Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Isolation and Identification
2.2. Inoculum Cultivation
2.3. Growth of Chlorella Vulgaris S2 on Different Organic Carbon Sources
2.4. Effect of Concentration of Carbon Source on Biomass Yield
2.5. Phototrophic Cultivation
2.6. Effect of C:N Ratio on Growth and Lipid Content
2.7. Heterotrophic Fed-Batch Cultivation
2.8. Analytical Methods
2.8.1. Determination of Monosaccharide Concentration by Ultra-Performance Liquid Chromatography (UPLC)
2.8.2. Determination of Nitrate Concentration
2.8.3. Biomass Composition
Carbohydrate Content
Protein Content
Lipid Content and Fatty Acid Composition
3. Results and Discussion
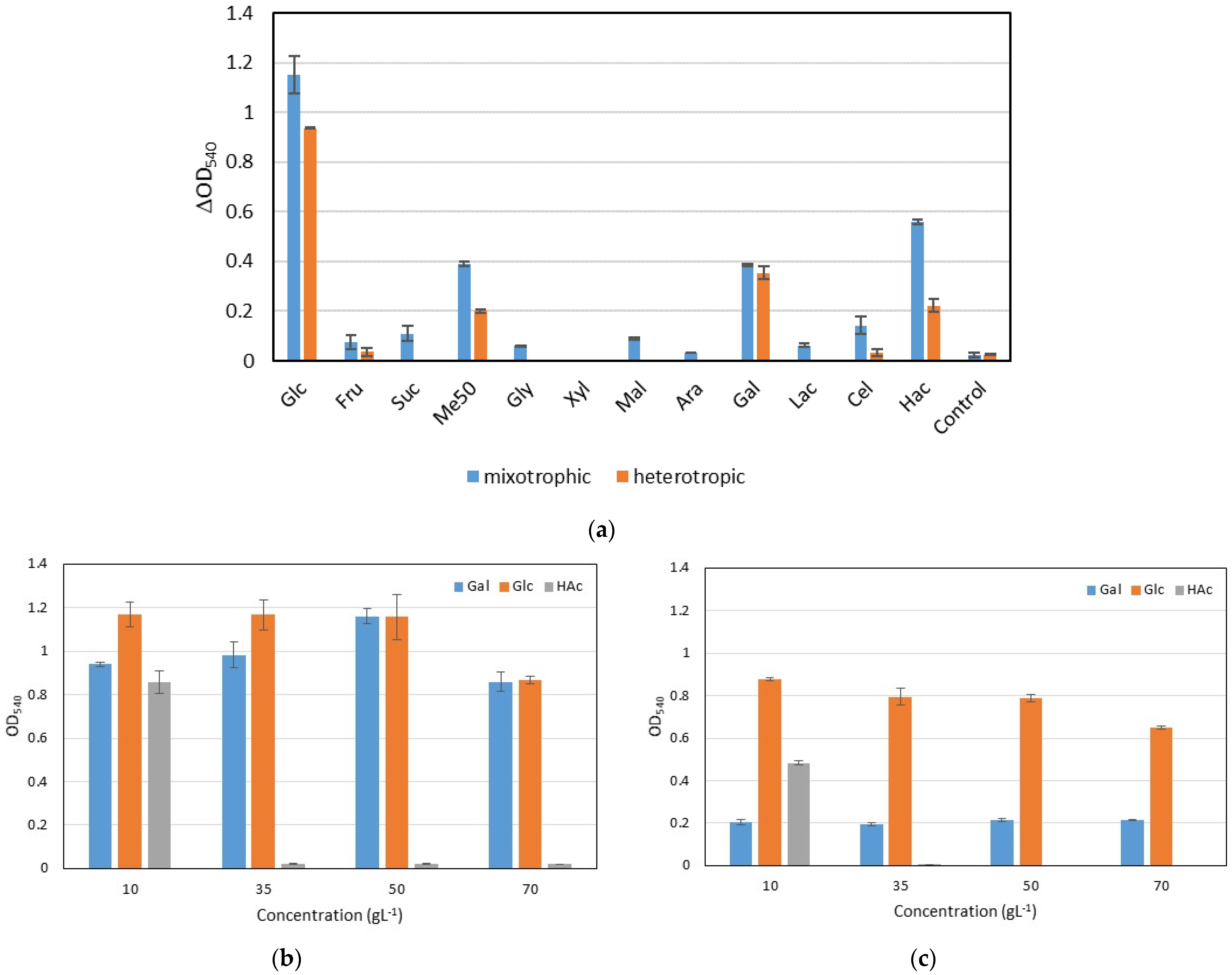
3.1. Utilisation of Different Carbon Sources and Effect of Carbon Concentration on Growth of Chlorella vulgaris S2
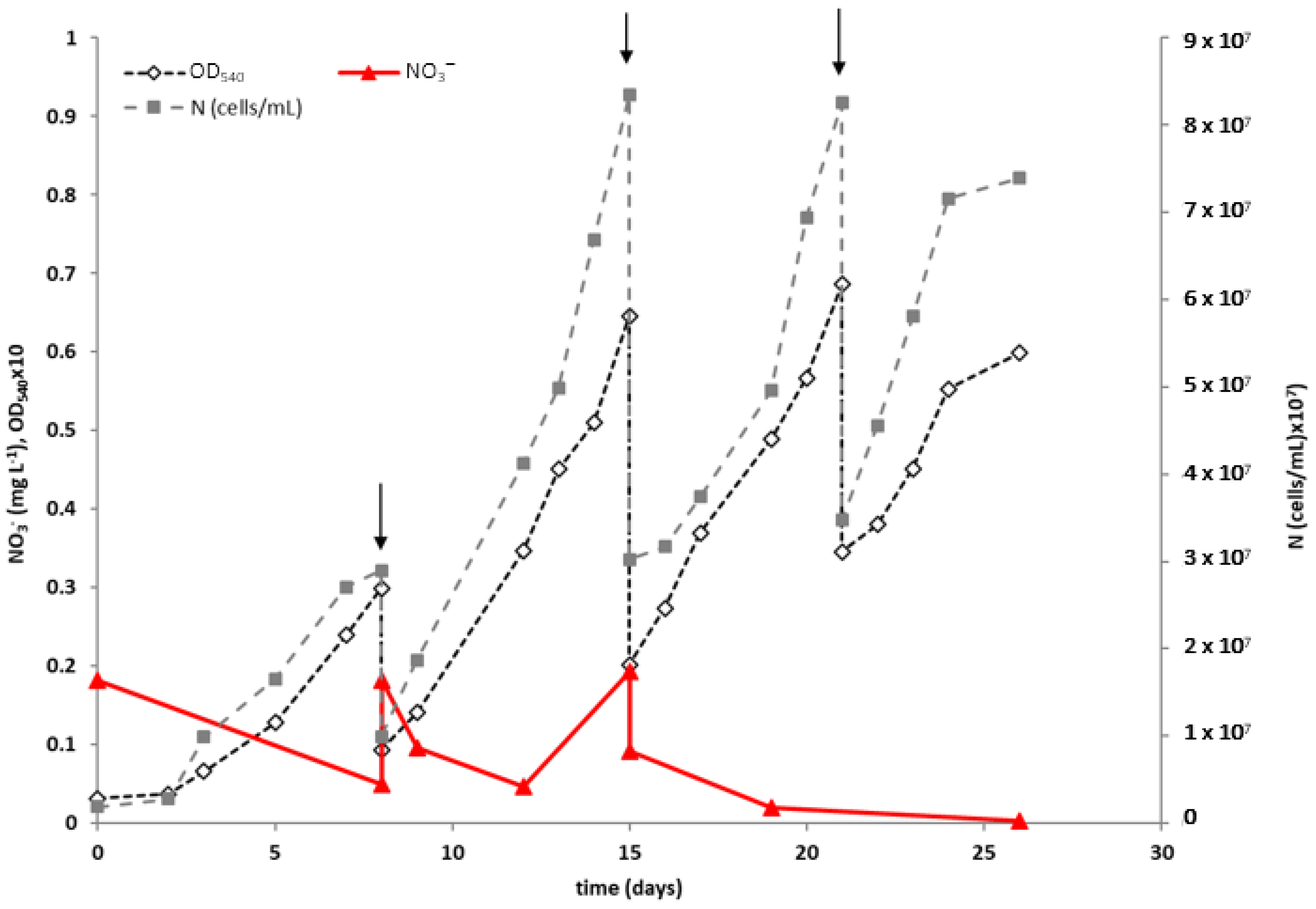
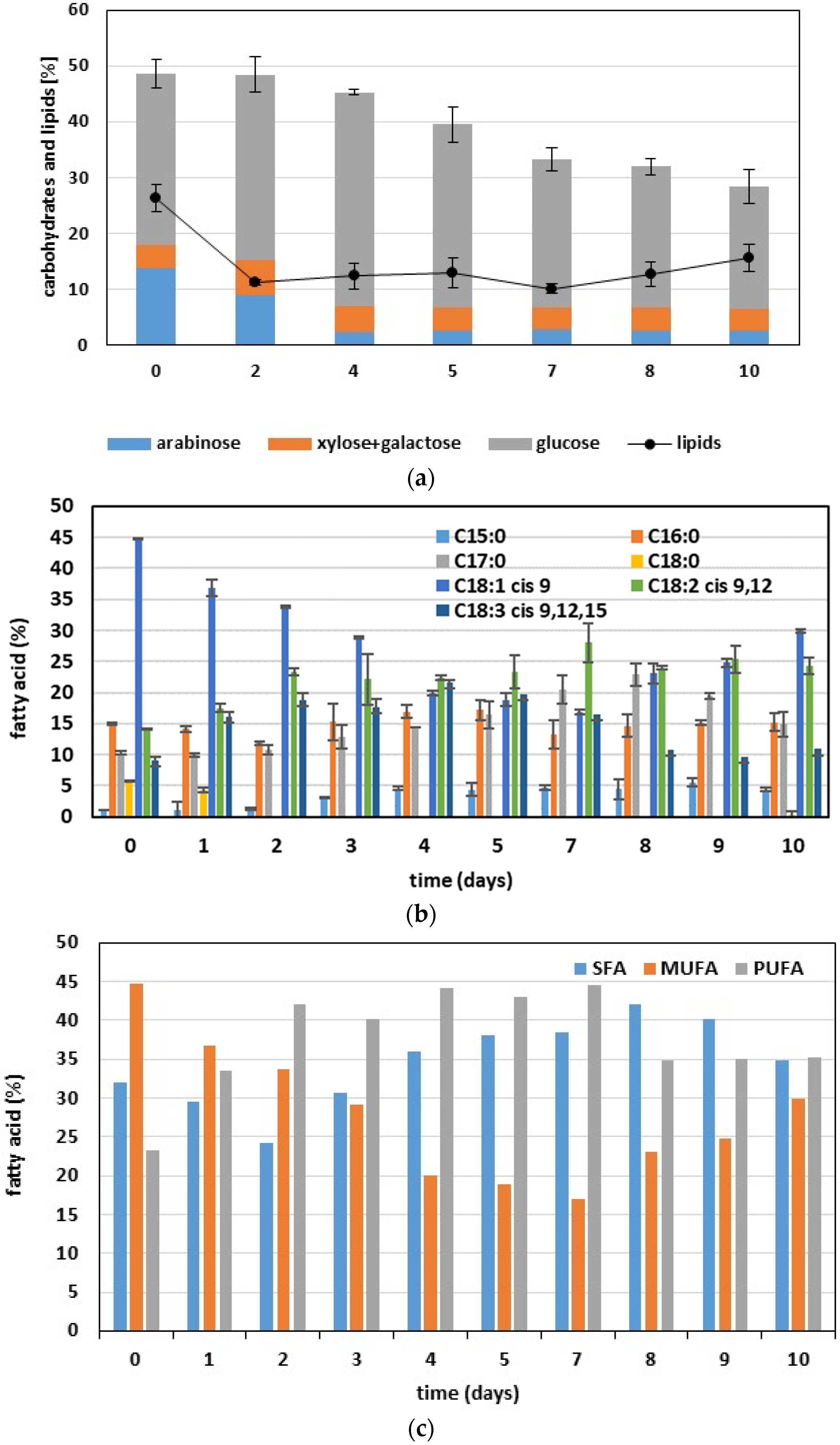
3.2. Fed-Batch Phototrophic Cultivation
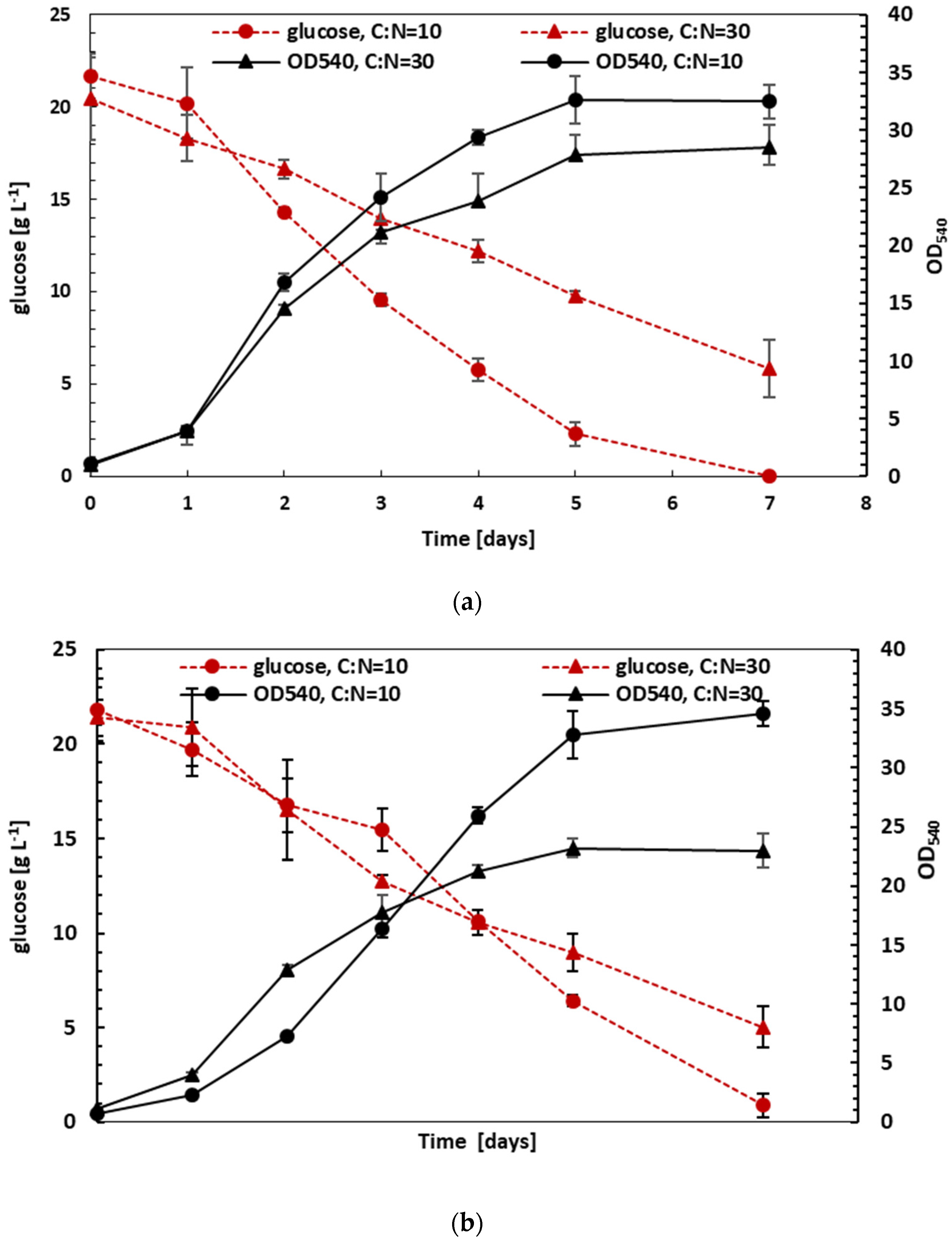
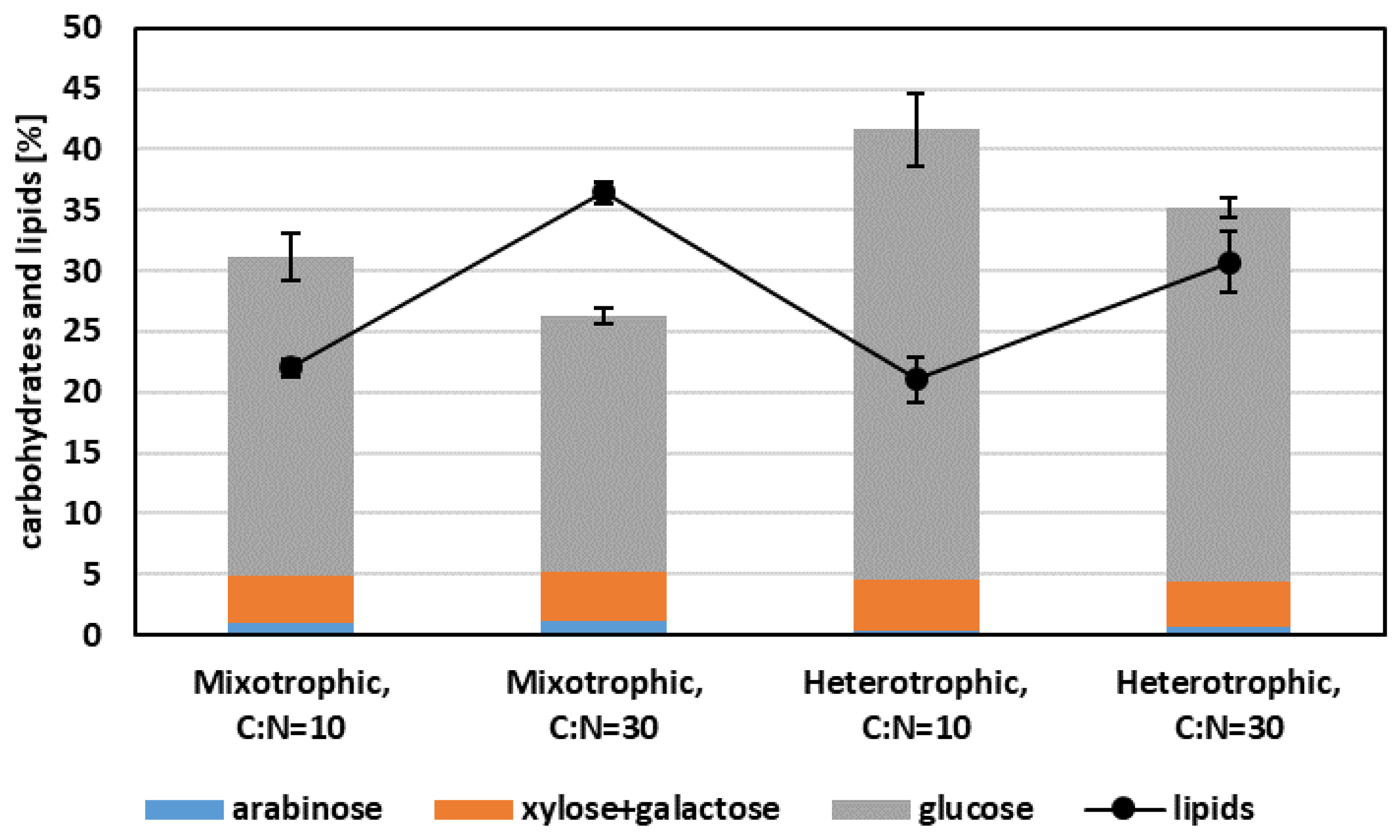
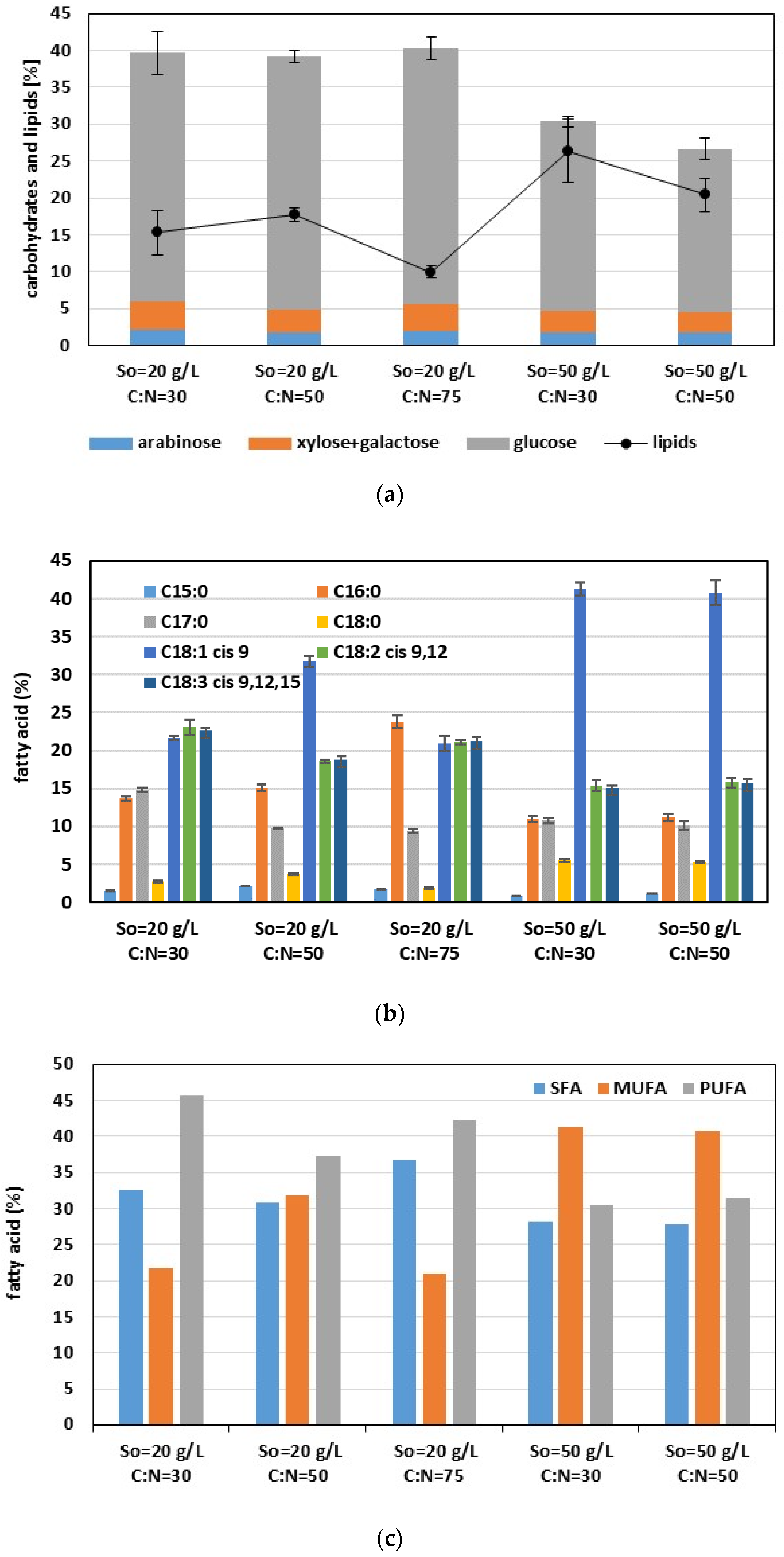
3.3. Effect of C:N Ratio on Growth and Lipid Production under Mixotrophic and Heterotrophic Conditions
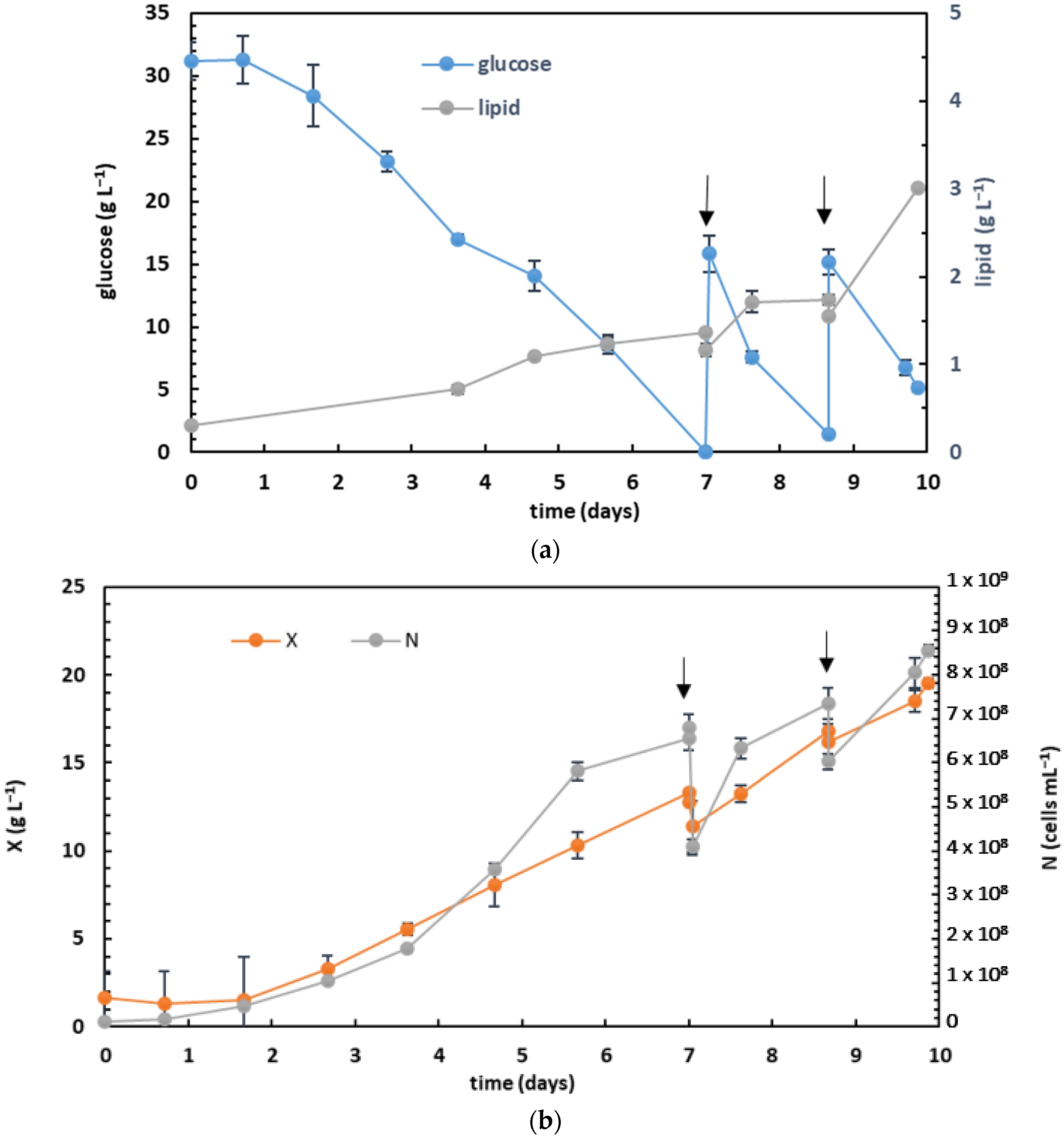
3.4. Fed-Batch Heterotrophic Cultivation on Glucose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dragone, G. Challenges and Opportunities to Increase Economic Feasibility and Sustainability of Mixotrophic Cultivation of Green Microalgae of the Genus Chlorella. Renew. Sustain. Energy Rev. 2022, 160, 112284. [Google Scholar] [CrossRef]
- Grubišić, M.; Ivančić Šantek, M.; Šantek, B. Potential of Microalgae for the Production of Different Biotechnological Products. Chem. Biochem. Eng. Q. 2019, 33, 161–181. [Google Scholar] [CrossRef]
- Debnath, C.; Kanti, T.; Bhunia, B.; Mishra, U. Microalgae: Sustainable Resource of Carbohydrates in Third-Generation Biofuel Production. Renew. Sustain. Energy Rev. 2021, 150, 111464. [Google Scholar] [CrossRef]
- Senroy, S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. Proc. Zool. Soc. 2014, 68, 1–8. [Google Scholar] [CrossRef]
- Krishna, A.; Wayne, K.; Rambabu, K.; Tao, Y.; Chu, D.; Show, P. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Martins, D.A.; Pereira, H.; Ben-hamadou, R.; Abu-salah, K.M.; Arabia, S. Alternative Sources of N-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of Starch and Lipids in Microalgae: Biosynthesis and Manipulation by Nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Talaei, M.; Prieto, A. A Review on Performance of Sustainable Microalgae Photobioreactor Façades Technology: Exploring Challenges and Advantages. Archit. Sci. Rev. 2024, 1–28. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Villanova, V.; Fortunato, A.E.; Singh, D.; Bo, D.D.; Conte, M.; Obata, T.; Jouhet, J.; Fernie, A.R.; Marechal, E.; Falciatore, A.; et al. Investigating Mixotrophic Metabolism in the Model Diatom Phaeodactylum tricornutum. Phil. Trans. R. Soc. B 2017, 372, 20160404. [Google Scholar] [CrossRef]
- Jiao, K.; Xiao, W.; Xu, Y.; Zeng, X.; Ho, S.H.; Laws, E.A.; Lu, Y.; Ling, X.; Shi, T.; Sun, Y.; et al. Using a Trait—Based Approach to Optimise Mixotrophic Growth of the Red Microalga Porphyridium purpureum towards Fatty Acid Production. Biotechnol. Biofuels 2018, 11, 273. [Google Scholar] [CrossRef]
- Tiong, I.; Ru, K.; Sung, Y.Y.; Jusoh, M.; Abdul, M.E.; Nagappan, T. Chlorella vulgaris: A Perspective on Its Potential for Combining High Biomass with High Value Bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Yun, H.; Kim, Y.; Yoon, H. Effect of Different Cultivation Modes (Photoautotrophic, Mixotrophic, and Heterotrophic) on the Growth of Chlorella sp. and Biocompositions. Front. Bioeng. Bioetchnol. 2021, 9, 774143. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Wang, H.; Huo, H.; Guo, B.; Liu, N.; Zhang, A.; Niu, S. Regulation of Biomass, Pigments, and Lipid Production by Chlorella vulgaris 31 through Controlling Trophic Modes and Carbon Sources. J. Appl. Phycol. 2020, 32, 1569–1579. [Google Scholar] [CrossRef]
- Alves, I.R.F.S.; Mahler, C.F.; Oliveira, L.B.; Reis, M.M.; Bassin, J.P. Assessing the Use of Crude Glycerol from Biodiesel Production as an Alternative to Boost Methane Generation by Anaerobic Co-Digestion of Sewage Sludge. Biomass Bioenergy 2020, 143, 105831. [Google Scholar] [CrossRef]
- Sun, C.; Ren, H.; Sun, F.; Hu, Y.; Liu, Q.; Song, G.; Abdulkhani, A.; Show, P.L. Glycerol Organosolv Pretreatment Can Unlock Lignocellulosic Biomass for Production of Fermentable Sugars: Present Situation and Challenges. Bioresour. Technol. 2022, 344, 126264. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Ivančić Šantek, M.; Grubišić, M.; Galić Perečinec, M.; Beluhan, S.; Šantek, B. Lipid Production by Mortierella Isabellina from Pretreated Corn Cobs and Effect of Lignocellulose Derived Inhibitors on Growth and Lipid Synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.; Li, C.; Peng, Y.; Lu, M.; Jin, W.; Bao, J.; Guo, Y. Effect of Organic Carbon to Nitrogen Ratio in Wastewater on Growth, Nutrient Uptake and Lipid Accumulation of a Mixotrophic Microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Lacroux, J.; Seira, J.; Trably, E.; Bernet, N.; Steyer, J.; Lis, R.V. Mixotrophic Growth of Chlorella sorokiniana on Acetate and Butyrate: Interplay between substrate, C:N Ratio and PH. Front. Microbiol. 2021, 12, 703614. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the Physiology of Chlorella vulgaris Cultivated in Sweet Sorghum Bagasse Hydrolysate for Sustainable Algal Biomass and Lipid Production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef]
- El, M.; Nejib, O.; Chourouk, T.; Kallel, A.; Hussain, S. Insights into the Use of Landfill Leachate to Grow Chlorella sp. for Lipid and Fatty Acids Production. Clean Technol. Environ. Policy 2023, 25, 1631–1642. [Google Scholar] [CrossRef]
- Grubišić, M. Biotechnological Potential of Microalgae Isolated from River Gacka and Adriatic Sea-Characterisation and Optimisation of Growth Conditions. Ph.D. Dissertation, University of Zagreb, Faculty of Food Technology and Biotechnology, Zagreb, Croatia, 2022. [Google Scholar]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Ivančić Šantek, M. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterisation of Biomass, Pigment, Lipid and Fatty Acid Composition, and Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina Polymorpha Gen. et Sp. Nov. H. J. Phycol. 1965, 38, 34–38. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Grubišić, M.; Šantek, B.; Kuzmić, M.; Čož-Rakovac, R.; Ivančić Šantek, M. Enhancement of Biomass Production of Diatom Nitzschia sp. S5 through Optimisation of Growth Medium Composition and Fed-Batch Cultivation. Mar. Drugs 2024, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Wychen, S.V.; Laurens, L.M.L. Determination of Total Carbohydrates in Algal Biomass Determination of Total Carbohydrates in Algal Biomass Laboratory Analytical Procedure (LAP); NREL/TP-5100-60957; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2015. [Google Scholar]
- Waterborg, J.H.; Matthews, H.R. Lowry Method for Protein Quantitation. In The Protein Protocols Handbook. Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–4. [Google Scholar]
- Grubišić, M.; Mihajlovski, K.; Gruičić, A.M.; Beluhan, S.; Šantek, B.; Ivančić Šantek, M. Strategies for Improvement of Lipid Production by Yeast Trichosporon Oleaginosus from Lignocellulosic Biomass. J. Fungi 2021, 7, 934. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Daum, G. Extraction of Yeast Lipids. Methods Mol. Biol. 2006, 313, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wychen, S.V.; Ramirez, K.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification Laboratory Analytical Procedure (LAP); NREL/TP-5100-60958; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2015. [Google Scholar]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and Lipid Productivities of Chlorella vulgaris under Autotrophic, Heterotrophic and Mixotrophic Growth Conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Angelini, F.; Bellini, E.; Marchetti, A.; Salvatori, G.; Villano, M.; Pontiggia, D.; Ferrari, S. Efficient Utilization of Monosaccharides from Agri-Food Byproducts Supports Chlorella vulgaris Biomass Production under Mixotrophic Conditions. Algal Res. 2024, 77, 103358. [Google Scholar] [CrossRef]
- Kong, W.; Hua, S.; Cao, H.; Mu, Y.; Yang, H.; Song, H.; Xia, C. Optimization of Mixotrophic Medium Components for Biomass Production and Biochemical Composition Biosynthesis by Chlorella vulgaris Using Response Surface Methodology. J. Taiwan Inst. Chem. Eng. 2012, 43, 359–366. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, W.; Zhang, P.; Sun, H.; Chen, M.; Lu, S.; Li, P. Effects of Various Organic Carbon Sources on the Growth and Biochemical Composition of Chlorella pyrenoidosa. Bioresour. Technol. 2014, 173, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Stadler, R.; Wolf, K.; Hilgarth, C.; Tanner, W.; Sauer, N. Subcellular Localization of the Lnducible Chlorella HUPl Monosaccharide-H+ Symporter and Cloning of a Co-Lnduced Galactose-H+ Symporter. Plant Physiol. 1992, 107, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Liu, L.; Wu, B.; Li, J.; He, M.; Hu, G. Simultaneous Production of Algal Biomass and Lipid by Heterotrophic Cultivation of Linoleic Acid-Rich Oleaginous Microalga Chlorella sorokiniana Using High Acetate Dosage. Bioresour. Technol. 2024, 399, 130566. [Google Scholar] [CrossRef] [PubMed]
- Perez-garcia, O.; Escalante, F.M.E.; Luz, E.; Bashan, Y. Heterotrophic Cultures of Microalgae: Metabolism and Potential Products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. Another Explanation for the Toxicity of Fermentation Acids at Low PH: Anion Accumulation versus Uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; de Jong, H. Acetate Metabolism and the Inhibition of Bacterial Growth by Acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhong, Y.; Jiang, Y.; Chen, F. Differential Lipid and Fatty Acid Profiles of Photoautotrophic and Heterotrophic Chlorella zofingiensis: Assessment of Algal Oils for Biodiesel Production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Aggelis, G. Biochemical Activities in Chlorella sp. and Nannochloropsis salina during Lipid and Sugar Synthesis in a Lab-Scale Open Pond Simulating Reactor. J. Biotechnol. 2013, 164, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of Phosphorus on Lipid Accumulation in Freshwater Microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Rohit, M.V.; Mohan, S.V. Quantum Yield and Fatty Acid Profile Variations with Nutritional Mode during Microalgae Cultivation. Front. Bioeng. Bioetchnol. 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Direct Mixotrophic Cultivation of a Chlorella sorokiniana Strain for Enhanced Biomass and Lipid Production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Chen, F.; Johns, M.R. Effect of C / N Ratio and Aeration on the Fatty Acid Composition of Heterotrophic Chlorella sorokiniana. J. Appl. Phycol. 1991, 3, 203–209. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Altimari, P.; Trabucco, F.; Toro, L. Mixotrophic Growth of Chlorella vulgaris and Nannochloropsis oculata: Interaction between Glucose and Nitrate. J. Chem. Technol. Biotechnol. 2013, 89, 652–661. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Zhang, D.; Liu, J.; Nanqi, R.; Yujie, F. Combined Effects of Carbon, Phosphorus and Nitrogen on Lipid Accumulation of Chlorella vulgaris in Mixotrophic Culture. J. Chem. Technol. Biotechnol. 2016, 91, 680–684. [Google Scholar] [CrossRef]
- Jin, W.; Chen, L.; Hu, M.; Sun, D.; Li, A.; Li, Y.; Hu, Z.; Zhou, S.; Tu, Y.; Xia, T.; et al. Tween-80 Is Effective for Enhancing Steam-Exploded Biomass Enzymatic Saccharification and Ethanol Production by Specifically Lessening Cellulase Absorption with Lignin in Common Reed. Appl. Energy 2016, 175, 82–90. [Google Scholar] [CrossRef]
- Bouyam, S.; Choorit, W.; Sirisansaneeyakul, S.; Chisti, Y. Heterotrophic Production of Chlorella sp. TISTR 8990—Biomass Growth and Composition under Various Production Conditions. Biotechnol. Prog. 2017, 33, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, A.D.S.; Riaño-pachón, D.M.; Mattiello, L.; Albuquerque, L. Analysis of Autotrophic, Mixotrophic and Heterotrophic Phenotypes in the Microalgae Chlorella vulgaris Using Time-Resolved Proteomics and Transcriptomics Approaches. Algal Res. 2020, 51, 102060. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and Morphological Changes Triggered by Nitrogen Stress in the Oleaginous Microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Vítová, M.; Bišová, K.; Kawano, S.; Zachleder, V. Accumulation of Energy Reserves in Algae: From Cell Cycles to Biotechnological Applications. Biotechnol. Adv. 2015, 33, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Gawa, K.; Arai, M.; Naganawa, H.; Ikeda, Y.; Kondo, S. A New β-D-Galactan Having 3-ο-Methyl-D-Galactose from Chlorella vulgaris. J. Appl. Glycosci. 2001, 48, 325–330. [Google Scholar] [CrossRef]
- Pieper, S.; Unterieser, I.; Mann, F.; Mischnick, P. A New Arabinomannan from the Cell Wall of the Chlorococcal Algae Chlorella vulgaris. Carbohydr. Res. 2012, 352, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Ikeda, Y.; Kondo, S. A New Trisaccharide, α-d-Glucopyranuronosyl-(1→3)-α-l-Rhamnopyranosyl-(1→2)-α-l-Rhamnopyranose from Chlorella vulgaris. Carbohydr. Res. 1999, 321, 128–131. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Ferreira, S.S.; Correia, A.; Vilanova, M.; Silva, T.H.; Coimbraa, A.M.; Nunesa, C. Reserve, Structural and Extracellular Polysaccharides of Chlorella vulgaris: A Holistic Approach. Algal Res. 2020, 45, 101757. [Google Scholar] [CrossRef]
- Li, T.; Gargouri, M.; Feng, J.; Park, J.; Gao, D.; Miao, C.; Dong, T.; Gang, D.R.; Chen, S. Regulation of Starch and Lipid Accumulation in a Microalga Chlorella sorokiniana. Bioresour. Technol. 2015, 180, 250–257. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Jiang, X.; Wang, X.; Liang, W. Lipid Accumulation of Chlorella pyrenoidosa under Mixotrophic Cultivation Using Acetate and Ammonium. Bioresour. Technol. 2018, 262, 342–346. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Deschênes, J.; Tremblay, R.; Jolicoeur, M. Glucose Feeding Recalibrates Carbon Flux Distribution and Favours Lipid Accumulation in Chlorella protothecoides through Cell Energetic Management. Algal Res. 2016, 14, 83–91. [Google Scholar] [CrossRef]
- Nagle, N.; Lemke, P. Production of Methyl Ester Fuel from Microalgae. Appl. Biochem. Biotechnol. 1990, 24–25, 355–361. [Google Scholar] [CrossRef]
- Williams, P.J.L.B.; Laurens, L.M. Microalgae as Biodiesel & Biomass Feedstocks: Review & Analysis of the Biochemistry, Energetics & Economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Baroni, É.G.; Yap, K.Y.; Webley, P.A.; Scales, P.J.; Martin, G.J.O. The Effect of Nitrogen Depletion on the Cell Size, Shape, Density and Gravitational Settling of Nannochloropsis salina, Chlorella sp. (Marine) and Haematococcus pluvialis. Algal Res. 2019, 39, 101454. [Google Scholar] [CrossRef]
- Verma, R.; Mehan, L.; Kumar, R.; Kumar, A.; Srivastava, A. Computational Fluid Dynamic Analysis of Hydrodynamic Shear Stress Generated by Different Impeller Combinations in Stirred Bioreactor. Biochem. Eng. J. 2019, 151, 107312. [Google Scholar] [CrossRef]
- Knothe, G. Fuel Properties of Highly Polyunsaturated Fatty Acid Methyl Esters. Prediction of Fuel Properties of Algal Biodiesel. Energy Fuels 2012, 26, 5265–5273. [Google Scholar] [CrossRef]

| Time (Days) | X (g L−1) | L (g L−1) | wL (%) | wP (%) | wCHO (%) | PrX (g L−1 d−1) | PrL (g L−1 d−1) |
|---|---|---|---|---|---|---|---|
| 8 | 0.500 ± 0.011 | 0.060 ± 0.004 | 12.05 ± 0.35 | 53.79 | - | 0.053 | 0.0090 |
| 15 | 0.780 ± 0.023 | 0.077 ± 0.004 | 9.86 ± 0.14 | 67.88 | - | 0.103 | 0.0102 |
| 21 | 1.026 ± 0.026 | 0.107 ± 0.010 | 10.42 ± 0.85 | 52.54 | - | 0.099 | 0.01038 |
| 26 | 0.788 ± 0.009 | 0.081 ± 0.006 | 10.24 ± 0.37 | 47.86 | 8.78 | 0.049 | 0.00545 |
| Cultivation | C:N (mol mol−1) | X (g L−1) | L (g L−1) | μ (day−1) | YX/S (g g−1) | YL/S (g g−1) | PrX (g L−1 d−1) | PrL (g L−1 d−1) |
|---|---|---|---|---|---|---|---|---|
| M | 10 | 8.58 ± 0.89 | 1.88 ± 0.12 | 7.84 | 0.396 | 0.087 | 1.226 | 0.270 |
| M | 30 | 7.48 ± 0.24 | 2.73 ± 0.15 | 6.86 | 0.512 | 0.187 | 1.069 | 0.390 |
| H | 10 | 10.26 ± 071 | 2.15 ± 0.06 | 7.59 | 0.491 | 0.103 | 1.465 | 0.308 |
| H | 30 | 6.92 ± 025 | 2.12 ± 0.14 | 4.04 | 0.423 | 0.130 | 0.988 | 0.303 |
| So (g L−1) | C:N (mol mol−1) | X (g L−1) | L (g L−1) | YX/S (g g−1) | YL/S (g g−1) | PrX (g L−1 d−1) | PrL (g L−1 d−1) |
|---|---|---|---|---|---|---|---|
| 20 | 30 | 10.10 ± 0.75 | 1.55 ± 0.21 | 0.499 | 0.077 | 1.443 * | 0.221 * |
| 20 | 50 | 12.51 ± 0.61 | 2.23 ± 0.13 | 0.818 | 0.146 | 1.390 ** | 0.248 ** |
| 20 | 75 | 6.84 ± 0.26 | 0.68 ± 0.17 | 0.787 | 0.079 | 0.760 ** | 0.075 ** |
| 50 | 30 | 14.49 ± 1.31 | 3.82 ± 0.22 | 0.406 | 0.108 | 1.610 ** | 0.424 ** |
| 50 | 50 | 20.14 ± 2.34 | 4.12 ± 0.27 | 0.658 | 0.135 | 2.238 ** | 0.458 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubišić, M.; Peremin, I.; Djedović, E.; Šantek, B.; Ivančić Šantek, M. Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition. Fermentation 2024, 10, 270. https://doi.org/10.3390/fermentation10060270
Grubišić M, Peremin I, Djedović E, Šantek B, Ivančić Šantek M. Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition. Fermentation. 2024; 10(6):270. https://doi.org/10.3390/fermentation10060270
Chicago/Turabian StyleGrubišić, Marina, Ines Peremin, Elvis Djedović, Božidar Šantek, and Mirela Ivančić Šantek. 2024. "Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition" Fermentation 10, no. 6: 270. https://doi.org/10.3390/fermentation10060270
APA StyleGrubišić, M., Peremin, I., Djedović, E., Šantek, B., & Ivančić Šantek, M. (2024). Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition. Fermentation, 10(6), 270. https://doi.org/10.3390/fermentation10060270








