Abstract
This research investigates how different fermentation techniques using non-Saccharomyces yeast (Candida ethanolica Ce, Hanseniaspora guilliermondii Hg, Hanseniaspora thailandica Ht) and Saccharomyces cerevisiae (Sc) affect the synthesis of hesperidin, nobiletin, and other flavonoid and aromatic substances, which play a vital role in improving the overall quality of fruit wines due to their various biological properties. The combination of Sc:(Ce.Ht)-1:100 (Ce 0.5 × 107 CFU/mL, Ht 0.5 × 107 CFU/mL, Sc 1 × 105 CFU/mL) yielded the highest hesperidin content at 4.12 ± 0.08 mg/L, followed by the Sc:(Ce.Hg)-1:1 (Ce 0.5 × 107 CFU/mL, Hg 0.5 × 107 CFU/mL, Sc 1 × 107 CFU/mL) combination at 4.08 ± 0.06 mg/L. The highest nobiletin content was achieved by the (Hg.Ht)-10-Sc (Hg 0.5 × 107 CFU/mL, Ht 0.5 × 107 CFU/mL, Sc 1 × 107 CFU/mL) combination, reaching 1.04 ± 0.05 mg/L, which was significantly higher than other multi-strain combinations. Additionally, the hesperidin content produced by the (Hg.Ht)-10-Sc combination was relatively high at 4.04 ± 0.02 mg/L, demonstrating a richness and complexity of aroma superior to that of fermentation with commercial yeast strains alone. The findings suggest that the (Hg.Ht)-10-Sc combination is the most effective multi-strain combination for increasing the levels of nobiletin and hesperidin in citrus wine, thereby enhancing the overall quality of the wine. These experimental results offer a promising approach for enhancing the quality of citrus wines and other fruit wines.
1. Introduction
Citrus fruits, being widely consumed and produced globally, are among the most popular fruit crops [1]. Consequently, the utilization of microbial fermentation in processing citrus fruits into wine products not only introduces new market opportunities in the citrus product processing industry but also addresses the increasing demand for a variety of citrus products. This methodology aids in alleviating challenges within the citrus industry, including concentrated ripening periods, inadequate storability, disconnect between production and sales, processing delays, and environmental pollution [2].
Presently, the predominant strains utilized for the production of citrus fruit wines are mature commercial wine yeasts commonly used in pure-culture fermentations, leading to fruit wines characterized by stable quality yet relatively simplistic flavor profiles [3]. Recent research has indicated that non-Saccharomyces yeast derived from the fruits themselves contributes significantly to the fermentation process of fruit wines [3]. The utilization of co-fermentation involving non-Saccharomyces and Saccharomyces cerevisiae (Sc) has been shown to significantly augment the synthesis of aroma compounds such as esters, phenolic acids, and flavonoids, while simultaneously decreasing ethanol levels and modifying the color profile of the resulting wine [4]. This trend is reflective of a burgeoning consumer preference for fruit wines exhibiting greater complexity in flavor profiles and unique regional attributes. Consequently, there is a rising demand for fruit wines that have been fermented using specific phenotypic non-Saccharomyces yeast in conjunction with commercially available wine yeasts, a practice that has the potential to enhance the marketability and competitiveness of fruit wines. Non-Saccharomyces yeast isolated from citrus is more adapted to the fermentation environment compared to exogenous non-Saccharomyces yeast, offering unique flavors and nutritional values [5].
Secondary metabolites found in citrus fruits, including flavonoids, alkaloids, limonoids, carotenoids, phenolic acids, and essential oils, are important for improving human health due to their diverse bioactive properties [6]. These include antioxidant, anti-inflammatory, and anticancer activities, as well as cardiovascular and neuroprotective effects. Research has shown that microbial fermentation can produce a variety of novel bioactive compounds, such as aromatic components, flavonoids, phenolic acids, lactic acid, ethyl esters, and tetramethylpyrazine, among other metabolites [7,8]. These newly synthesized bioactive substances have been found to possess enhanced biological activities, particularly in compounds like nobiletin and hesperidin [8]. Nobiletin, also known as nobiletine, tangeretin, and 5,6,7,8,3′,4′-hexamethoxyflavone, is a polymethoxyflavonoid compound primarily derived from the dried young or immature fruit of citrus plants and their cultivated varieties. It demonstrates a wide range of medicinal properties, supported by several research studies [9,10]. These properties encompass anti-cancer, anti-inflammatory, antioxidant, tissue fibrosis prevention, blood pressure regulation, blood sugar management, and cholesterol-lowering effects [9,10,11]. Hesperidin is mainly used in the central nervous system to treat delayed motor disorders like Parkinson’s disease [12]. Recent research indicates that hesperidin is instrumental in suppressing tumor growth and treating conditions that impact various vital physiological systems. Its mechanisms include inducing apoptosis with a wide-ranging anti-cancer effect, particularly in lung, stomach, breast, and oral cancers [13,14]. Additionally, hesperidin has been found to lower blood pressure, regulate blood lipids, address arrhythmias, and combat arteriosclerosis in the cardiovascular system [15].
The intricate process of fruit wine production via natural fermentation entails intricate interactions between multiple yeast strains [16], yielding a final product with elevated levels of bioactive compounds in comparison to those generated using commercial wine yeasts, albeit with unpredictable outcomes. Consequently, the amalgamation of non-Saccharomyces yeast with Sc to replicate the advantageous outcomes of natural fermentation has the potential to harness the unique functional attributes of individual strains for the production of premium-quality goods. Identifying combinations of fermentation methods that enhance fruit wine quality represents a promising avenue of research.
Our previous research identified non-Saccharomyces yeast with favorable characteristics in naturally fermented Nanfeng tangerine fruit vinegar, laying a solid foundation for this study. Furthermore, it was observed that co-fermentation of these non-Saccharomyces yeasts and Sc could enhance the flavor and total flavonoid content of fruit wine, although the levels of hesperidin and nobiletin did not show an increase [17]. It has been reported that multi-strain fermentation can elevate the flavonoid content in Goji Juice [18]. The aim of this research is to utilize three non-Saccharomyces yeast varieties (Candida ethanolica Ce, Hanseniaspora guilliermondii Hg, and Hanseniaspora thailandica Ht) obtained from the spontaneous fermentation of Nanfeng tangerine wine, known for their impressive fermentation abilities, along with commercial wine yeast, in various mixtures to ferment Nanfeng tangerines. The analysis of nobiletin and hesperidin content, as well as aromatic components, in fruit wines produced through various fermentation combinations will be conducted to establish a foundation for the creation of functional fruit wines with high levels of bioactive compounds.
2. Materials and Methods
2.1. Yeast Strains and Raw Materials
S. cerevisiae (Angel yeast) was obtained from Angel Yeast Co., Ltd. (Yichang, Hubei, China) and stored at −80 °C before usage in our laboratory. Three non-Saccharomyces yeast strains, Ce, Hg, and Ht, were isolated and identified from the natural fermentation of Nanfeng tangerine wine in preliminary laboratory experiments and were stored at −80 °C.
Nanfeng (NF) tangerines (Citrus reticulata Blanco) from NF county (N 27°12′48″ and E 116°31′31″), Jiangxi Province, China, were harvested at optimal maturity, washed three times with deionized water (Milli-Q Advantage A10 Water System, Millipore, Billerica, MA, USA), blotted dry with sterilized filter paper, and squeezed with a sterilized hand-press juicer (Guangzhou Onoke Food Equipment Co., Ltd., Guangzhou, China) to obtain the juice. A total of 2 L of tangerine juice was poured into a 5 L sterilized mechanical agitation tank fermenter (BIOTECH-5JG, Shanghai Baoxin Co., Ltd., Shanghai, China) for spontaneous alcoholic fermentation (SF), hermetically closed, and maintained at 26–28 °C for 5 days. The raw material was obtained from fresh Nanfeng tangerines, which were peeled and juiced. The basic physicochemical parameters of the mandarin juice were as follows: pH of 4.13, soluble solids content of 14.6 Brix, initial sugar content of 130.86 g/L, and total acidity of 8.96 g/L. K2S2O5 and sucrose were used to set the levels of SO2 and total sugar at 50 mg/L and 200 g/L, respectively. The Nanfeng tangerine juice was mixed thoroughly, pasteurized at 95 °C for 7 min, and then rapidly cooled to room temperature.
2.2. Fermentation Process
The inoculation rates and fermentation temperatures of the fermentation process were adapted from a previously described method [17]. Sc and three non-Saccharomyces yeast strains (Ce, Hg, and Ht) were cultured separately in aseptic YPD medium at 28 °C and 160 rpm (Shanghai Yiheng Scientific Instrument Co., Ltd., THZ-300, Shanghai, China) for 24 h by shock for two consecutive times. The seed culture was centrifuged at 4000 rpm for 5 min to collect the yeast cells, which were then used for sequential and mixed fermentation of Nanfeng tangerine wine. In the context of pure fermentation, a concentration of 107 CFU/mL of a singular yeast strain was introduced to Nanfeng tangerine juice. In the case of sequential fermentation, Sc was inoculated into the tangerine juice for 48 h subsequent to the introduction of non-Saccharomyces yeast. At this juncture, the residual sugar content measured 115 g/L. Four different combinations (Ce.Hg)-10-Sc, (Ce.Ht)-10-Sc, (Hg.Ht)-10-Sc, and (Ce.Hg.Ht)-10-Sc were used, with an inoculum ratio of 1:1 and a cell quantity of 107 CFU/mL, respectively. In mixed fermentation, Sc was inoculated concurrently with non-Saccharomyces yeast species at the same cell concentration of 107 CFU/mL (Sc:(Ce.Hg)-1:1, Sc:(Ce.Ht)-1:1, Sc:(Hg.Ht)-1:1, and Sc:(Ce.Hg.Ht)-1:1). To minimize Sc competition and the effect of high ethanol concentrations, as well as to reduce the workload in comparison with that of sequential fermentation, we performed mixed fermentation with an inoculum ratio of 1:100 105 CFU/mL Sc plus 107 CFU/mL non-Saccharomyces. Four combinations were grouped (Sc:(Ce.Hg)-1:100, Sc:(Ce.Ht)-1:100, Sc:(Hg.Ht)-1:100, and Sc:(Ce.Hg.Ht)-1:100). The experimental groups are listed in Table 1.

Table 1.
The fermentation groups.
The experiment was conducted with three repetitions, maintaining a fermentation temperature of 28 °C. The progress of tangerine wine fermentation was monitored by daily measurements of residual sugar concentrations and yeast cell counts. Fermentation completion was determined by observing constant residual sugar concentrations. Following fermentation, both the tangerine juice and wines underwent centrifugation at 4500 rpm for 10 min to remove yeast cells and precipitates. The resulting supernatants were stored at 80 °C until further analysis.
2.3. Physicochemical Analysis
The determination of total sugars was performed with slight modifications to the method as described previously [17]. The anthrone colorimetric method was utilized to determine the remaining sugar levels in Nanfeng tangerine wine by diluting a 1 mL sample to the correct concentration, then placing it in an ice water bath for 5 min before adding 4 mL of anthrone reagent (0.1% anthrone in concentrated sulfuric acid, Sinopharm Group Chemical Reagent, Beijing, China). After being placed in a boiling water bath for 10 min, the mixture was cooled in cold water, and the absorbance was measured at 620 nm with a spectrophotometer (Perkin Elmer, Lambda365, Boston, MA, USA). The results were expressed in mg/mL of glucose. Monitoring the antioxidant activity and physicochemical properties.
The acidity level was measured through titration with sodium hydroxide (Xilong Chemical, Shantou, China) following the guidelines of GB 12456-2021, assessing the total acidity of Nanfeng tangerine juice and Nanfeng tangerine wine in terms of g/L citric acid. A pH meter (Shanghai Yi Electrical Scientific Instrument, PHS-3C, Shanghai, China) was used to measure the pH level. All experiments were conducted in triplicate.
Ethanol content was analyzed following the guidelines of the national standard GB/T 15038-2006 ‘Analytical methods of wine and fruit wine’ [19]. Anhydrous ethanol (chromatographic grade, Xilong Chemical Co., Ltd., Shantou, China) volumes of 2, 4, 7, 9, 11, and 15 mL were diluted to volume in 100 mL volumetric flasks with distilled water. Before injection into the GC system (Scion, GC 456C, Shanghai, China), a 1.5 mL ethanol standard sample was passed through a 0.22 µm pore-size filter membrane for filtration. The chromatographic parameters included an injector temperature of 210 °C, a column flow rate of 1.8 mL/min, and a split ratio of 20:1. The temperature protocol was established to maintain at 42 °C for 1 min, then rise to 70 °C with a speed of 3 °C per minute and maintain for 1 min, followed by an increase to 200 °C at a rate of 15 °C per minute and a hold for 4 min. The flame ionization detector was set to a temperature of 210 °C, with a flow rate of 40 mL/min for hydrogen and 400 mL/min for air.
DPPH radical scavenging activity was determined with minor adjustments to the techniques outlined as described previously [20]. After diluting the samples to the desired concentration using anhydrous methanol, a mixture of 200 µL of the diluted sample and 3.8 mL of 0.1 mM DPPH (Sinopharm Group Chemical Reagent, Beijing, China) solution was prepared. Following a 30-min incubation in darkness, the absorbance was recorded at a wavelength of 517 nm. Every sample underwent testing three times. To determine the percentage of the DPPH radical scavenging rate, the following formula was used:
DPPH radical scavenging rate (%) = (1 − AS/Ab) × 100%
AS represents the absorbance of the sample, while Ab represents the absorbance of the blank.
The determination of the ABTS radical scavenging method was carried out with slight modifications to the methods as described previously [20]. A mixture of ABTS solution (7.0 mM, 10 mL, Sinopharm Group Chemical Reagent, Beijing, China) and potassium persulfate (140 mM, 176 µL, Sinopharm Group Chemical Reagent, Beijing, China) was left to incubate at room temperature in the dark for 14 h. An appropriate quantity of ethanol was added to the ABTS solution to reach an absorbance of 0.70 ± 0.02 at 734 nm. After diluting the samples with anhydrous ethanol to the desired concentration, 200 µL of the diluted sample was mixed with 3.8 mL of the ABTS+·solution. Following a 6-min incubation period at ambient temperature, the absorption was recorded at a wavelength of 734 nm. Every sample underwent testing three times. To determine the rate of ABTS+·scavenging, use the following formula:
ABTS radical scavenging rate (%) = (1 − AS/Ab) × 100%
AS represents the absorbance of the sample, while Ab represents the absorbance of the blank.
2.4. Determination of Organic Acids and Polyphenolic Compounds
Nanfeng tangerine wine was analyzed for organic acids using HPLC (Agilent Technologies, 1260 Infinity, Palo Alto, CA, USA) and an Ultimate AQ-C18 column (4.6 mm × 250 mm, 5 µm), following a modified version of a previously established research protocol [17]. The mobile phase was a mixture of 0.025% trifluoroacetic acid solution and methanol in a ratio of 95:5 (V/V). A flow rate of 0.8 mL/min was established. The gradient elution protocol is described in Table 2, using a detection wavelength of 210 nm and injecting 10 µL. Nine organic acid standards—oxalic acid, malic acid, vitamin C, lactic acid, acetic acid, citric acid, succinic acid, and fumaric acid—were used to create standard curves for measuring organic acids in Nanfeng tangerine wine through the external standard technique.

Table 2.
HPLC mobile phase gradient elution procedure.
Phenolic acid levels in Nanfeng tangerine wine were examined by utilizing an Ultimate AQ-C18 column (4.6 mm × 250 mm, 5 µm), following a previously established procedure with slight adjustments [17]. The HPLC flow rate was adjusted to 1.0 mL/min using a gradient elution program outlined in Table 2. The temperature of the column was kept at 40 degrees Celsius, with an injection volume of 10 microliters. Quantification wavelengths were 260 nm and 320 nm, with a scanning wavelength range of 200 to 400 nm. Retention time was utilized for qualitative analysis, while quantification was achieved through the external standard method. Protocatechuic acid and p-hydroxybenzoic acid were quantified at 260 nm, whereas chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid were quantified at 320 nm for their peak areas. HPLC chromatograms and standard curve equations for the phenolic acid standards were obtained using HPLC.
The level of flavonoids in Nanfeng tangerine wine was analyzed by HPLC using an Agilent Eclipse XDB-C18 column (4.6 mm × 250 mm, 5 µm), following a modified version of a previously established research protocol [17]. Table 3 indicates a gradient elution program was established with a flow rate of 1.0 mL/min. The temperature of the column was held constant at 30 °C, with measurement wavelengths set at 283 nm and 330 nm and a scan range from 200 to 400 nm. A 10 µL volume was injected for analysis. Retention time was used for qualitative analysis, while quantification was performed through the external standard method to generate chromatograms and standard curve equations for the flavonoid standards. At 283 nm, analysis included naringin, hesperidin, neohesperidin, eriocitrin, didymin, poncirin, and hesperetin, while at 330 nm, analysis included nobiletin, tangeretin, and sinensetin.

Table 3.
Analysis of conventional physical and chemical properties and antioxidant activity (ABTS and DPPH radical scavenging activity) of Nanfeng tangerine wine.
2.5. Electronic Nose Measurement
The aroma composition of Nanfeng tangerine wine was analyzed using a Gemini electronic nose (Alpha MOS, FOX4000, Toulouse, France). Following a modified research method [17], 5 mL of Nanfeng tangerine wine sample was transferred into a 20 mL headspace vial and closed with a PTFE rubber cap. The Nanfeng tangerine wine sample was left to enrich at ambient temperature for half an hour, allowing the volatile components to evenly disperse in the vial’s headspace. A syringe was then used to withdraw 2.5 mL of headspace gas for electronic nose measurement. Air was used as the carrier gas at a flow rate of 150 mL/min. Electronic nose settings: acquisition time was 90 s, sampling interval was 0.5 s, data acquisition delay was 250 s, and cleaning time was 300 s. Each sample was measured in triplicate. The data were recorded by the electronic nose software (Germany Airsense PEN 3.5) and used for subsequent principal component analysis.
2.6. Determination of Aroma Components
The volatile aroma components of Nanfeng tangerine wine were analyzed using HS-SPME-GC-MS (Supelco, Bellefonte, PA, USA), based on previous research methods with some modifications [17]. A precise 5 mL sample of Nanfeng tangerine wine was taken into a 20 mL headspace extraction vial, to which 1.5 g of NaCl and 5 µL of a 10 mg/mL cyclohexanone solution as an internal standard were added. Following a 20-min equilibration at 40 °C, a mature DVB/CAR/PDMS extraction fiber from Supelco in Bellefonte, PA, USA, was placed in the vial, with the fiber protruding 2 cm above the liquid sample, and absorbed at 40 °C for 52 min. The sample was gently vortexed at 750 rpm using a magnetic stirrer during the equilibration and adsorption phases. The extraction fiber was then quickly removed and inserted into the injection port of the gas chromatograph-mass spectrometer at 250 °C for desorption for 3 min, initiating data collection simultaneously. Gas chromatography was performed using an HP-5MS capillary column with dimensions of 30 m × 250 µm × 0.25 µm. The carrier gas used was high purity helium with a flow rate of 1.2 mL/min and a split ratio of 5:1. The temperature was initially set to 40 °C for 2 min, then increased to 180 °C at a rate of 4 °C per minute, where it was maintained for 2 min, before being raised to 250 °C at a rate of 10 °C per minute. The detector and ion source temperatures were adjusted to 250 °C and 200 °C, respectively, with an electron energy of 70 eV and a mass scan range of 30 to 500 amu. Retention indices (RI) were used to qualitatively identify volatile components by comparing them with the NIST 14.L database. Volatile components were quantified by utilizing cyclohexanone as an internal reference, determining the volatile compound content through normalization of GC peak areas compared to the internal standard, and calculating compound equivalents based on the internal standard concentration. The odor activity value (OAV), which is the ratio of the concentration of aroma compounds to their odor thresholds (OT), is widely used to quantify the contribution of aroma compounds. Aroma compounds with an OAV of 1 or higher are viewed as distinctive, with a higher OAV suggesting a more significant role in the aroma profile of Nanfeng tangerine wine.
2.7. Statistical Analysis
The experimental data underwent statistical analysis with SPSS 26.0 software to conduct one-way ANOVA and Duncan’s test (p < 0.05), identifying significant variances at p < 0.05. The data are displayed as the average plus or minus the standard deviation. SIMCA 13.0 was utilized to perform principal component analysis (PCA) on the chemical constituents throughout various fermentation procedures. Systematic cluster analysis and heatmap visualization analysis (Z-score) of the volatile aroma components of wines produced by different fermentation methods were performed using Origin 2021 software.
3. Results
3.1. Changes in Residual Sugar Content during the Fermentation of Nanfeng Tangerine Wine
The impact of the fermentation method and inoculation rate on sugar consumption during the fermentation process with multiple strains was evaluated by monitoring changes in residual sugar content. Sc exhibited good fermentation performance. As shown in Figure 1, pure culture fermentation with Sc maintained its excellent fermentation performance, with sugar in the juice being completely consumed within 72 h, reaching the endpoint of fermentation. The multi-strain combination fermentation methods, except for the sequential fermentation of Hg-10-Sc, which took 96 h, reached the fermentation endpoint after 48 or 72 h. This confirms previous research findings that non-Saccharomyces yeast have weaker fermentation performance compared to Sc [21]. The sugar consumption rate was faster in mixed fermentations with combinations of non-Saccharomyces and Sc than in pure culture and sequential fermentations; this result is consistent with previous reports [22]. This is due to the complex interactions and vigorous metabolism between microbes in combined fermentations, which accelerate sugar consumption. The reduced sugar consumption rate observed in the later stages of multi-strain combination fermentation can be attributed to the lower alcohol tolerance of non-Saccharomyces yeasts. Utilizing a blend of multiple yeast strains, including non-Saccharomyces varieties and Sc, the fermentation process was accelerated, possibly leading to a shorter duration for wine fermentation. In comparison to non-Saccharomyces yeast mixed fermentation, Sc demonstrated superior fermentation performance. Conversely, non-Saccharomyces yeast mixed fermentation exhibited a significantly higher fermentation rate when compared to non-Saccharomyces yeast fermentation alone. The fermentation rates were ranked as follows: Ce:Ht-1:1 > Ce:HG-1:1 > Ce:Hg:Ht-1:1:1.
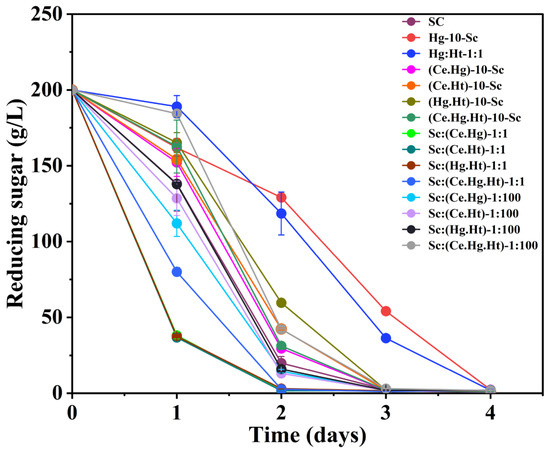
Figure 1.
Sugar consumption kinetics and growth kinetics of yeast strains during fermentations. Abbreviations: Sc, S. cerevisiae; Ce, C. ethanolica; Hg, H. guilliermondii; Ht, H. thailandica. Data shown are the mean ± SD of triplicates.
3.2. Physicochemical Properties and Antioxidant Activity of Nanfeng Tangerine Wine
The diversity of microbial strains in the multi-strain combination fermentation system and the complex interactions between strains can produce Nanfeng tangerine wines with different characteristics. The physicochemical properties and antioxidant activities of different Nanfeng tangerine wines were obtained through chemical and instrumental analysis, as shown in Table 3. Pure culture fermentation with Sc exhibited the strongest fermentation performance and the highest ethanol production, reaching 11.13% (v/v), consistent with previous research findings [23,24]. The use of multiple strains in the fermentation process led to a notable decrease in the alcohol level of the mandarin wine, as non-Saccharomyces yeast have a less efficient fermentation ability for ethanol and a lower conversion efficiency of sugar into ethanol [25]. The ethanol levels from (Ce.Hg)-10-Sc, (Ce.Ht)-10-Sc, and (Hg.Ht)-10-Sc sequential fermentation methods were notably higher than other multi-strain combination fermentation methods, with similar results to Hg-10-Sc sequential fermentation (Table 3). This suggests that sequential fermentation methods enhanced ethanol production in this experiment. With the exception of Sc(Ce.Hg.Ht)-1:1, the remaining sugar levels in multi-strain combination fermentation techniques were notably lower compared to Sc and Hg-10-Sc groups. This could be attributed to the intricate microbial fermentation process that led to the conversion of sugar into substances other than ethanol. In comparison to fermentation using only Sc in pure culture, multi-strain combination fermentation methods showed a significant increase in total acidity content, which can be attributed to the metabolic processes of non-Saccharomyces yeast converting sugars into acidic compounds during fermentation. The antioxidant capacity of wines from multi-strain combination fermentation was not weakened compared to the juice, indicating that multi-strain fermentation does not affect the antioxidant capacity of the wine. The 1:1 multi-strain combination fermentation group had significantly lower ethanol content than Sc, making it suitable for the production of low-alcohol fruit wines, and the antioxidant activity was not significantly reduced, making it appealing to a broader audience.
3.3. Enhancement of Hesperidin and Nobiletin Content in Wine through Multi-Strain Combination Fermentation
Seven types of flavonoids were detected in the wine produced by multi-strain combination fermentation methods (Figure 2), including naringin, neohesperidin, hesperidin, neohesperidin, eriocitrin, hesperetin, and nobiletin. Notably, hesperetin and nobiletin are flavonoids newly formed after alcohol fermentation, not present in the juice, and are derived from the microbial transformation of flavonoids such as hesperidin. The highest content of hesperetin was produced by the Sc:(Ce.Ht)-1:100 combination, reaching 4.12 ± 0.08 mg/L, followed by the Sc:(Ce.Hg)-1:1 combination with a content of 4.08 ± 0.06 mg/L. The highest content of nobiletin was in the (Hg.Ht)-10-Sc combination, reaching 1.04 ± 0.05 mg/L, significantly higher than other multi-strain combinations, and the hesperetin content produced by the (Hg.Ht)-10-Sc combination was also relatively high (4.04 ± 0.02 mg/L), making it the optimal combination among these multi-strain fermentations for enhancing the content of hesperetin and nobiletin in wine. The potential mechanism involves the enzymatic deglucosidation of new hesperidin and hesperidin in fruit juice by strains during the multi-strain joint fermentation process of Nanfeng Tangerine fruit wine. The expression of these enzymes varies among different multi-strain combinations, leading to varying levels of hesperidin and hesperidin in the final product. Fermentation of alcohol has the potential to enhance the levels of beneficial active substances in citrus-based wine. Further PCA analysis of flavonoid compounds revealed that different fermentation processes produce unique concentrations of flavonoid compounds, presenting different functional characteristics in citrus wine (Figure 2C). The change in phenolic profile is the result of using different fermentation methods. This confirms that citrus wine’s flavonoid content can be increased through different microbes or fermentation methods, contributing to improved wine quality.
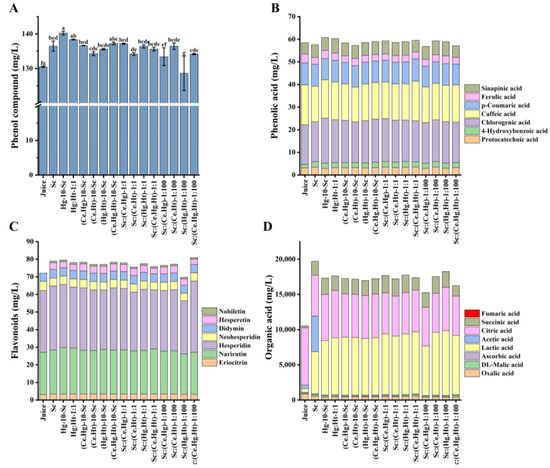
Figure 2.
The content of polyphenols (phenolic acid and flavonoids) and organic acids in Nanfeng tangerine juice and Nanfeng tangerine wines. (A) is the content of total polyphenols; (B) is the content of phenolic acid. (C) is the content of flavonoids, and (D) is the content of organic acids detected by HPLC. Data shown are the mean ± SD of triplicate, values with different Roman letters in the same row indicating significant differences at p < 0.05 (Duncan’s test).
The overall amount of polyphenols in wines made using multi-strain combination fermentation techniques was notably less than in wines produced through Hg-10-Sc sequential fermentation. Among these methods, the Sc:(Ce.Hg)-1:100 fermentation resulted in the lowest total polyphenol content in Nanfeng tangerine wine (Figure 2A, Table 4). Seven phenolic acids were identified, including protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid, with chlorogenic and caffeic acids being the most prevalent. Chlorogenic acid content significantly increased in multi-strain fermentation combinations, excluding pure culture fermentation with Sc. Studies have shown that chlorogenic acid (CGA) exhibits beneficial protective effects on liver diseases related to human signaling pathways [26,27,28]. The multi-strain combination of fermented wines (Figure 2D) contained eight different organic acids: oxalic acid, malic acid, vitamin C, lactic acid, acetic acid, citric acid, succinic acid, and fumaric acid. Table 5 demonstrates that the overall amount of organic acid in Sc:(Ce.Hg)-1:100 was notably less compared to the other fermentation groups, primarily because of its reduced lactic acid output. The citric acid content in wines from the 1:1 and 1:100 multi-strain combination fermentation methods was up to 3000 mg/L lower compared to the juice, and the reduction of citric acid can decrease the tartness of the wine. There was no production of acetic acid in the multi-strain combination fermentation methods. Notably, except for Sc:(Ce.Hg)-1:100, the lactic acid content in the 1:1 and 1:100 multi-strain combination fermentation methods was significantly higher than in all other fermentation groups in this study, with no significant change in malic acid content. This may be due to a more favorable environment for malolactic fermentation, suggesting that the 1:1 and 1:100 multi-strain combination fermentation methods promote malolactic fermentation, thereby improving the smoothness of Nanfeng tangerine wine. The succinic acid content significantly increased in the multi-strain combination fermented wines, which is beneficial for antibacterial and detoxification effects and enhancing human immune function. The multi-strain combination fermentation methods can exploit the advantages of non-Saccharomyces yeast in producing secondary metabolites, which are beneficial for increasing the content of beneficial components in the wine and improving wine quality by reducing undesirable substances.

Table 4.
The content of polyphenols (phenolic acid and flavonoids) in Nanfeng tangerine juice and Nanfeng tangerine wines.

Table 5.
The content of organic acids in Nanfeng tangerine juice and Nanfeng tangerine wines.
3.4. Composition and Content Analysis of Volatile Aroma Components
Natural fermentation is the most traditional method in fruit wine brewing due to the complex interactions among diverse microbial communities in the fermentation system, which results in wine with rich and complex flavors [29]. This study aimed to simulate the complex fermentation system present in natural fermentation by using multi-strain combination fermentation methods to brew Nanfeng tangerine wine in hopes of achieving a wine with distinctive flavors. Nanfeng tangerine wine, created through fermentation with multiple strains, was examined for its volatile aromatic compounds and concentrations using HS-SPME-GC-MS and an electronic nose, as depicted in Figure 3A. In the fermented Nanfeng tangerine wine with multiple strains, 31 volatile substances were found, which is consistent with earlier studies that found numerous terpenes and aldehydes in citrus wine, such as d-limonene, linalool, terpineol, geraniol, and decanal [30,31,32]. In a prior investigation, it was discovered that the sequential fermentation of Hg-10-Sc (549.31 mg/L) had a beneficial impact on the aroma of Nanfeng tangerine wine. Compared to these, the multi-strain combination fermentation methods, Sc:(Ce.Hg.Ht)-1:1 (511.64 mg/L) and (Hg.Ht)-10-Sc (500.66 mg/L), had relatively higher richness in aroma compounds (Figure 3A, Table S1). Odor active compound and electronic nose PCA analysis results show that different combinations of multi-strain fermentation methods have distinct flavors and aroma characteristic compounds (Figure 3B,C, Table S2), thus producing Nanfeng tangerine wines with diverse sensory qualities.
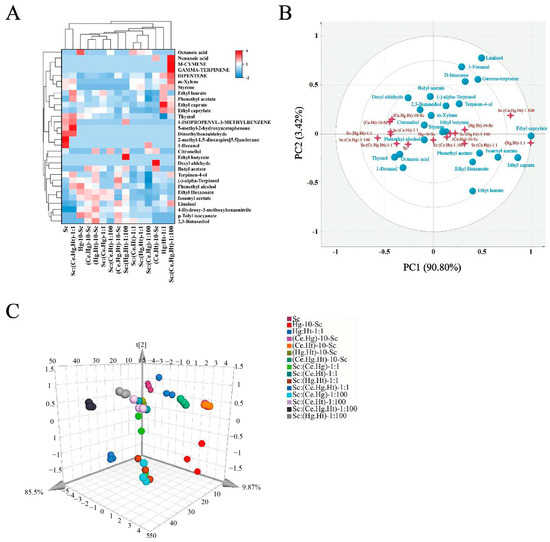
Figure 3.
The volatile aroma compounds in Nanfeng tangerine wines. (A) is the hierarchical cluster analysis of volatile odor-active aroma compounds; (B) is the principal component analysis of volatile aroma compounds (OAV ≥ 1); and (C) is the principal component analysis of E-nose datasets.
4. Conclusions
This study demonstrated that multi-strain combination fermentation methods can effectively enhance the content of hesperidin and nobiletin in citrus wine, with the (Hg.Ht)-10-Sc combination emerging as the optimal multi-strain combination for increasing the content of these flavonoids. Compared to commercial yeast monoculture fermentation, the content of nobiletin was increased by 16% and hesperidin by 5%. Additionally, the alcohol level in Nanfeng tangerine wine created using a combination of multiple strains for fermentation was notably reduced compared to that made solely with Sc fermentation, rendering it better suited for today’s popular consumer market. The fermentation process involving multiple strains showcased a diverse microbiome, active metabolic processes, fast sugar utilization, and rapid fermentation speed, despite a slightly reduced efficiency in converting sugar to ethanol. However, this method has the potential to enhance the taste and beneficial compounds in the wine. The aroma richness and complexity were better in Sc:(Ce.Hg.Ht)-1:1 and (Hg.Ht)-10-Sc. During fermentation, Sc, Hg, and Ht are known to produce β-glucosidase, with non-Saccharomyces cerevisiae strains typically exhibiting higher enzymatic activity compared to Saccharomyces cerevisiae. Additionally, Ce displays tannase activity, facilitating the degradation of cell walls and promoting the release of compounds contained within. The utilization of a 1:1 and sequential multi-strain combination fermentation method may have the potential to enhance the co-fermentation process of multiple strains with minimal mutual influence compared to alternative fermentation approaches. This approach may effectively leverage the strengths of individual strains and ultimately enhance the flavor profile of Nanfeng tangerine wine to a considerable extent. The 1:1 and 1:100 multi-strain combination fermentation methods facilitated malolactic fermentation, significantly reducing the content of malic acid and increasing the content of lactic and succinic acids, enhancing the smoothness of Nanfeng tangerine wine and its health-promoting properties.
Overall, the simulated complex microbial system in this experiment requires further exploration, as it did not achieve the complexity and richness of aroma similar to natural fermentation. This may be due to inappropriate inoculation ratios or the selection of strains not suited for multi-strain combination fermentation. The co-fermentation of different strains produces complex metabolites, but the interactions between strains may also hinder their development and adversely affect wine fermentation. Therefore, selecting appropriate strains and fermentation methods is crucial. Further investigation into the metabolic and molecular mechanisms underlying the promotion of bioactive substances by different strain combinations during fruit wine fermentation could be achieved by monitoring the biotransformation process.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fermentation10050238/s1, Figure S1: The concentration of volatile aroma compounds identified in tangerine wines. Table S1. Chemical composition of tangerine juice and tangerine wines (means ± SD). Table S2: The standard curve, validation range, and coefficient of determination (r2) for the non-volatile aroma compounds in tangerine wines.
Author Contributions
S.Z.: conceptualization, methodology, validation, investigation, and writing—original draft preparation. Y.O.: investigation, methodology, validation, and writing review and editing. L.X.: data curation and software. J.L.: conceptualization, writing—review and editing, visualization, project administration, and funding acquisition. Y.W.: investigation and methodology. B.G.: investigation. D.Z.: conceptualization, methodology, project administration, and funding acquisition. Y.X.: resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Jiangxi Provincial Natural Science Foundation (20224BAB205043) and the Key Research and Development Program Key Project of Jiangxi Province, China (No. 20171ACF60007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are available within the manuscript and are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gupta, A.K.; Pathak, U.; Tongbram, T.; Medhi, M.; Terdwongworakul, A.; Magwaza, L.S.; Mditshwa, A.; Chen, T.; Mishra, P. Emerging approaches to determine maturity of citrus fruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 5245–5266. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Q.; Tian, B.; Zhu, S.; Du, F.; Mao, R.; Li, S.; Liu, L.; Zhu, Y. Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. J. Fungi 2022, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, Y.; Zhong, K.; Luo, D.; Wu, Y.; Gao, H. Discovering the effect of co-fermentation involving Saccharomyces cerevisiae and Schizosaccharomyces pombe on the sensory quality improvement of mandarin wine based on metabolites and transcriptomic profiles. J. Sci. Food Agric. 2023, 103, 7932–7940. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Arellano-Plaza, M.; Kirchmayr, M.; Barrera-Martínez, I.; Gschaedler-Mathis, A. Preservation of non-Saccharomyces yeasts: Current technologies and challenges. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3464–3503. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xu, C.; Gao, X.; Zhang, W.; Yao, Z.; Wang, T.; Feng, X.; Wang, Y. Comparative study on secondary metabolites from different citrus varieties in the production area of Zhejiang. Front. Nutr. 2023, 10, 1159676. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Xiaojuan, T.; Fahad, S. The consequences of fermentation metabolism on the qualitative qualities and biological activity of fermented fruit and vegetable juices. Food Chem. X 2024, 21, 101209. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef]
- Wang, H.-H.; Sun, Y.-N.; Qu, T.-Q.; Sang, X.-Q.; Zhou, L.-M.; Li, Y.-X.; Ren, F.-Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef]
- Moazamiyanfar, R.; Rezaei, S.; Ali Ashrafzadeh, H.; Rastegar-Pouyani, N.; Jafarzadeh, E.; Mouludi, K.; Khodamoradi, E.; Zhaleh, M.; Taeb, S.; Najafi, M. Nobiletin in Cancer Therapy; Mechanisms and Therapy Perspectives. Curr. Pharm. Des. 2023, 29, 1713–1728. [Google Scholar] [CrossRef] [PubMed]
- Kesh, S.; Kannan, R.R.; Sivaji, K.; Balakrishnan, A. Hesperidin downregulates kinases lrrk2 and gsk3β in a 6-OHDA induced Parkinson’s disease model. Neurosci. Lett. 2021, 740, 135426. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gong, H.; Wei, Y.; Xu, Y.; Zhou, X.; Wang, Z.; Feng, F. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine 2023, 112, 154680. [Google Scholar] [CrossRef] [PubMed]
- Pla-Pagà, L.; Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Domenech-Coca, C.; Canela, N.; del Bas, J.M.; Caimari, A.; et al. Effect of the consumption of hesperidin in orange juice on the transcriptomic profile of subjects with elevated blood pressure and stage 1 hypertension: A randomized controlled trial (CITRUS study). Clin. Nutr. 2021, 40, 5812–5822. [Google Scholar] [CrossRef]
- Garofalo, C.; Tristezza, M.; Grieco, F.; Spano, G.; Capozzi, V. From grape berries to wine: Population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J. Microbiol. Biotechnol. 2016, 32, 59. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Xiao, Y.; He, Z.; Liu, J.; Wang, Y.; Gao, B.; Chang, J.; Zhu, D. Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines. J. Fungi 2022, 8, 128. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by Multiple Bacterial Strains Improves the Production of Bioactive Compounds and Antioxidant Activity of Goji Juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16159-1996; Analytical Methods of Wine and Fruit Wine. 2006. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=4CFF012592D9DE362A765DD3ED1F9C26 (accessed on 25 April 2024).
- Tokumaru, O.; Shuto, Y.; Ogata, K.; Kamibayashi, M.; Bacal, K.; Takei, H.; Yokoi, I.; Kitano, T. Dose-dependency of multiple free radical-scavenging activity of edaravone. J. Surg. Res. 2018, 228, 147–153. [Google Scholar] [CrossRef]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione production by non-Saccharomyces yeasts and its impact on winemaking: A review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, C.; Yang, D.; Liu, H.; Xue, J.; Duan, C.; Yan, G. Effects of three indigenous non-Saccharomyces yeasts and their pairwise combinations in co-fermentation with Saccharomyces cerevisiae on volatile compounds of Petit Manseng wines. Food Chem. 2022, 368, 130807. [Google Scholar] [CrossRef] [PubMed]
- Eliodório, K.P.; e Cunha, G.C.D.G.; Müller, C.; Lucaroni, A.C.; Giudici, R.; Walker, G.M.; Alves, S.L., Jr.; Basso, T.O. Advances in yeast alcoholic fermentations for the production of bioethanol, beer and wine. Adv. Appl. Microbiol. 2019, 109, 61–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of chemical constitution and aroma properties of kiwi wines obtained from pure and mixed fermentation with Wickerhamomyces anomalus and Saccharomyces cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.; Mehlomakulu, N.N.; Nortje, S.; Beukes, L.; Hoff, J.; Booyse, M.; Erten, H. Non-Saccharomyces yeast for lowering wine alcohol levels: Partial aeration versus standard conditions. FEMS Yeast Res. 2022, 22, foac002. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singla, R.K.; Pandey, A.K. Chlorogenic Acid: A Dietary Phenolic Acid with Promising Pharmacotherapeutic Potential. Curr. Med. Chem. 2023, 30, 3905–3926. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Jahn, L.J.; Rekdal, V.M.; Sommer, M.O.A. Microbial foods for improving human and planetary health. Cell 2023, 186, 469–478. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef]
- Bruna-Maynou, F.J.; Castro, R.; Rodríguez-Dodero, M.C.; Barroso, C.G.; Durán-Guerrero, E. Flavored Sherry vinegar with citric notes: Characterization and effect of ultrasound in the maceration of orange peels. Food Res. Int. 2020, 133, 109165. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Y.; Yang, Y.; Jin, Z.; Luo, X. Effect of fermentation modes on nutritional and volatile compounds of Huyou vinegar. J. Food Sci. Technol. 2018, 55, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).