Differences in Volatile Profiles and Sensory Characteristics in Plum Spirits on a Production Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Plum Brandy
2.2. Chemicals and Reagents
2.3. Instruments
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results
3.1. Analytical Profile
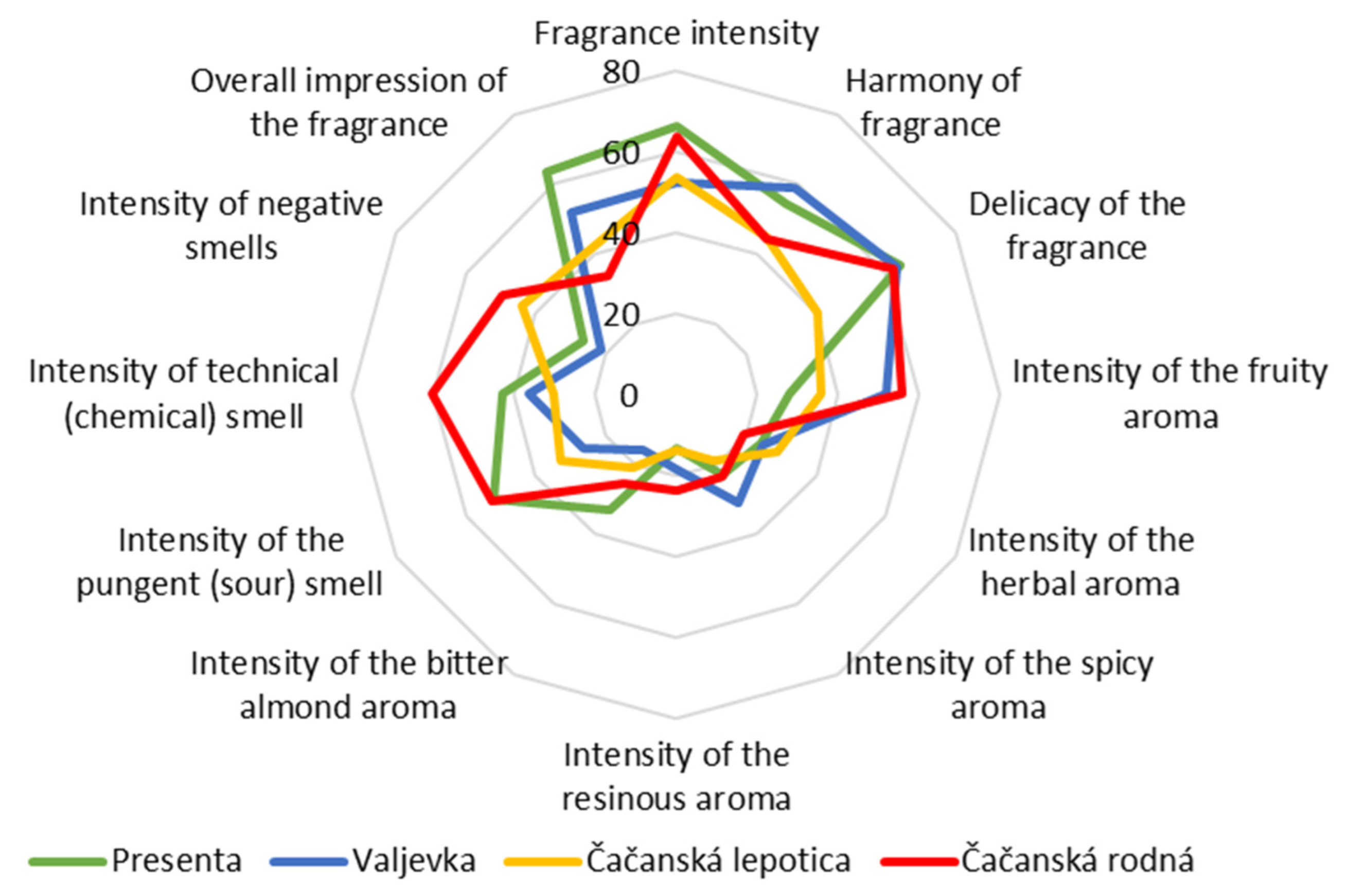
3.2. Sensory Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christoph, N.; Bauer-Christoph, C. Flavour of Spirit Drinks: Raw Materials, Fermentation, Distillation, and Ageing. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–239. [Google Scholar]
- Querol, A.; Fleet, G.H. Yeasts in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Ismail, H.M.; Williams, A.A.; Tucknott, O.G. The flavour of plums (Prunus domestica L.). An examination of the aroma components of plum juice from the cultivar victoria. J. Sci. Food Agric. 1981, 32, 613–619. [Google Scholar] [CrossRef]
- Ismail, H.M.; Williams, A.A.; Tucknott, O.G. The flavour components of plums: An examination of the aroma components present in the headspace above four cultivars of intact plums, Marjorie’s seedling, Merton Gem, NA 10 and Victoria. J. Sci. Food Agric. 1981, 32, 498–502. [Google Scholar] [CrossRef]
- Blumenthal, P.; Steger, M.C.; Einfalt, D.; Rieke-Zapp, J.; Quintanilla Bellucci, A.; Sommerfeld, K.; Schwarz, S.; Lachenmeier, D.W. Methanol mitigation during manufacturing of fruit spirits with special consideration of novel coffee cherry spirits. Molecules 2021, 26, 2585. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008; FAO: Rome, Italy, 2019. [Google Scholar]
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.-M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Spaho, N.; Dürr, P.; Grba, S.; Velagić-Habul, E.; Blesić, M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J. Inst. Brew. 2013, 119, 48–56. [Google Scholar] [CrossRef]
- Nikićević, N. Effects of some production factors on chemical composition and sensory qualities of Williams pear brandy. J. Agric. Sci. 2005, 50, 193–206. [Google Scholar]
- Satora, P.; Kostrz, M.; Sroka, P.; Tarko, T. Chemical profile of spirits obtained by spontaneous fermentation of different varieties of plum fruits. Eur. Food Res. Technol. 2017, 243, 489–499. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Singh, V.; Murphy, N.R.; Balasubramanian, V.; Mainland, J.D. Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc. Natl. Acad. Sci. USA 2019, 116, 9598–9603. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xie, T.; Xie, J.; Chen, C.; Ai, L.; Tian, H. Aroma perceptual interactions of benzaldehyde, furfural, and vanillin and their effects on the descriptor intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef] [PubMed]
- Solís-Solís, H.; Calderón-Santoyo, M.; Schorr-Galindo, S.; Luna-Solano, G.; Ragazzo-Sánchez, J. Characterization of aroma potential of apricot varieties using different extraction techniques. Food Chem. 2007, 105, 829–837. [Google Scholar] [CrossRef]
- Pino, J.A.; Quijano, C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375. [Google Scholar] [CrossRef] [PubMed]
- Nikićević, N.; Velickovic, M.; Jadranin, M.; Vučković, I.; Novaković, M.; Vujisić, L.V.; Stanković, M.; Urosevic, I.; Tešević, V. The effects of the cherry variety on the chemical and sensorial characteristics of cherry brandy. J. Serbian Chem. Soc. 2011, 76, 1219–1228. [Google Scholar] [CrossRef]
- Zierer, B.; Schieberle, P.; Granvogl, M. Aroma-active compounds in Bartlett pears and their changes during the manufacturing process of Bartlett pear brandy. J. Agric. Food Chem. 2016, 64, 9515–9522. [Google Scholar] [CrossRef] [PubMed]
- Butac, M.; Bozhkova, V.; Zhivondov, A.; Milosevic, N.; Bellini, E.; Nencetti, V.; Blazek, J.; Balsemin, E.; Lafarque, B.; Kaufmane, E. Overview of plum breeding in Europe. In Proceedings of the II Balkan Symposium on Fruit Growing 981, Pitesti, Romania, 5–7 September 2011; pp. 91–98. [Google Scholar]
- Popović, B.T.; Mitrović, O.V.; Leposavić, A.P.; Paunović, S.A.; Jevremović, D.R.; Nikićević, N.J.; Tesević, V.V. Chemical and sensory characterization of plum spirits obtained from cultivar Čačanska Rodna and its parent cultivars. J. Serbian Chem. Soc. 2019, 84, 1381–1390. [Google Scholar] [CrossRef]
- Logistica Fruit. European Statistics Handbook 2024; Fruit Logistica: Berlin, Germany, 2024. [Google Scholar]
- Vyviurska, O.; Matura, F.; Furdíková, K.; Špánik, I. Volatile fingerprinting of the plum brandies produced from different fruit varieties. J. Food Sci. Technol. 2017, 54, 4284–4301. [Google Scholar] [CrossRef]
- Heller, D.; Einfalt, D. Reproducibility of Fruit Spirit Distillation Processes. Beverages 2022, 8, 20. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A.; Saraiva, J.; Rocha, S.M. Study of the volatile components of a candied plum and estimation of their contribution to the aroma. Food Chem. 2008, 111, 897–905. [Google Scholar] [CrossRef]
- Niimi, J.; Guixer, B.; Splivallo, R. Odour active compounds determined in the headspace of yellow and black plum wines (Prunus domestica L.). LWT 2020, 130, 109702. [Google Scholar] [CrossRef]
- Ivanović, S.; Simić, K.; Tešević, V.; Vujisić, L.; Ljekočević, M.; Gođevac, D. GC-FID-MS based metabolomics to access plum brandy quality. Molecules 2021, 26, 1391. [Google Scholar] [CrossRef]
- Popović, B.; Mitrović, O.; Nikićević, N.; Tešević, V.; Urošević, I.; Miletić, N.; Milojević, S. Influence of Different Pre-Distillation Steps on Aromatic Profile of Plum Spirits Produced by Traditional and Modified Methods. Processes 2023, 11, 863. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Dziekońska-Kubczak, U.; Strąk, E. The effect of distillation conditions and alcohol content in ‘heart’fractions on the concentration of aroma volatiles and undesirable compounds in plum brandies. J. Inst. Brew. 2017, 123, 452–463. [Google Scholar] [CrossRef]
- Skotniczny, M.; Satora, P.; Pańczyszyn, K.; Cioch-Skoneczny, M. Growth dynamics and diversity of yeasts during spontaneous plum mash fermentation of different varieties. Foods 2020, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Liebminger, A.; Philipp, C.; Sari, S.; Holstein, M.; Dietrich, V.; Goessinger, M. In-line conductivity measurement to select the best distillation technique for improving the quality of apricot brandies. Eur. Food Res. Technol. 2021, 247, 1987–1997. [Google Scholar] [CrossRef]
- Filatova, M.; Bechynska, K.; Hajslova, J.; Stupak, M. A comprehensive characterization of volatile profiles of plum brandies using gas chromatography coupled to high resolution mass spectrometry. LWT 2022, 167, 113864. [Google Scholar] [CrossRef]
- Botelho, G.; Anjos, O.; Estevinho, L.M.; Caldeira, I. Methanol in Grape Derived, Fruit and Honey Spirits: A Critical Review on Source, Quality Control, and Legal Limits. Processes 2020, 8, 1609. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spaho, N. Distillation techniques in the fruit spirits production. In Distillation: Innovative Applications and Modeling; IntechOpen: London, UK, 2017; pp. 129–152. [Google Scholar]
- Suomalainen, H. Yeast esterases and aroma esters in alcoholic beverages. J. Inst. Brew. 1981, 87, 296–300. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Luo, Y.; Xiao, H.; Zhao, H. Utilizing an endogenous pathway for 1-butanol production in Saccharomyces cerevisiae. Metab. Eng. 2014, 22, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bečvářová, H.; Hanč, O.; Macek, K. Course of transformation of benzaldehyde by Saccharomyces cerevisiae. Folia Microbiol. 1963, 8, 165–169. [Google Scholar] [CrossRef]
- Delfini, C.; Gaia, P.; Bardi, L.; Mariscalco, G.; Contiero, M.; Pagliara, A. Production of benzaldehyde, benzyl alcohol and benzoic acid by yeasts and Botrytis cinerea isolated from grape musts and wines. Vitis 1991, 30, 253–263. [Google Scholar]
- Velíšek, J.; Pudil, F.; Davídek, J.; Kubelka, V. The neutral volatile components of Czechoslovak plum brandy. Z. Lebensm.-Unters. Forsch. 1982, 174, 463–466. [Google Scholar] [CrossRef]
- Miličević, B.; Lukić, I.; Babić, J.; Šubarić, D.; Miličević, R.; Ačkar, Đ.; Miličević, D. Aroma and sensory characteristics of Slavonian plum brandy. Technol. Acta 2012, 5, 1–7. [Google Scholar]
- Gomez, E.; Ledbetter, C.A.; Hartsell, P.L. Volatile compounds in apricot, plum, and their interspecific hybrids. J. Agric. Food Chem. 1993, 41, 1669–1676. [Google Scholar] [CrossRef]
- Krammer, G.; Winterhalter, P.; Schwab, M.; Schreier, P. Glycosidically bound aroma compounds in the fruits of Prunus species: Apricot (P. armeniaca, L.), peach (P. persica, L.), yellow plum (P. domestica, L. ssp. Syriaca). J. Agric. Food Chem. 1991, 39, 778–781. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Patelski, P.; Dziekońska-Kubczak, U. Influence of yeast on the yield of fermentation and volatile profile of ‘Węgierka Zwykła’plum distillates. J. Inst. Brew. 2016, 122, 612–623. [Google Scholar] [CrossRef]
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The flavour of fruit spirits and fruit liqueurs: A review. Flavour Fragr. J. 2015, 30, 197–207. [Google Scholar] [CrossRef]
- Bajer, T.; Hill, M.; Ventura, K.; Bajerová, P. Authentification of fruit spirits using HS-SPME/GC-FID and OPLS methods. Sci. Rep. 2020, 10, 18965. [Google Scholar] [CrossRef]
- Schnabel, K.-O.; Belitz, H.-D.; von Ranson, C. Untersuchungen zur Struktur-Aktivität-Beziehung bei Geruchsstoffen. 1. Mitteilung: Wahrnehmungsschwellenwerte und Geruchsqualitäten von gesättigten aliphatischen und alicyclischen Verbindungen mit Sauerstoff-Funktion. Z. Lebensm.-Unters. Forsch. 1988, 187, 215–223. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Nguyen, N.T.H.; Wang, W.-Y.; Huang, W.-L.; Huang, C.-L.; Chiang, T.-Y. Metagenomics analyses of microbial dynamics associated with putative flavor development in mash fermentation of sake. LWT 2022, 163, 113570. [Google Scholar] [CrossRef]
- Gschaedler, A. Contribution of non-conventional yeasts in alcoholic beverages. Curr. Opin. Food Sci. 2017, 13, 73–77. [Google Scholar] [CrossRef]
- Baleiras-Couto, M.M.; Caldeira, I.; Gomes, F.; Botelho, G.; Duarte, F.L. Saccharomyces cerevisiae Diversity in Arbutus unedo L. Fermentations in Association with the Volatile and Sensory Similarities of the Distillates. Foods 2022, 11, 1916. [Google Scholar] [CrossRef] [PubMed]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic Fermentation as a Source of Congeners in Fruit Spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Russmayer, H.; Marx, H.; Sauer, M. Microbial 2-butanol production with Lactobacillus diolivorans. Biotechnol. Biofuels 2019, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.A.; Flouros, A.I.; Demertzis, P.G.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control 2005, 16, 157–164. [Google Scholar] [CrossRef]

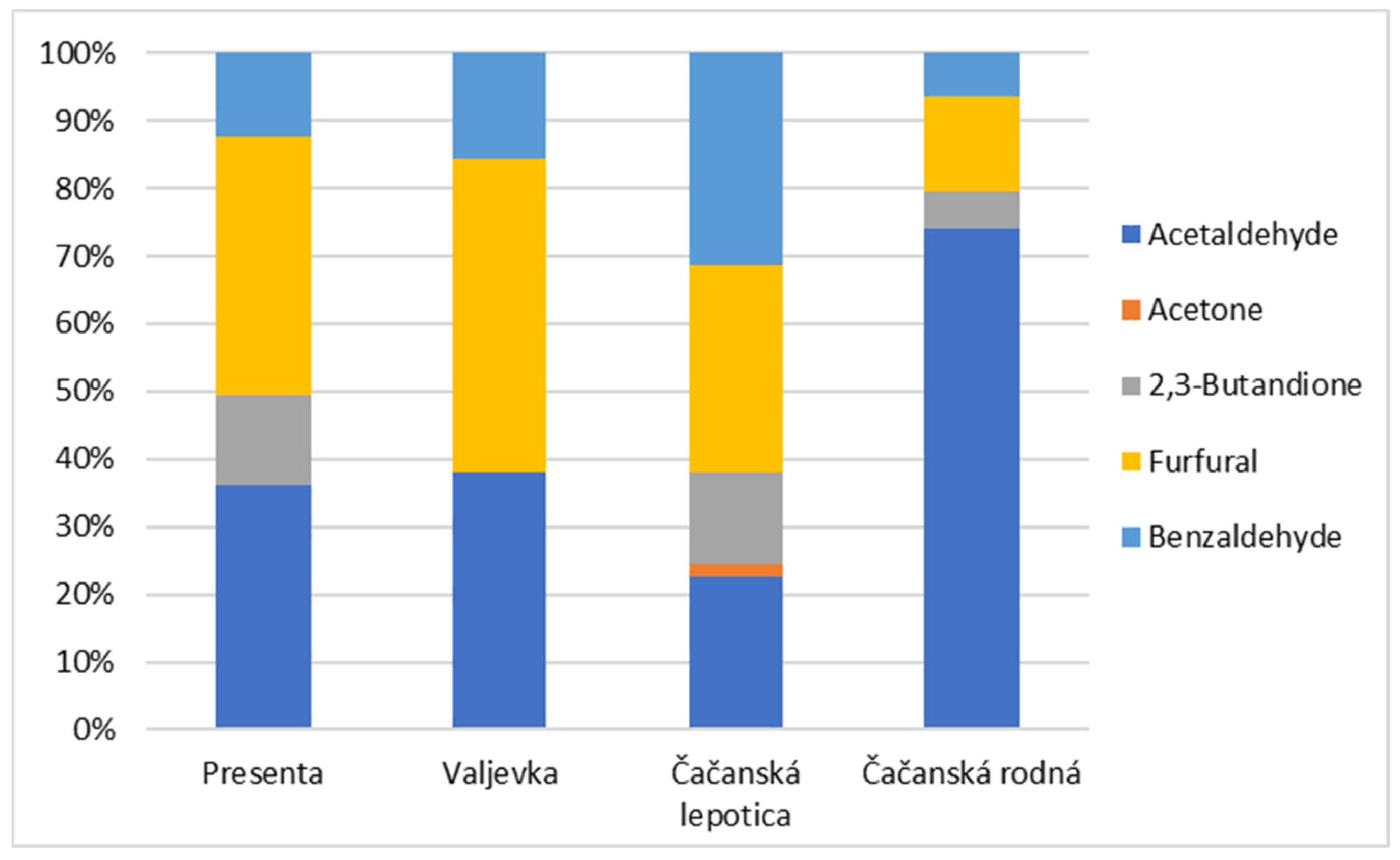
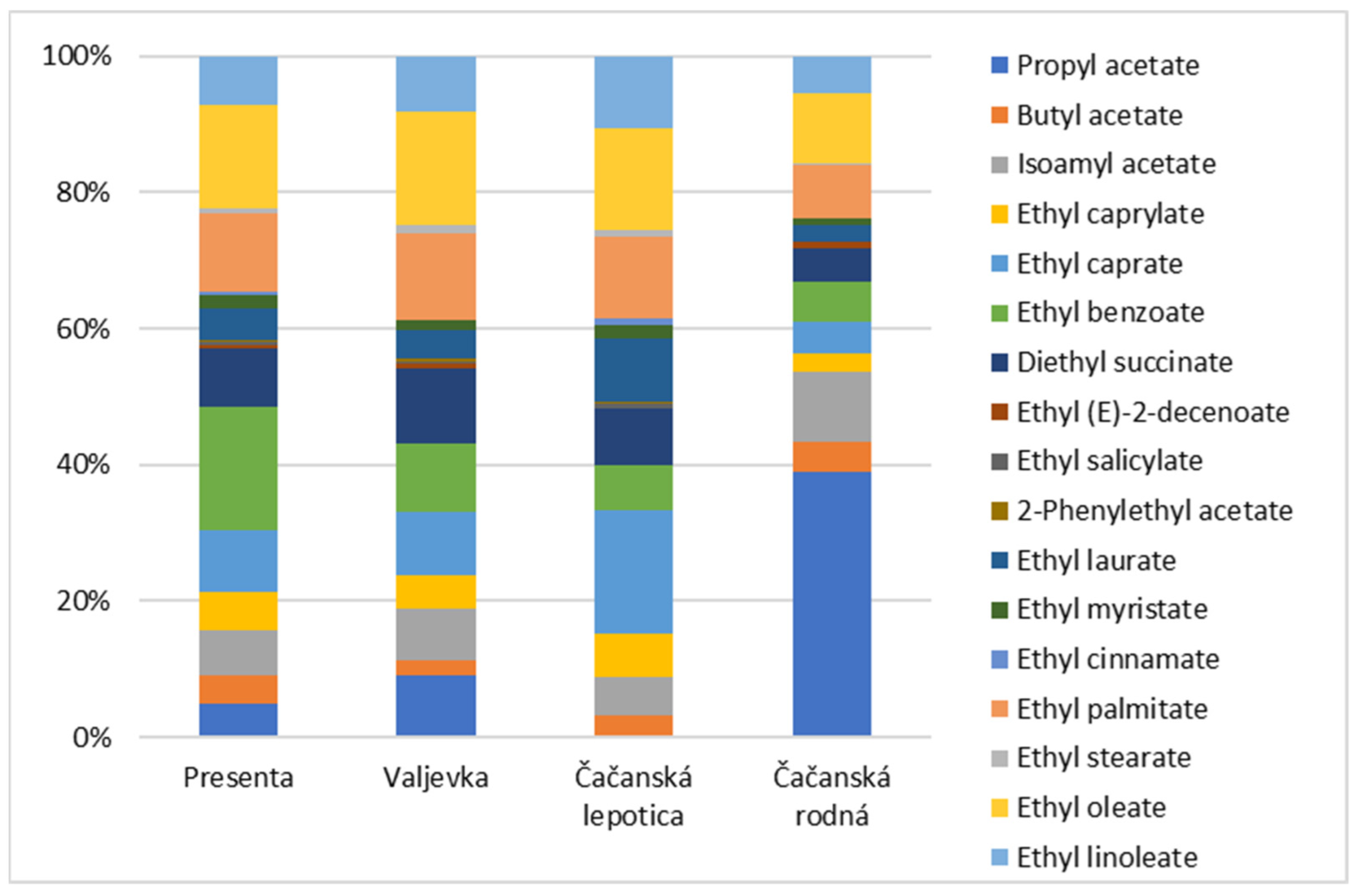
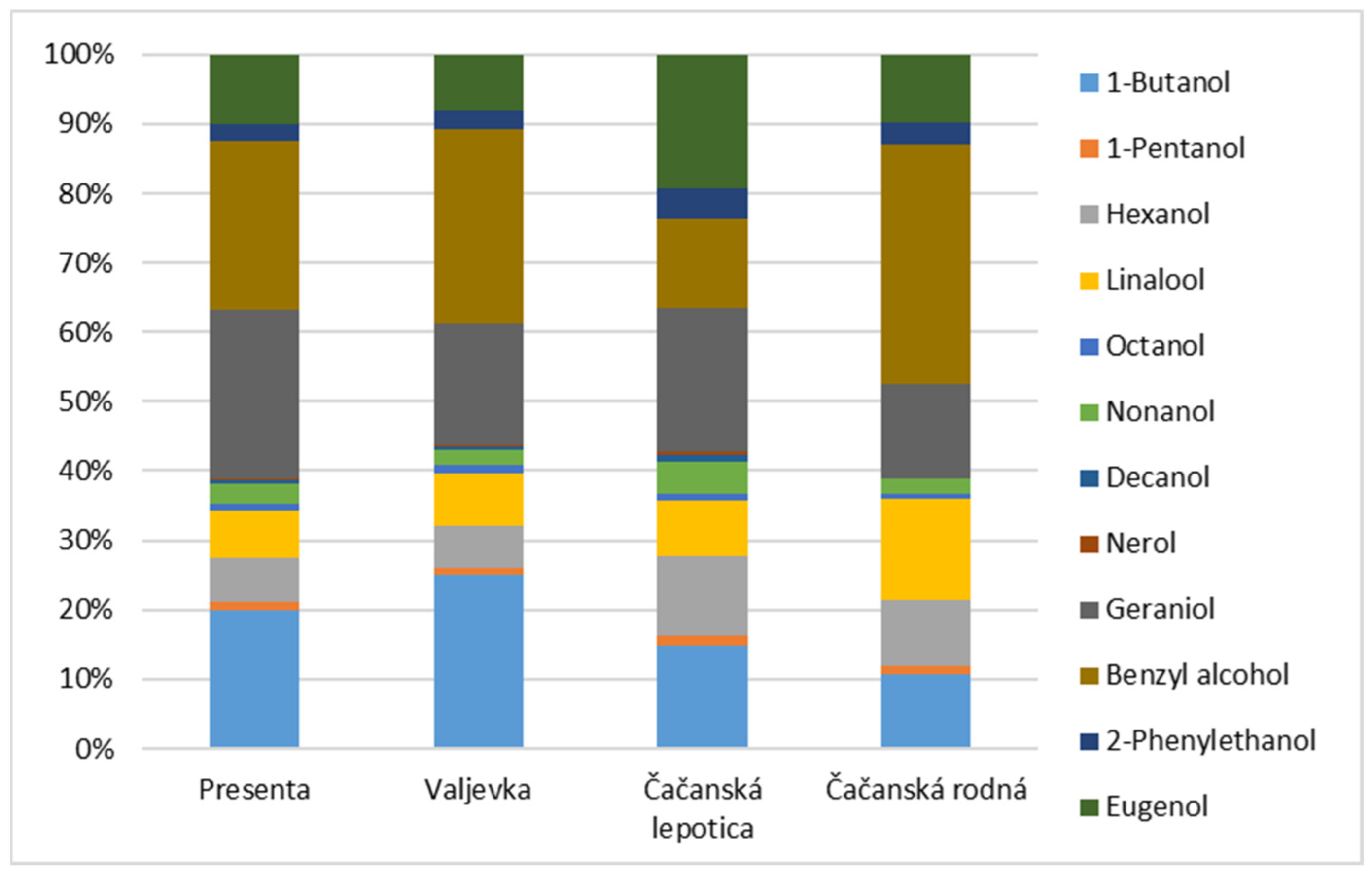
| Major Compounds | RT (min) | p | Minor Esters | RT (min) | p |
|---|---|---|---|---|---|
| Methyl acetate | 4.813 | 0.678 | Methyl acetate | 4.813 | 0.732 |
| Ethyl acetate | 6.019 | 0.725 | Propyl acetate | 9.29 | 0.047 * |
| Methanol | 6.305 | 0.341 | Butyl acetate | 13.509 | 0.824 |
| 2-Butanol | 11.47 | 0.379 | Isoamyl acetate | 15.257 | 0.052 |
| 1-Propanol | 12.058 | 0.013 * | Ethyl caprylate | 25.167 | 0.240 |
| Isobutanol | 14.305 | 0.041 * | Ethyl caprate | 30.258 | 0.259 |
| Isoamyl alcohol | 18.404 | 0.237 | Ethyl benzoate | 31.092 | 0.026 * |
| Ethyl (-)-L-lactate | 22.692 | 0.240 | Diethyl succinate | 31.224 | 0.021 * |
| Acetic acid | 25.590 | 0.374 | Ethyl (E)-2-decenoate | 33.136 | 0.065 |
| Minor alcohols | Ethyl salicylate | 34.352 | 0.034 * | ||
| 1-Butanol | 16.216 | 0.283 | 2-Phenylethyl acetate | 34.407 | 0.053 |
| 1-Pentanol | 18.404 | 0.233 | Ethyl laurate | 34.82 | 0.238 |
| 1-Hexanol | 22.88 | 0.605 | Ethyl myristate | 38.975 | 0.082 |
| Linalool | 28.034 | 0.620 | Ethyl cinnamate | 40.744 | 0.214 |
| 1-Octanol | 28.282 | 0.045 * | Ethyl palmitate | 42.794 | 0.058 |
| 1-Nonanol | 30.723 | 0.0004 * | Ethyl stearate | 46.322 | 0.027 * |
| 1-Decanol | 33.034 | 0.001 * | Ethyl oleate | 46.717 | 0.336 |
| Nerol | 33.949 | 0.298 | Ethyl linoleate | 47.478 | 0.086 |
| Geraniol | 34.923 | 0.098 | Carbonyl compounds | ||
| Benzyl alcohol | 35.661 | 0.118 | Acetaldehyde | 3.538 | 0.019 * |
| 2-Phenylethanol | 36.392 | 0.181 | Acetone | 4.615 | 0.144 |
| Eugenol | 41.381 | 0.107 | 2,3-Butandione | 9.591 | 0.365 |
| Furfural | 26.135 | 0.298 | |||
| Benzaldehyde | 27.682 | 0.283 |
| Compounds Categories | Fragrance Intensity | Harmony of Fragrance | Delicacy of Fragrance | Intensity of Fruity Aroma | Intensity of Herbal Aroma | Intensity of Spicy Aroma | Intensity of Resinous Aroma | Intensity of Bitter Almond Aroma | Intensity of Pungent Smell | Intensity of Technical Smell | Intensity of Negative smells | Overall Impression of Fragrance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher alcohols | 0.355 | −0.474 | 0.342 | 0.776 | −0.864 | 0.077 | 0.945 | 0.011 | 0.466 | 0.899 | 0.612 | −0.723 |
| Total esters | 0.753 | 0.455 | 0.927 | −0.029 | −0.677 | 0.399 | 0.176 | 0.625 | 0.643 | 0.577 | −0.418 | 0.517 |
| Acetic acid | 0.471 | −0.927 | −0.459 | −0.222 | −0.088 | −0.914 | 0.027 | 0.503 | 0.603 | 0.353 | 0.867 | −0.488 |
| Terpenes | 0.610 | 0.420 | 0.383 | −0.768 | 0.049 | −0.086 | −0.611 | 0.791 | 0.472 | −0.057 | −0.525 | 0.873 |
| Aldehydes and ketones | 0.177 | −0.846 | −0.753 | −0.448 | 0.332 | −0.991 | −0.306 | 0.342 | 0.294 | −0.066 | 0.737 | −0.360 |
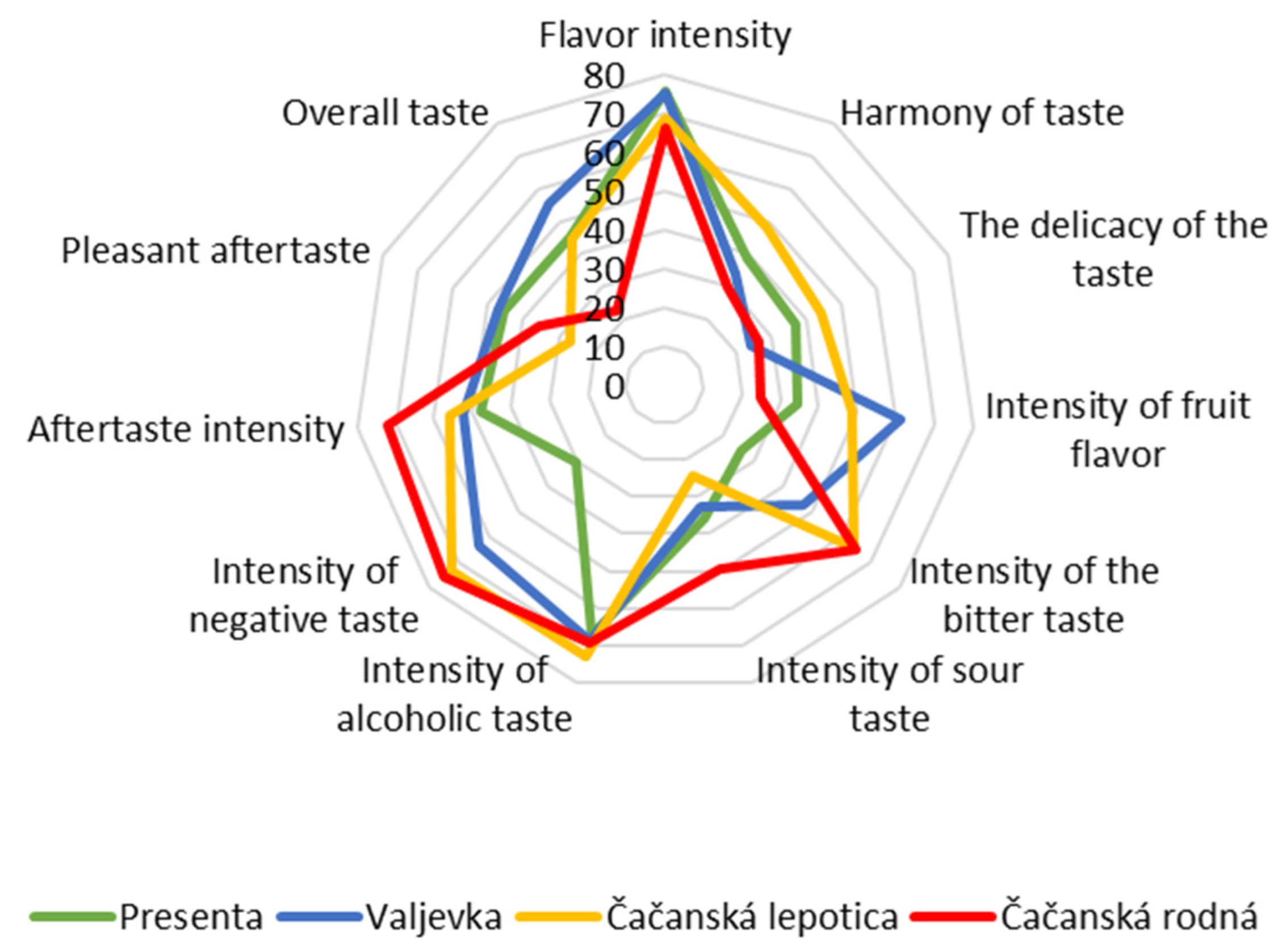
| Compounds Categories | Flavor Intensity | Harmony of Taste | Delicacy of the Taste | Intensity of Fruit Flavor | Intensity of Bitter Taste | Intensity of Sour Taste | Intensity of Alcoholic Taste | Intensity of Negative Taste | Aftertaste Intensity | Pleasant Aftertaste | Overall Taste | |
| Higher alcohols | 0.100 | −0.274 | −0.057 | −0.872 | 0.862 | 0.682 | 0.396 | 0.947 | 0.858 | 0.257 | −0.534 | |
| Total esters | 0.908 | 0.219 | 0.076 | −0.082 | −0.090 | 0.639 | 0.261 | 0.130 | 0.484 | 0.991 | 0.662 | |
| Acetic acid | 0.124 | 0.648 | 0.845 | 0.109 | 0.743 | 0.547 | 0.883 | 0.613 | 0.558 | −0.090 | −0.667 | |
| Terpenes | 0.818 | 0.711 | 0.482 | 0.696 | −0.481 | 0.266 | 0.294 | −0.402 | −0.021 | 0.673 | 0.775 | |
| Aldehydes and ketones | −0.112 | 0.660 | 0.814 | 0.393 | 0.468 | 0.174 | 0.669 | 0.268 | 0.165 | −0.385 | −0.623 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balak, J.; Drábová, L.; Maťátková, O.; Doležal, M.; Marsík, D.; Jarosova Kolouchova, I. Differences in Volatile Profiles and Sensory Characteristics in Plum Spirits on a Production Scale. Fermentation 2024, 10, 235. https://doi.org/10.3390/fermentation10050235
Balak J, Drábová L, Maťátková O, Doležal M, Marsík D, Jarosova Kolouchova I. Differences in Volatile Profiles and Sensory Characteristics in Plum Spirits on a Production Scale. Fermentation. 2024; 10(5):235. https://doi.org/10.3390/fermentation10050235
Chicago/Turabian StyleBalak, Josef, Lucie Drábová, Olga Maťátková, Marek Doležal, Dominik Marsík, and Irena Jarosova Kolouchova. 2024. "Differences in Volatile Profiles and Sensory Characteristics in Plum Spirits on a Production Scale" Fermentation 10, no. 5: 235. https://doi.org/10.3390/fermentation10050235
APA StyleBalak, J., Drábová, L., Maťátková, O., Doležal, M., Marsík, D., & Jarosova Kolouchova, I. (2024). Differences in Volatile Profiles and Sensory Characteristics in Plum Spirits on a Production Scale. Fermentation, 10(5), 235. https://doi.org/10.3390/fermentation10050235








