of yeast activity and thus readiness for the bottling process. In the unsulfurized wine maturation process, the biomass concentration of wine yeast was 8.6% higher, and its metabolic activity lasted six months longer due to the less reductive maturation environment. Spontaneous autolysis of yeast cells was pronounced in the case of unsulfurized wine maturation. In this case, the biomass concentration of wine yeasts was 8.6% higher than in the sulfurized wine. In this case, the activity of the yeast biomass lasted six months longer due to the less reductive maturation environment [
25].
The
sur-lies maturation process in barrels without additional sulfur is the recommended process for aging wine. The concentration of amino acid sulfur released from dead cells was high enough during the maturation of unsulfurized wine to protect the wine until final bottling [
24].
In the research of Dikanović-Lučan and Palić (1995) [
23], it was found out that wine stored in wooden casks shows higher redox potential and oxygen content (Eh 365–380 m V and 3.1–4.7 mgO
2/L, respectively) compared with bottled wine (Eh 275–280 m V and 2.8–3.4 mgO
2/L). The period of red wines’ aging in wooden casks generally is from 1 to 2 years longer than that of bottled ones. During this period, oxygen continuously dissolves; thus, it influences the increase in the redox potential in wine [
16,
26].
5. Conclusions
Redox potential in wine technology represents a fast, accurate, and reliable measurement, the results of which give an insight into wine yeast metabolic activity and therefore the oxidation as well as the reduction ability of wine. Although many inorganic and organic redox couples are present in the chemistry of grape must fermentation, the metabolism of wine yeasts is the factor that is clearly reflected in the redox potential and has the strongest influence on its measurements [
11]. Redox potential as a parameter is also extremely sensitive to process changes, reflecting automatic and manual operations of fermentation conditions; therefore, it is an insight parameter of yeast metabolism [
30,
31]. It is influenced by temperature, mixing, oxygenation, or carbon dioxide inlet. Using these parameters, it is possible to balance and steer the whole fermentation process according to a previously determined redox potential of high-yielding fermentation. In this way, better fermentation efficiency can be achieved.
The presented results of the measurements of the redox potential of wine maturation in barrels can also represent a useful tool for monitoring the maturation process. In this case, redox potential is a suitable indicator of the effects of micro-oxidation and yeast deactivation. From the measurements, it was shown that yeast activity in the barrel is a much longer process than that imagined. Different layers of redox measurements, which at the end of this process are unified into the same redox potential value, are indicators of the maturation process in the barrel.