The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives
Abstract
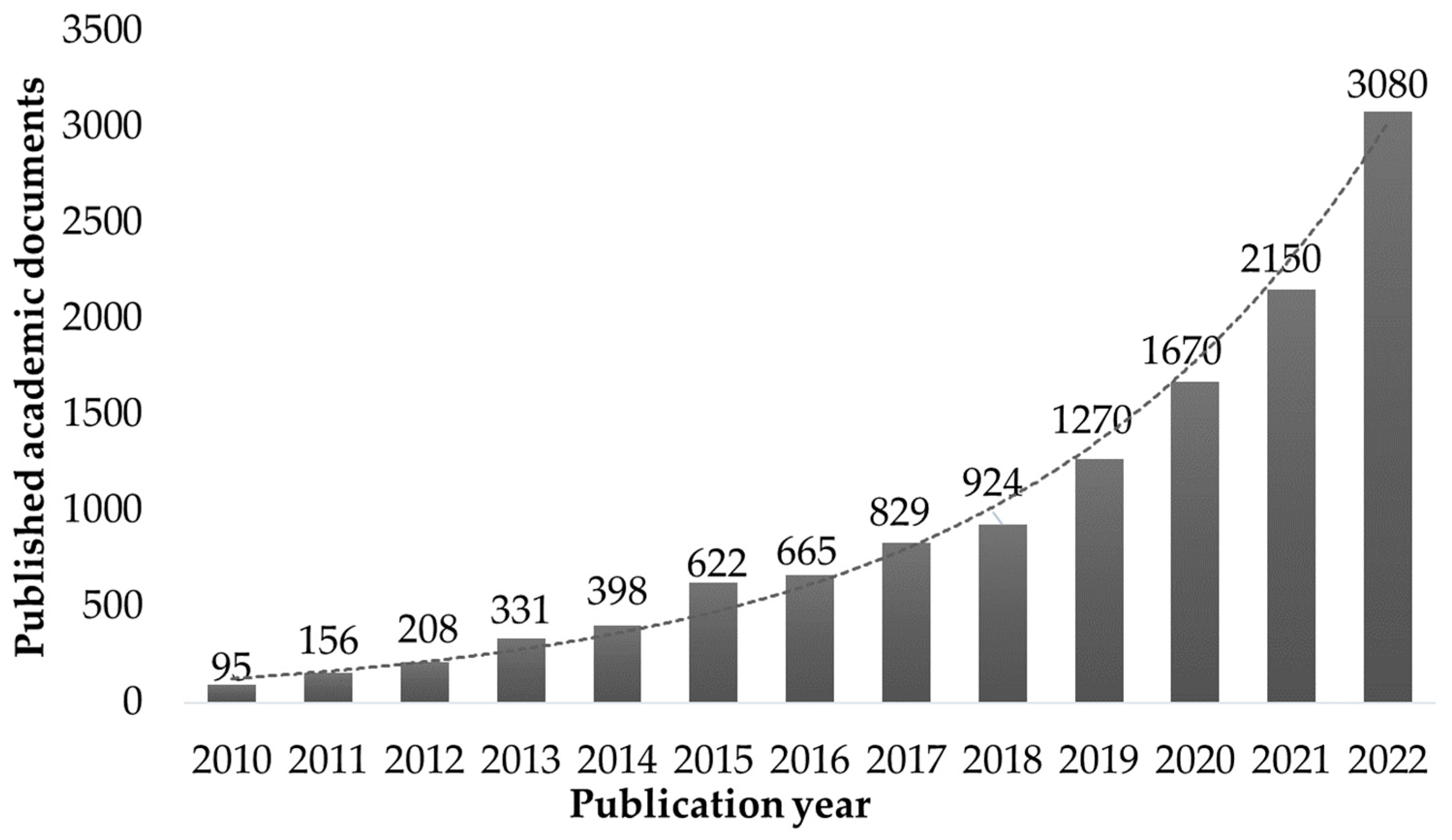
1. Introduction
2. Classification of Renewable Raw Materials
2.1. Raw Material of the 1st and 2nd Generations
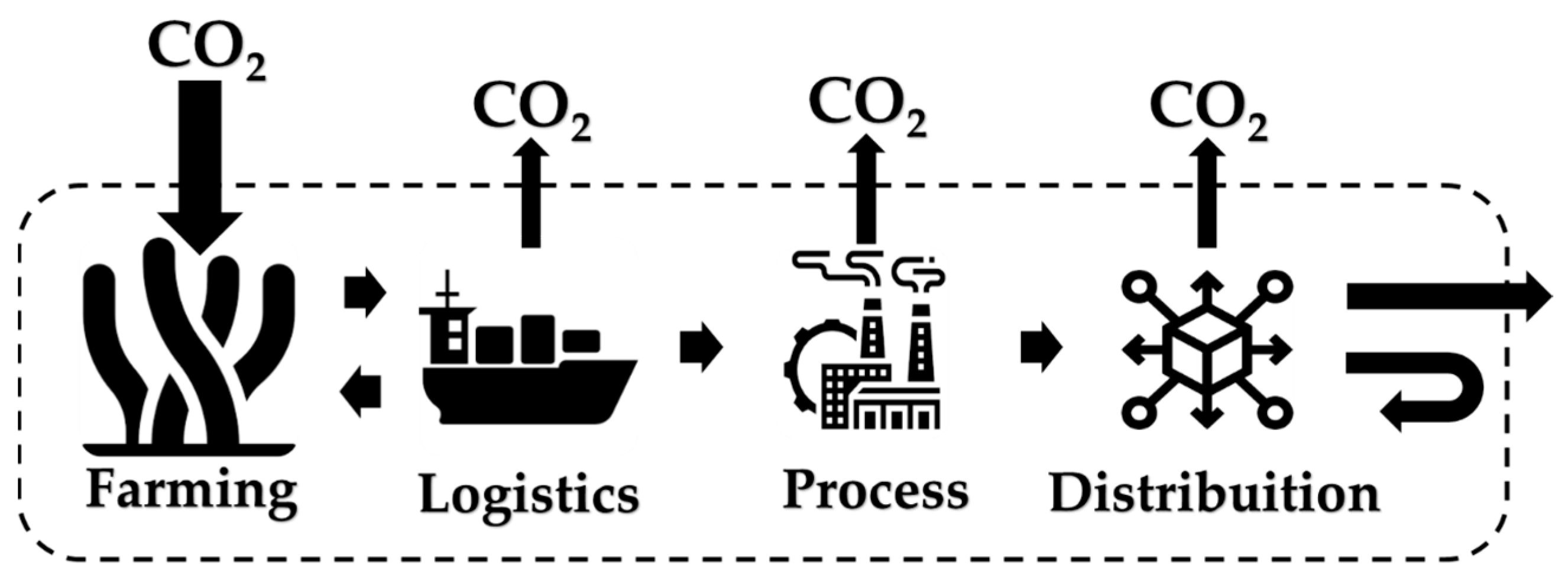
2.2. Macroalgae: Third-Generation Raw Materials
2.3. Kappaphycus alvarezii
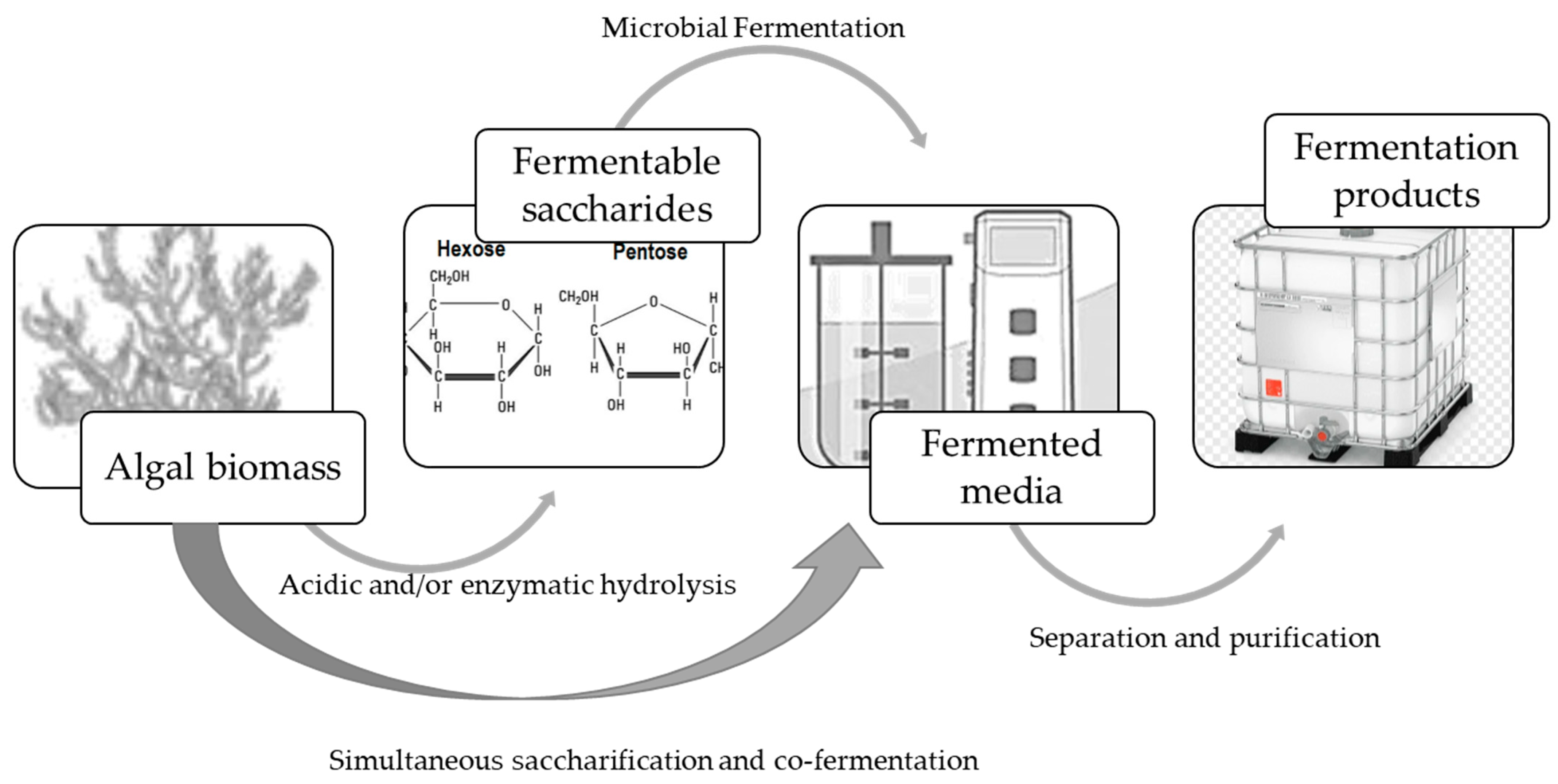
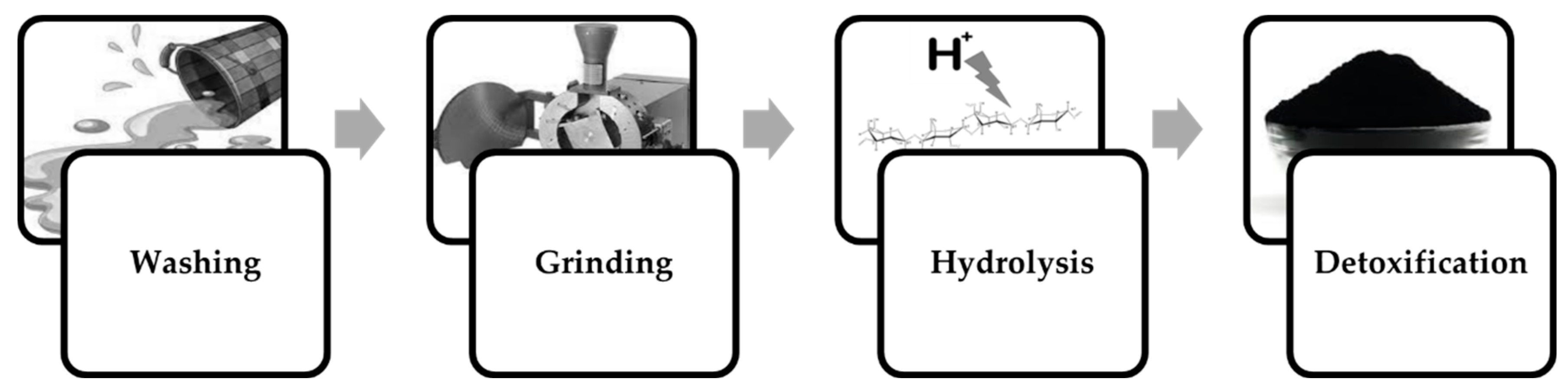
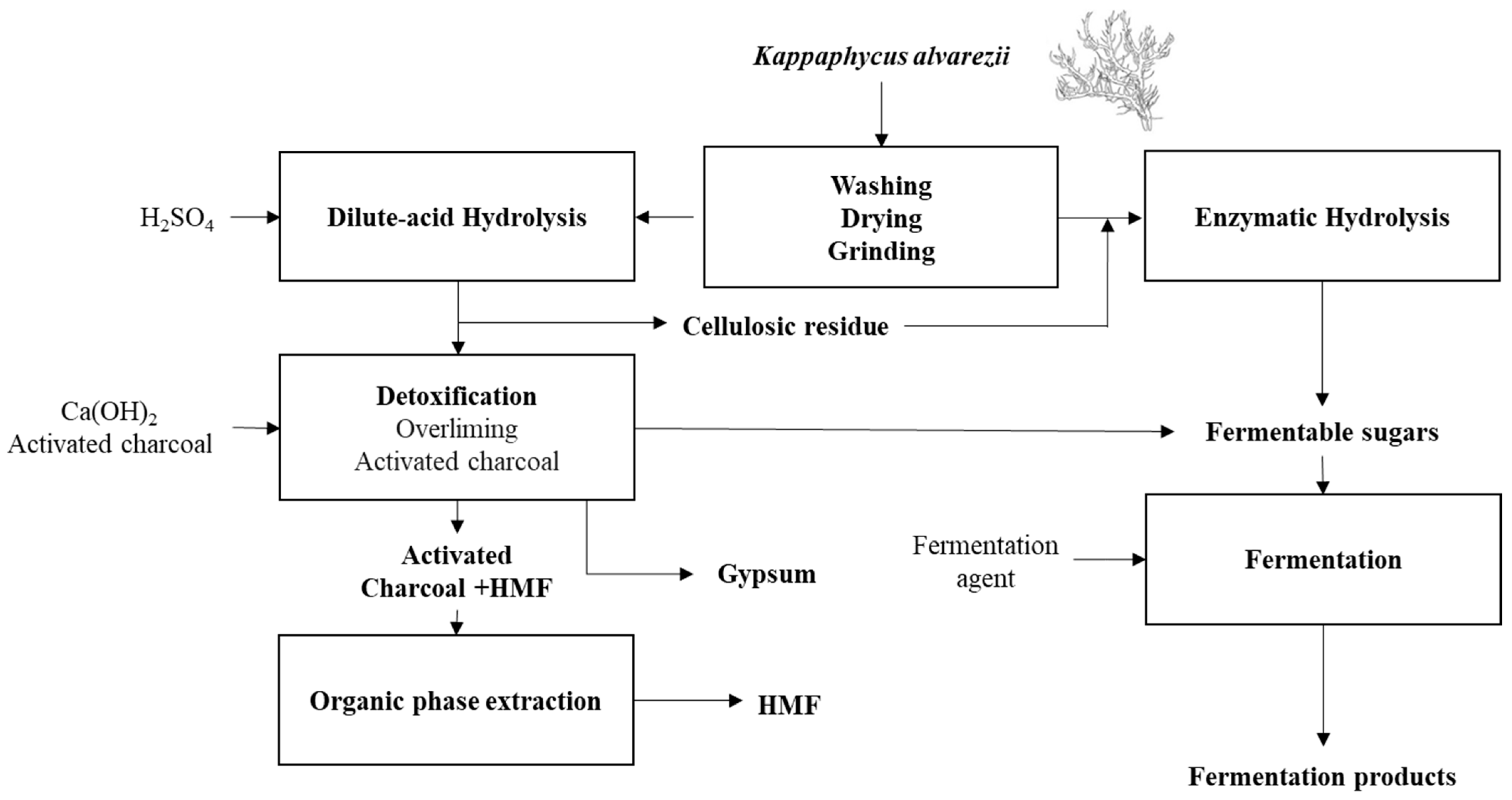
3. Processing K. alvarezii Biomass for Fermentation Processes
3.1. Enabling Dilute-Acid Hydrolysate Fermentation
3.1.1. HMF Removal from K. alvarezii Hydrolysates
3.1.2. Cellular Acclimation to Hydrolysate
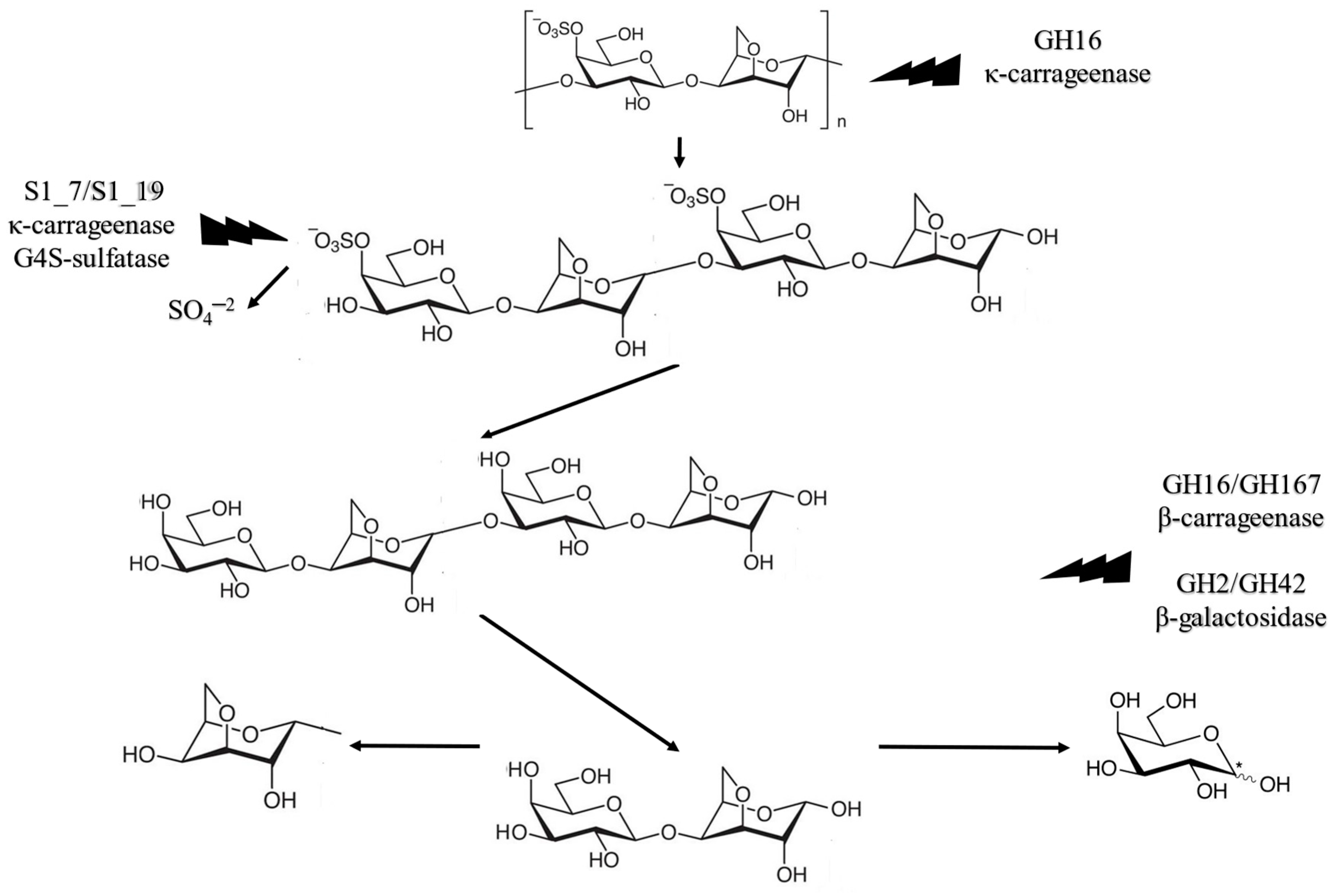
3.2. Enzymatic Hydrolysis Using Carrageenases-Type Enzymes
4. The Fermentation of Rhodophyta-Type Saccharides
4.1. Metabolic Pathways for Galactose Fermentation
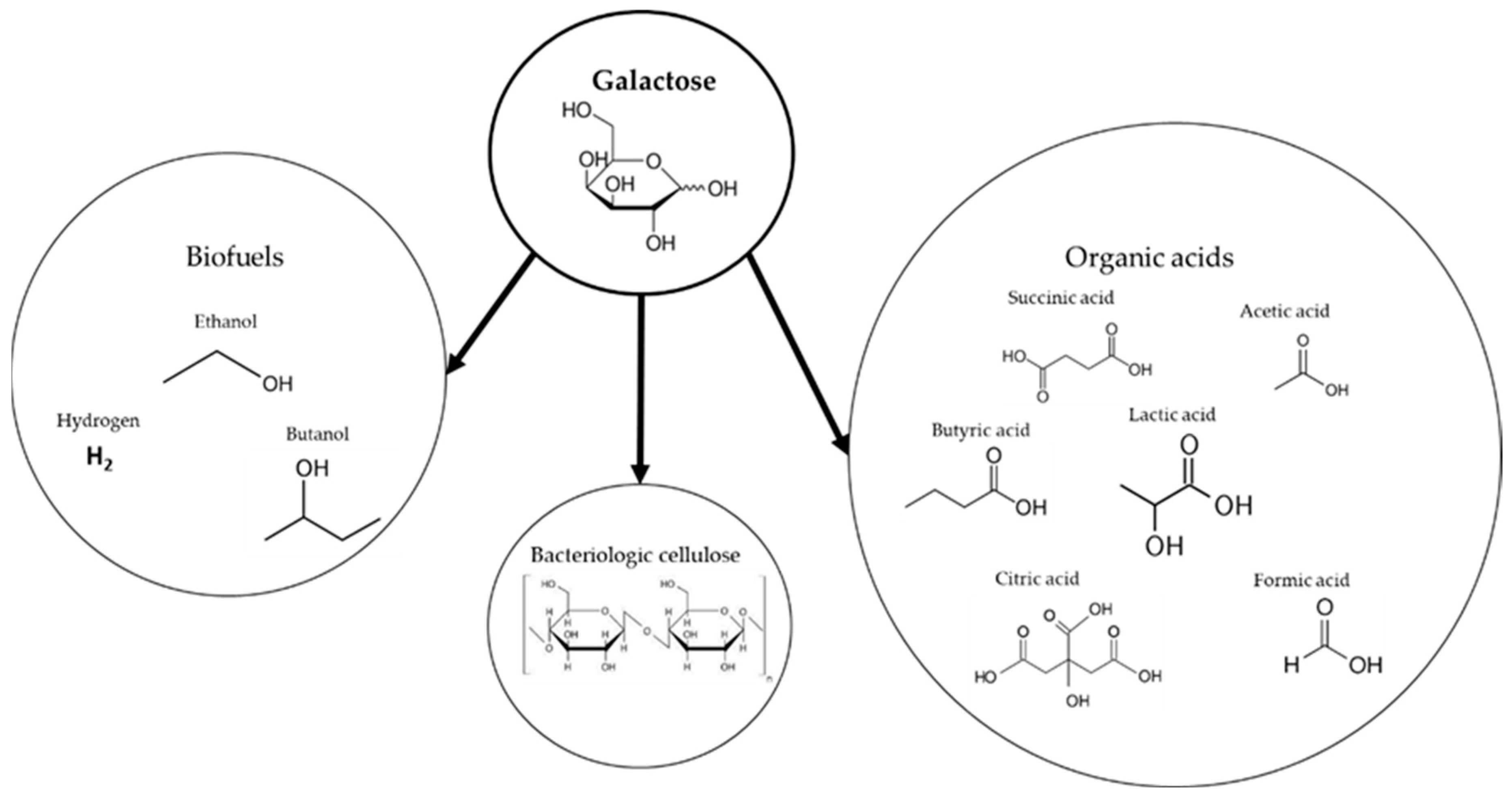
4.2. Products from Galactose Fermentation
4.2.1. Biofuels
4.2.2. Organic Acids
4.2.3. Bacterial Cellulose
5. K. alvarezii Fermentation Biorefinery Scheme
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Shi, S.; Yin, J. Global Research on Carbon Footprint: A Scientometric Review. Environ. Impact Assess. Rev. 2021, 89, 106571. [Google Scholar] [CrossRef]
- Yaacob, N.F.F.; Mat Yazid, M.R.; Abdul Maulud, K.N.; Ahmad Basri, N.E. A Review of the Measurement Method, Analysis and Implementation Policy of Carbon Dioxide Emission from Transportation. Sustainability 2020, 12, 5873. [Google Scholar] [CrossRef]
- Lackner, M. 3rd-Generation Biofuels: Bacteria and Algae as Sustainable Producers and Converters. In Handbook of Climate Change Mitigation and Adaptation; Springer: New York, NY, USA, 2015; pp. 1–32. [Google Scholar]
- Paraschiv, S.; Paraschiv, L.S. Trends of Carbon Dioxide (CO2) Emissions from Fossil Fuels Combustion (Coal, Gas and Oil) in the EU Member States from 1960 to 2018. Energy Rep. 2020, 6, 237–242. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring Global Carbon Emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- ElHady, S.; Amin, O.; Elhussieny, A.; Fahim, I.S. Bioplastics, Biodegradable Plastics, and Degradation in Natural Environments. In Biodegradability of Conventional Plastics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 47–67. [Google Scholar]
- Tukker, A. Life Cycle Assessment as a Tool in Environmental Impact Assessment. Environ. Impact Assess. Rev. 2000, 20, 435–456. [Google Scholar] [CrossRef]
- Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life Cycle Assessment of Carbon Dioxide Removal Technologies: A Critical Review. Energy Environ. Sci. 2021, 14, 1701–1721. [Google Scholar] [CrossRef]
- Fiorese, G.; Catenacci, M.; Verdolini, E.; Bosetti, V. Advanced Biofuels: Future Perspectives from an Expert Elicitation Survey. Energy Policy 2013, 56, 293–311. [Google Scholar] [CrossRef]
- Ziolkowska, J.; Simon, L.; Zilberman, D. Capturing Uncertainties in Evaluation of Biofuels Feedstocks: A Multi-Criteria Approach for the US; ETH Zurich: Zurich, Switzerland, 2011. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Successful Approaches for a Red Seaweed Biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef]
- Lange, L.; Bak, U.G.; Hansen, S.C.B.; Gregersen, O.; Harmsen, P.; Karlsson, E.N.; Meyer, A.; Mikkelsen, M.D.; Van Den Broek, L.; Hreggviðsson, G.Ó. Opportunities for Seaweed Biorefinery. In Sustainable Seaweed Technologies: Cultivation, Biorefinery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–31. ISBN 9780128179437. [Google Scholar]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An Overview of Marine Macroalgae as Bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Hung, L.D.; Hori, K.; Nang, H.Q.; Kha, T.; Hoa, L.T. Seasonal Changes in Growth Rate, Carrageenan Yield and Lectin Content in the Red Alga Kappaphycus alvarezii Cultivated in Camranh Bay, Vietnam. J. Appl. Phycol. 2009, 21, 265–272. [Google Scholar] [CrossRef]
- Campbell, R.; Hotchkiss, S. Carrageenan Industry Market Overview. In Tropical Seaweed Farming Trends, Problems and Opportunities; Springer International Publishing: Cham, Switzerland, 2017; pp. 193–205. [Google Scholar]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative Analysis of Key Technologies for Cellulosic Ethanol Production from Brazilian Sugarcane Bagasse at a Commercial Scale. Biofuels Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Nogueira, M.C.F.; Henriques, M.B. Large-Scale versus Family-Sized System Production: Economic Feasibility of Cultivating Kappaphycus alvarezii along the Southeastern Coast of Brazil. J. Appl. Phycol. 2020, 32, 1893–1905. [Google Scholar] [CrossRef]
- Nass, L.L.; Pereira, P.A.A.; Ellis, D. Biofuels in Brazil: An Overview. Crop Sci. 2007, 47, 2228–2237. [Google Scholar] [CrossRef]
- Paz-Cedeno, F.R.; Solórzano-Chávez, E.G.; de Oliveira, L.E.; Gelli, V.C.; Monti, R.; de Oliveira, S.C.; Masarin, F. Sequential Enzymatic and Mild-Acid Hydrolysis of By-Product of Carrageenan Process from Kappaphycus alvarezii. Bioenergy Res. 2019, 12, 419–432. [Google Scholar] [CrossRef]
- Hargreaves, P.I.; Barcelos, C.A.; da Costa, A.C.A.; Pereira, N. Production of Ethanol 3G from Kappaphycus alvarezii: Evaluation of Different Process Strategies. Bioresour. Technol. 2013, 134, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-Generation Biorefineries as the Means to Produce Fuels and Chemicals from CO2. Nat. Catal. 2020, 3, 274–288. [Google Scholar] [CrossRef]
- Tabacof, A.; Calado, V.; Pereira, N. Third Generation Lactic Acid Production by Lactobacillus pentosus from the Macroalgae Kappaphycus alvarezii Hydrolysates. Fermentation 2023, 9, 319. [Google Scholar] [CrossRef]
- Baig, M.Z.; Dharmadhikari, S.M. Optimization of Detoxification with Over Liming and Charcoal Treatment for Increasing the Fermentability of Cotton Stalk Hydrolyzate. Indian. J. Appl. Res. 2014, 5, 453–455. [Google Scholar] [CrossRef]
- Chi, Z.; Rover, M.; Jun, E.; Deaton, M.; Johnston, P.; Brown, R.C.; Wen, Z.; Jarboe, L.R. Overliming Detoxification of Pyrolytic Sugar Syrup for Direct Fermentation of Levoglucosan to Ethanol. Bioresour. Technol. 2013, 150, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, A.; Ruth, M.; Schell, D.J. Conditioning Hemicellulose Hydrolysates for Fermentation: Effects of Overliming PH on Sugar and Ethanol Yields. Process Biochem. 2006, 41, 1806–1811. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Likozar, B. A Review of Bio-Refining Process Intensification in Catalytic Conversion Reactions, Separations and Purifications of Hydroxymethylfurfural (HMF) and Furfural. Chem. Eng. J. 2022, 429, 132325. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Bäumgen, M.; Dutschei, T.; Bornscheuer, U.T. Marine Polysaccharides: Occurrence, Enzymatic Degradation and Utilization. ChemBioChem 2021, 22, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Rudke, A.R.; de Andrade, C.J.; Ferreira, S.R.S. Kappaphycus Alvarezii Macroalgae: An Unexplored and Valuable Biomass for Green Biorefinery Conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- Louw, J.; Dogbe, E.S.; Yang, B.; Görgens, J.F. Prioritisation of Biomass-Derived Products for Biorefineries Based on Economic Feasibility: A Review on the Comparability of Techno-Economic Assessment Results. Renew. Sustain. Energy Rev. 2023, 188, 113840. [Google Scholar] [CrossRef]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-Generation Biorefineries: A Sustainable Platform for Food, Clean Energy, and Nutraceuticals Production. Biomass Convers. Biorefinery 2022, 12, 4215–4230. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Dutta, K.; Daverey, A.; Lin, J.-G. Evolution Retrospective for Alternative Fuels: First to Fourth Generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic Engineering of Algae for Fourth Generation Biofuels Production. Energy Environ. Sci. 2011, 4, 2451. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Mondal, S.; Kumar, V.; Singh, S.P. Roadmap to Sustainable Carbon-Neutral Energy and Environment: Can We Cross the Barrier of Biomass Productivity? Environ. Sci. Pollut. Res. 2021, 28, 49327–49342. [Google Scholar] [CrossRef] [PubMed]
- Barboza-Rodríguez, R.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Rosales Aguado, M.L.; Ruiz, H.A. Photobioreactor Configurations in Cultivating Microalgae Biomass for Biorefinery. Bioresour. Technol. 2024, 394, 130208. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S.; Abomohra, A.E.-F. Integrated Microalgal Biorefinery—Routes, Energy, Economic and Environmental Perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–325. [Google Scholar]
- Ho, D.P.; Ngo, H.H.; Guo, W. A Mini Review on Renewable Sources for Biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, D.; Finco, A.; Bacchi, M. Interdependencies between Biofuel, Fuel and Food Prices: The Case of the Brazilian Ethanol Market. Energies 2016, 9, 464. [Google Scholar] [CrossRef]
- Bonomi, A.; Cavalett, O.; da Cunha, M.P.; Lima, M.A.P. The Virtual Sugarcane Biorefinery Concept. In Virtual Biorefinery: An Optimization Strategy for Renewable Carbon Valorization; Springer: Cham, Switzerland, 2016; pp. 5–11. [Google Scholar]
- Soares, G.A. O Avanço Das Tecnologias de Segunda Geração e Seus Impactos Na Indústria Do Etanol. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Harper, A.B.; Powell, T.; Cox, P.M.; House, J.; Huntingford, C.; Lenton, T.M.; Sitch, S.; Burke, E.; Chadburn, S.E.; Collins, W.J.; et al. Land-Use Emissions Play a Critical Role in Land-Based Mitigation for Paris Climate Targets. Nat. Commun. 2018, 9, 2938. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Johnston, M.; Foley, J.A.; Holloway, T.; Monfreda, C.; Ramankutty, N.; Zaks, D. Carbon Payback Times for Crop-Based Biofuel Expansion in the Tropics: The Effects of Changing Yield and Technology. Environ. Res. Lett. 2008, 3, 034001. [Google Scholar] [CrossRef]
- Spera, S.A.; Galford, G.L.; Coe, M.T.; Macedo, M.N.; Mustard, J.F. Land-use Change Affects Water Recycling in Brazil’s Last Agricultural Frontier. Glob. Chang. Biol. 2016, 22, 3405–3413. [Google Scholar] [CrossRef]
- Plevin, R.J.; Jones, A.D.; Torn, M.S.; Gibbs, H.K. Greenhouse Gas Emissions from Biofuels’ Indirect Land Use Change Are Uncertain but May Be Much Greater than Previously Estimated. Environ. Sci. Technol. 2010, 44, 8015–8021. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, S.E.; Ramírez, A. When Are Negative Emissions Negative Emissions? Energy Environ. Sci. 2019, 12, 1210–1218. [Google Scholar] [CrossRef]
- Pangestuti, M.B.; Suhartini, S.; Hidayat, N. Life Cycle Assessment of Bioenergy Production from Macroalgae: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012070. [Google Scholar] [CrossRef]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Brockmann, D.; Pradinaud, C.; Champenois, J.; Benoit, M.; Hélias, A. Environmental Assessment of Bioethanol from Onshore Grown Green Seaweed. Biofuels Bioprod. Biorefining 2015, 9, 696–708. [Google Scholar] [CrossRef]
- Langlois, J.; Sassi, J.; Jard, G.; Steyer, J.; Delgenes, J.; Hélias, A. Life Cycle Assessment of Biomethane from Offshore-cultivated Seaweed. Biofuels Bioprod. Biorefining 2012, 6, 387–404. [Google Scholar] [CrossRef]
- Seghetta, M.; Hou, X.; Bastianoni, S.; Bjerre, A.-B.; Thomsen, M. Life Cycle Assessment of Macroalgal Biorefinery for the Production of Ethanol, Proteins and Fertilizers—A Step towards a Regenerative Bioeconomy. J. Clean. Prod. 2016, 137, 1158–1169. [Google Scholar] [CrossRef]
- Amponsah, L.; Chuck, C.; Parsons, S. Life Cycle Assessment of a Marine Biorefinery Producing Protein, Bioactives and Polymeric Packaging Material. Int. J. Life Cycle Assess. 2023, 29, 174–191. [Google Scholar] [CrossRef]
- Rose, D. Life Cycle of Carbon in Macroalgae for Various Products; U.S. Department of Energy: Richland, WA, USA, 2021.
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal Biomass as a Potential Resource for Lactic Acid Fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef]
- Fasahati, P.; Dickson, R.; Saffron, C.M.; Woo, H.C.; Liu, J.J. Seaweeds as a Sustainable Source of Bioenergy: Techno-Economic and Life Cycle Analyses of Its Biochemical Conversion Pathways. Renew. Sustain. Energy Rev. 2022, 157, 112011. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- El-Sheek, M.; Abomhohra, A.E.-F. Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Rupert, R.; Rodrigues, K.F.; Thien, V.Y.; Yong, W.T.L. Carrageenan From Kappaphycus Alvarezii (Rhodophyta, Solieriaceae): Metabolism, Structure, Production, and Application. Front. Plant Sci. 2022, 13, 859635. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.; Umanzor, S.; Díaz-Larrea, J.; Araújo, P.G. Kappaphycus alvarezii (Rhodophyta): New Record of an Exotic Species for the Caribbean Coast of Costa Rica. Am. J. Plant Sci. 2019, 10, 1888–1902. [Google Scholar] [CrossRef]
- Castelar, B.; de Siqueira, M.F.; Sánchez-Tapia, A.; Reis, R.P. Risk Analysis Using Species Distribution Modeling to Support Public Policies for the Alien Alga Kappaphycus alvarezii Aquaculture in Brazil. Aquaculture 2015, 446, 217–226. [Google Scholar] [CrossRef]
- Masarin, F.; Cedeno, F.R.P.; Chavez, E.G.S.; de Oliveira, L.E.; Gelli, V.C.; Monti, R. Chemical Analysis and Biorefinery of Red Algae Kappaphycus Alvarezii for Efficient Production of Glucose from Residue of Carrageenan Extraction Process. Biotechnol. Biofuels 2016, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Lechat, H.; Amat, M.; Mazoyer, J.; Gallant2, D.J.; Buléon, A.; Lahaye, M. Cell Wall Composition of the Carrageenophyte Kappaphycus alvarezii (Gigartinales, Rhodophyta) Partitioned by Wet Sieving. Hydrobiologia 1997, 9, 565–572. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The System of Galactans of the Red Seaweed, Kappaphycus alvarezii, with Emphasis on Its Minor Constituents. Carbohydr. Res. 2004, 339, 2575–2592. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Ganesan, K.; Selvaraj, K.; Subba Rao, P.V. Studies on the Functional Properties of Protein Concentrate of Kappaphycus alvarezii (Doty) Doty—An Edible Seaweed. Food Chem. 2014, 153, 353–360. [Google Scholar] [CrossRef]
- Indriatmoko; Heriyanto; Limantara, L.; Brotosudarmo, T.H.P. Composition of Photosynthetic Pigments in a Red Alga Kappaphycus alvarezi Cultivated in Different Depths. Procedia Chem. 2015, 14, 193–201. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Comparison of Sulfuric and Hydrochloric Acids as Catalysts in Hydrolysis of Kappaphycus alvarezii (Cottonii). Bioprocess. Biosyst. Eng. 2012, 35, 123–128. [Google Scholar] [CrossRef]
- Ra, C.H.; Nguyen, T.H.; Jeong, G.T.; Kim, S.K. Evaluation of Hyper Thermal Acid Hydrolysis of Kappaphycus alvarezii for Enhanced Bioethanol Production. Bioresour. Technol. 2016, 209, 66–72. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Gandhi, M.R.; Thampy, S.; Maiti, P.; Brahmbhatt, H.; Eswaran, K.; Ghosh, P.K. Kappaphycus alvarezii as a Source of Bioethanol. Bioresour. Technol. 2012, 103, 180–185. [Google Scholar] [CrossRef]
- Tabacof, A.; Calado, V.; Pereira, N. Lactic Acid Fermentation of Carrageenan Hydrolysates from the Macroalga Kappaphycus alvarezii: Evaluating Different Bioreactor Operation Modes. Polysaccharides 2023, 4, 256–270. [Google Scholar] [CrossRef]
- Abd-Rahim, F.; Wasoh, H.; Rafein, M.; Ariff, A. Food Hydrocolloids Production of High Yield Sugars from Kappaphycus alvarezii Using Combined Methods of Chemical and Enzymatic Hydrolysis. Food Hydrocoll. 2014, 42, 309–315. [Google Scholar] [CrossRef]
- Puspawati, S.; Wagiman; Ainuri, M.; Nugraha, D.A. Haslianti The Production of Bioethanol Fermentation Substrate from Eucheuma Cottonii Seaweed through Hydrolysis by Cellulose Enzyme. Agric. Agric. Sci. Procedia 2015, 3, 200–205. [Google Scholar] [CrossRef]
- Dave, N.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. A Critical Review on Production of Bioethanol from Macroalgal Biomass. Algal Res. 2019, 42, 101606. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, J.; Na, J.G.; Oh, Y.K.; Chang, Y.K. Production of 5-Hydroxymethylfurfural from Agarose by Using a Solid Acid Catalyst in Dimethyl Sulfoxide. RSC Adv. 2015, 5, 47983–47989. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Detoxification of Acidic Catalyzed Hydrolysate of Kappaphycus alvarezii (Cottonii). Bioprocess. Biosyst. Eng. 2012, 35, 93–98. [Google Scholar] [CrossRef]
- Santos, D.H.d.S.; Xiao, Y.; Chaukura, N.; Hill, J.M.; Selvasembian, R.; Zanta, C.L.P.S.; Meili, L. Regeneration of Dye-Saturated Activated Carbon through Advanced Oxidative Processes: A Review. Heliyon 2022, 8, e10205. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption- and Decomposition-Based Techniques for the Regeneration of Activated Carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Carratalá-Abril, J.; Lillo-Ródenas, M.A.; Linares-Solano, A.; Cazorla-Amorós, D. Regeneration of Activated Carbons Saturated with Benzene or Toluene Using an Oxygen-Containing Atmosphere. Chem. Eng. Sci. 2010, 65, 2190–2198. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Humin: Its Composition and Importance in Soil Organic Matter. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 143, pp. 47–138. [Google Scholar]
- Xu, Z.; Yang, Y.; Yan, P.; Xia, Z.; Liu, X.; Zhang, Z.C. Mechanistic Understanding of Humin Formation in the Conversion of Glucose and Fructose to 5-Hydroxymethylfurfural in [BMIM]Cl Ionic Liquid. RSC Adv. 2020, 10, 34732–34737. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.C.V.; Arora, J.S.; Mushrif, S.H. Mechanistic Investigation into the Formation of Humins in Acid-Catalyzed Biomass Reactions. ACS Omega 2022, 7, 44786–44795. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in Understanding the Humins: Formation, Prevention and Application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Sumerskii, I.V.; Krutov, S.M.; Zarubin, M.Y. Humin-like Substances Formed under the Conditions of Industrial Hydrolysis of Wood. Russ. J. Appl. Chem. 2010, 83, 320–327. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Gao, Q.; Wang, L.; Zhang, J. Characterization of Bulk Soil Humin and Its Alkaline-Soluble and alkaline-Insoluble Fractions. Rev. Bras. Cienc. Solo 2015, 39, 120–126. [Google Scholar] [CrossRef]
- Körner, P.; Jung, D.; Kruse, A. Influence of the PH Value on the Hydrothermal Degradation of Fructose. ChemistryOpen 2019, 8, 1121–1132. [Google Scholar] [CrossRef]
- Xia, J.; Qiu, Z.; Ma, S.; Liu, Q.; Han, R.; Liu, X.; Xu, J. Efficient Polymalic Acid Production from Corn Straw Hydrolysate by Detoxification of Phenolic Inhibitors. Front. Bioeng. Biotechnol. 2023, 11, 1339982. [Google Scholar] [CrossRef]
- Wang, T.; Meng, Y.; Qin, Y.; Feng, W.; Wang, C. Removal of Furfural and HMF from Monosaccharides by Nanofiltration and Reverse Osmosis Membranes. J. Energy Inst. 2018, 91, 473–480. [Google Scholar] [CrossRef]
- Anburajan, P.; Pugazhendhi, A.; Park, J.-H.; Kumar, G.; Choi, C.-S.; Kim, S.-H. Inhibitory Effect of 5-Hydroxymethylfurfural on Continuous Hydrogen Fermentation by Mixed Culture in a Fixed Bed Reactor. Int. J. Hydrogen Energy 2017, 42, 27570–27576. [Google Scholar] [CrossRef]
- Dessie, W.; Xin, F.; Zhang, W.; Zhou, J.; Wu, H.; Ma, J.; Jiang, M. Inhibitory Effects of Lignocellulose Pretreatment Degradation Products (Hydroxymethylfurfural and Furfural) on Succinic Acid Producing Actinobacillus succinogenes. Biochem. Eng. J. 2019, 150, 107263. [Google Scholar] [CrossRef]
- Lohr, D.; Venkov, P.; Zlatanova, J. Transcriptional Regulation in the Yeast GAL Gene Family: A Complex Genetic Network. FASEB J. 1995, 9, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Zhang, R.K.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Microbial Adaptation to Enhance Stress Tolerance. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced Biotransformation of Furfural and Hydroxymethylfurfural by Newly Developed Ethanologenic Yeast Strains. Appl. Biochem. Biotechnol. 2005, 121, 0451–0460. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Yang, C.-F.; Huang, C.-R. Biotransformation of 5-Hydroxy-Methylfurfural into 2,5-Furan-Dicarboxylic Acid by Bacterial Isolate Using Thermal Acid Algal Hydrolysate. Bioresour. Technol. 2016, 214, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sana, B. Marine Microbial Enzymes: Current Status and Future Prospects. In Hb25_Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 905–917. [Google Scholar]
- Gurpilhares, D.d.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A., Jr.; Sette, L.D. Marine Prebiotics: Polysaccharides and Oligosaccharides Obtained by Using Microbial Enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Suriya, J.; Bharathiraja, S.; Krishnan, M.; Manivasagan, P.; Kim, S.-K. Extremozymes from Marine Actinobacteria. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2016; pp. 43–66. [Google Scholar]
- Parrilli, E.; Tedesco, P.; Fondi, M.; Tutino, M.L.; Lo Giudice, A.; de Pascale, D.; Fani, R. The Art of Adapting to Extreme Environments: The Model System Pseudoalteromonas. Phys. Life Rev. 2021, 36, 137–161. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and Carrageenases: Versatile Polysaccharides and Promising Marine Enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, W.; Wu, H.; Guang, C.; Zhang, W.; Mu, W. An Overview of D-Galactose Utilization through Microbial Fermentation and Enzyme-Catalyzed Conversion. Appl. Microbiol. Biotechnol. 2021, 105, 7161–7170. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Jacob, A.; Herrmann, C.; Murphy, J.D. Fermentative Bio-Hydrogen Production from Galactose. Energy 2016, 96, 346–354. [Google Scholar] [CrossRef]
- Fonseca, B.C.; Guazzaroni, M.-E.; Reginatto, V. Fermentative Production of H2 from Different Concentrations of Galactose by the New Isolate Clostridium beijerinckii Br21. Int. J. Hydrogen Energy 2016, 41, 21109–21120. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, S.-H.; Yoon, J.-J.; Kim, S.-H.; Park, H.-D. Predominance of Cluster I Clostridium in Hydrogen Fermentation of Galactose Seeded with Various Heat-Treated Anaerobic Sludges. Bioresour. Technol. 2014, 157, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Bioproduction of Butanol from Biomass: From Genes to Bioreactors. Curr. Opin. Biotechnol. 2007, 18, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Olajuyin, A.M.; Yang, M.; Liu, Y.; Mu, T.; Tian, J.; Adaramoye, O.A.; Xing, J. Efficient Production of Succinic Acid from Palmaria Palmata Hydrolysate by Metabolically Engineered Escherichia Coli. Bioresour. Technol. 2016, 214, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Dharani, G. Evaluation of Seaweed for the Production of Lactic Acid by Fermentation Using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Murad, A.E.-H.; Khalaf, S.A.-D. Citric Acid Production from Whey with Sugars and Additives by Aspergillus niger. Afr. J. Biotechnol. 2003, 2, 356–359. [Google Scholar] [CrossRef]
- Lazar, Z.; Gamboa-Meléndez, H.; Le Coq, A.-M.C.-; Neuvéglise, C.; Nicaud, J.-M. Awakening the Endogenous Leloir Pathway for Efficient Galactose Utilization by Yarrowia lipolytica. Biotechnol. Biofuels 2015, 8, 185. [Google Scholar] [CrossRef]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of Lignocellulosic Sugars to Acetic Acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef]
- He, F.; Qin, S.; Yang, Z.; Bai, X.; Suo, Y.; Wang, J. Butyric Acid Production from Spent Coffee Grounds by Engineered Clostridium tyrobutyricum Overexpressing Galactose Catabolism Genes. Bioresour. Technol. 2020, 304, 122977. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdallah, W.; Dahman, Y. Production of Green Biocellulose Nanofibers by Gluconacetobacter xylinus through Utilizing the Renewable Resources of Agriculture Residues. Bioprocess. Biosyst. Eng. 2013, 36, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Q.; Azzawi, I.D.J.; Sameen, A.Z.; Salman, H.M. Hydrogen Fuel Cell Vehicles: Opportunities and Challenges. Sustainability 2023, 15, 11501. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, X.; Zeng, J.; Zhang, J. Biotechnological Production of Succinic Acid: Current State and Perspectives. Biofuels Bioprod. Biorefining 2012, 6, 302–318. [Google Scholar] [CrossRef]
- Almqvist, H.; Pateraki, C.; Alexandri, M.; Koutinas, A.; Lidén, G. Succinic Acid Production by Actinobacillus succinogenes from Batch Fermentation of Mixed Sugars. J. Ind. Microbiol. Biotechnol. 2016, 43, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Lee, S.Y.; Chang, H.N. Succinic Acid Production by Anaerobiospirillum Succiniciproducens ATCC 29305 Growing on Galactose, Galactose/Glucose, and Galactose/Lactose. J. Microbiol. Biotechnol. 2008, 18, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Morales, M.; Gunnarsson, I.B.; Fotidis, I.A.; Vasilakou, E.; Lyberatos, G.; Angelidaki, I. Laminaria Digitata as a Potential Carbon Source for Succinic Acid and Bioenergy Production in a Biorefinery Perspective. Algal Res. 2015, 9, 126–132. [Google Scholar] [CrossRef]
- Marinho, G.S.; Alvarado-Morales, M.; Angelidaki, I. Valorization of Macroalga Saccharina latissima as Novel Feedstock for Fermentation-Based Succinic Acid Production in a Biorefinery Approach and Economic Aspects. Algal Res. 2016, 16, 102–109. [Google Scholar] [CrossRef]
- Gutierrez, N.A.; McKay, I.A.; French, C.E.; Brooks, J.D.; Maddox, I.S. Repression of Galactose Utilization by Glucose in the Citrate-Producing YeastCandida Guilliermondii. J. Ind. Microbiol. 1993, 11, 143–146. [Google Scholar] [CrossRef]
- Dahman, Y.; Jayasuriya, K.E.; Kalis, M. Potential of Biocellulose Nanofibers Production from Agricultural Renewable Resources: Preliminary Study. Appl. Biochem. Biotechnol. 2010, 162, 1647–1659. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of Different Carbon Sources on Bacterial Cellulose Production by Gluconacetobacter xylinus Strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef] [PubMed]
| Carrageenan | Cellulose | Ash | Proteins | Insoluble Aromatics | Sulfate Groups |
|---|---|---|---|---|---|
| 31.3 ± 0.8 | 13.5 ± 0.1 | 16.0 ± 0.2 | 2.5 ± 0.3 | 1.5 ± 0.2 | 10.1 ± 0.3 |
| Hydrolysis Conditions | Products | Ref. | ||
|---|---|---|---|---|
| Acid Hydrolysis | Enzymatic Hydrolysis | Sugars (g/L) | HMF (g/L) | |
| 10% (w/v), 130 °C, 0.2 M of H2SO4, 15 min | - | 38.5 Gal | 4–5 | [70] |
| 12% (w/v), 140 °C, 180 mM of H2SO4, 5 min | - | 38.3 Tot | <5.0 | [71] |
| 50% (w/v), 121 °C, 1.0% v/v of H2SO4, 60 min | Cellic CTec2 (45 FPU/g DW) 50 °C, pH 5 150 rpm, 24 h | 81.6 Gal | 20.7 | [22] |
| 26.2–30.6% (w/w), 100 °C, 0.9 N of H2SO4, 1 h | - | 54.0 Tot | N/R | [72] |
| 8.0 g/100 mL, 110 °C 0.2 N of H2SO4, 90 min | Celluclast® 1.5 L (150 FPU/g DW) 55 °C, pH 5.5, 150 rpm, 48 h | 50.0 Tot | N/R | [74] |
| - | Cellic CTec2 (10 FPU/g DW) 45 °C, pH 4.8, 120 rpm, 72 h | 13.7 Tot | N/R | [65] |
| - | Cellulase (36 AU) 50 °C, 100 rpm, 12 h | 8.0 Tot | N/R | [75] |
| Enzyme-processed hydrolysate at 60–80 °C, 0.5–2% H2SO4, 30–90 min | Cellic CTec2 (100 FPU/g DW) 45 °C, pH 4.8, 120 rpm, 72 h | 16.0 Tot | <0.01 | [21] |
| 0.4 M of H2SO4, 100 °C, 3 h | - | 4.08 mg Tot/g biomass | N/R | [76] |
| 30% (w/v), 111 °C, 1.0% v/v H2SO4, 45 min | - | 54.2 Gal | 12.51 | [24] |
| Step | Enzymes for κ-Carrageenan Processing * | Products |
|---|---|---|
| Initial fragmentation | GH16 κ-carrageenase | Oligomer of neo carrageenan |
| Sulphate group removal | S1_7/S1_19 κ-carrageenan-G4S-sulfatase | β-carrageenan |
| Disaccharide formation | GH16/GH167 β-carrageenase | d-galactose and 3,6-anydro-d-galactose dimer |
| Monosaccharide formation | GH127/GH129 3,6-anydro-d-galactosidase | d-galactose 3,6-anydro-d-galactose |
| Step | Substrate | Enzymes Involved | Products |
|---|---|---|---|
| Cell wall permeation | Galactose | Gal-PTS | Galactose-6-Phosphate |
| Intracellular metabolism | Galactose-6-phosphate | lacAB | Tagatose-6-Phosphate |
| Intracellular metabolism | Tagatose-6-phosphate | lacC | Tagatose-1,6-Diphosphate |
| Intracellular metabolism | Tagatose1,6-diphosphate | lacD | Dihydroxyacetone phosphate, Glyceraldyde-3-phosphate |
| Step | Substrate | Enzymes Involved | Products |
|---|---|---|---|
| Cell wall permeation | Galactose | galP | β-Galactose |
| Intracellular metabolism | β-Galactose | galM | Galactose-1-phosphate |
| Intracellular metabolism | Galactose-1-phosphate | galT, galE, galU | Glucose-1-phosphate |
| Intracellular metabolism | Glucose-1-phosphate | pgmB | Tagatose-1,6-diphosphate |
| Product | Microorganism | Fermentation Type | Origin of Galactose | Ref. |
|---|---|---|---|---|
| Ethanol | Saccharomyces cerevisiae | Anaerobic | K. alvarezii | [22,74] |
| Hydrogen | cluster I Clostridium Clostridium beijerinckii Br21 | Anaerobic | Synthetic | [105,106,107] |
| Butanol | C. beijerinckii BA101 C. acetobutylicum 824 | Anaerobic | Synthetic | [108] |
| Succinic acid | E. coli | Anaerobic | Palmaria palmata | [109] |
| Lactic acid | Lactobacillus pentosus Lactobacillus plantarum | Anaerobic | K. alvarezii | [73,110] |
| Citric acid | Aspergillus niger Yarrowia lipolytica Candida guilliermondii | Aerobic and Anaerobic | Whey protein Synthetic | [111,112] |
| Acetic acid | Moorella thermoacetica | Anaerobic | Lignocellulosic sugars | [113] |
| Butyric acid | Clostridium tyrobutyricum | Anaerobic | Spent coffee ground | [114] |
| Bacterial Cellulose | Gluconacetobacter xylinus | Aerobic | Wheat straw | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabacof, A.; Calado, V.; Pereira, N., Jr. The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives. Fermentation 2024, 10, 283. https://doi.org/10.3390/fermentation10060283
Tabacof A, Calado V, Pereira N Jr. The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives. Fermentation. 2024; 10(6):283. https://doi.org/10.3390/fermentation10060283
Chicago/Turabian StyleTabacof, Adam, Verônica Calado, and Nei Pereira, Jr. 2024. "The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives" Fermentation 10, no. 6: 283. https://doi.org/10.3390/fermentation10060283
APA StyleTabacof, A., Calado, V., & Pereira, N., Jr. (2024). The Macroalga Kappaphycus alvarezii as a Potential Raw Material for Fermentation Processes within the Biorefinery Concept: Challenges and Perspectives. Fermentation, 10(6), 283. https://doi.org/10.3390/fermentation10060283







