Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Processing of Apple Must
2.2.2. Processing of Cider
2.2.3. The Growth Curves of Yeast
2.2.4. Monitoring of Fermentation and Characterization of Ciders
2.2.5. Analysis of Volatile Compounds
2.2.6. Chromatographic Analysis of Amino Acids
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Yeast Growth Kinetics
3.2. Characterization of Ciders
3.3. Synthesis of Volatile Compounds
| Volatile Compounds mg/L | Different Inoculum and Volatile Composition after 10 Days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure | Mixed | Sequential | Mixed | Sequential | Odor Threshold | |||||||
| Sc | Hu | Hg | Hu + Sc | Hu + Sc 1d | Hu + Sc 2d | Hu + Sc 3d | Hg + Sc | Hg + Sc 1d | Hg + Sc 2d | Hg + Sc 3d | ||
| Ester | ||||||||||||
| Ethyl ethanoate | 26.9 gh ± 0.14 | 147 a ± 0.8 | 9.9 i ± 0.6 | 18 hi ± 2 | 78 d ± 7 | 91 c ± 9 | 133 b ± 13 | 32 g ± 3 | 43 f ± 4 | 48 ef ± 4 | 55 e ± 5 | 7.5 (1;2;3;6) |
| Ethyl butanoate | 0.50 b ± 0.01 | <LOD | 0.05 g ± 0.01 | 0.18 f ± 0.01 | 0.37 c ± 0.03 | 0.17 f ± 0.01 | 1.10 a ± 0.08 | 0.25 e ± 0.01 | 0.26 e ± 0.01 | 0.31 d ± 0.01 | 0.32 d ± 0.01 | 0.02 (1;2;3) |
| Isopentyl acetate | 2.88 ± 0.15 | 0.63 ± 0.03 | <LOD | 1.8 ±0.2 | 2.1 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 3.9 ± 0.3 | 3.1 ± 0.2 | 3.1 ± 0.2 | 2.4 ± 0.2 | 0.03 (1;2;3) |
| Hexyl ethanoate | 1.11 a ± 0.09 | 0.18 g ± 0.01 | <LOD | 0.13 g ± 0.01 | 0.25 f ± 0.02 | 0.35 e ± 0.03 | 0.26 f ± 0.03 | 0.43 d ± 0.01 | 0.56 cd ± 0.01 | 0.61 bc ± 0.01 | 0.61 bc ± 0.01 | 0.67 (2;3;6) |
| 2-hydroxyethyl propanoate | 26 c ± 2 | 48 a ± 4 | 45 a ± 4 | 3.8 g ± 0.1 | 10.5 de ± 0.4 | 7.5 ef ± 0.3 | 14.5 d ± 0.6 | 8.91 e ± 0.03 | 8.41 e ± 0.03 | 13.1 de ± 0.1 | 15.3 d ± 0.1 | 1.8 (3) |
| Ethyl octanoate | 2.1 b ± 0.2 | 0.10 g ± 0.01 | 0.02 g ± 0.01 | 0.5 e ± 0.03 | 0.54 b ± 0.04 | 0.54 e ± 0.04 | 0.28 f ± 0.02 | 2.3 a ± 0.2 | 1.12 d ± 0.09 | 1.3 c ± 0.1 | 0.96 d ± 0.08 | 0.002 (1;6) |
| Ethyl lactate | 0.85 a ± 0.08 | 0.29 cd ± 0.03 | 0.09 f ± 0.01 | 0.09 f ± 0.01 | 0.10 f ± 0.01 | 0.17 e ± 0.01 | 0.05 f ± 0.01 | 0.69 b ± 0.05 | 0.27 d ± 0.02 | 0.34 c ± 0.03 | 0.21 e ± 0.02 | 0.5 (1;6) |
| Diethyl succinate | 8.0 a ± 0.5 | 6.2 b ± 0.4 | 4.3 cd ± 0.2 | 1.72 h ± 0.02 | 4.51 c ± 0.05 | 2.12 g ± 0.02 | 3.45 e ± 0.04 | 4.12 d ± 0.01 | 3.14 f ± 0.01 | 3.33 ef ± 0.01 | 2.20 g ± 0.01 | 0.02 (1;2;3) |
| Ethyl dodecanoate | 4.7 ± 0.4 | 4.3 ± 0.4 | 2.9 ± 0.2 | 1.79 ± 0.01 | 3.01 ± 0.01 | 2.10 ± 0.01 | 3.50 ± 0.01 | 3.65 ± 0.08 | 2.90 ± 0.06 | 3.59 ± 0.08 | 4.7 ± 0.1 | 0.5 (1) |
| Aldehyde | ||||||||||||
| Ethanal | 28.1 b ± 0.4 | 38.0 a ± 0.6 | 21.5 c ± 0.6 | 9.9 i ± 0.9 | 19 d ± 2 | 12 egh ± 1 | 27 b ± 2 | 11.2 ehi ± 0.8 | 12.5 fg ± 0.9 | 14 f ± 1 | 18 d ± 1 | 0.5 (3) |
| Acid | ||||||||||||
| Butanoic acid | 10.7 a ± 0.6 | 4.8 b ± 0.2 | 3.3 c ± 0.3 | <LOD | <LOD | 2.86 d ± 0.04 | <LOD | 11.0 a ± 0.4 | <LOD | <LOD | <LOD | 0.24 (5) |
| Octanoic acid | 5.0 a ± 0.1 | <LOD | <LOD | 1.62 h ± 0.03 | 1.75 g ± 0.03 | 1.96 f ± 0.03 | 1.74 g ± 0.03 | 3.7 b ± 0.1 | 3.2 c ± 0.1 | 2.47 e ± 0.07 | 2.67 d ± 0.08 | 10 (1;6) |
| Higher alcohol | ||||||||||||
| 3-methyl-1-butanol | 116.1 a ± 0.8 | 22.0 h ± 0.1 | 12.4 h ± 0.4 | 60 f ± 5 | 64 ce ± 6 | 39 g ± 3 | 59 ef ± 5 | 112 a ± 10 | 76 bd ± 7 | 83 b ± 8 | 70 cd ± 6 | 30 (1;2;3) |
| 2-hexanol | 10.1 a ± 0.6 | 0.28 i ± 0.02 | 0.13 i ± 0.01 | 2.4 fg ± 0.2 | 2.6 f ± 0.2 | 2.3 gh ± 0.2 | 1.8 h ± 0.2 | 8.0 c ± 0.5 | 9.0 b ± 0.5 | 6.0 d ± 0.3 | 5.2 e ± 0.3 | 15 (3) |
| 1-hexanol | 0.78 f ± 0.01 | <LOD | <LOD | 0.38 g ± 0.01 | 1.39 c ± 0.03 | 0.85 e ± 0.02 | 1.40 c ± 0.03 | 1.88 a ± 0.06 | 1.10 d ± 0.04 | 1.56 b ± 0.05 | 1.58 b ± 0.05 | 0.11 (6) |
| 2-phenylethanol | 39 a ± 2 | <LOD | <LOD | 11.1 g ± 0.02 | 15.74 e ± 0.02 | 7.86 h ± 0.01 | 12.78 f ± 0.02 | 31.0 b ± 0.5 | 19.5 d ± 0.3 | 23.0 c ± 0.4 | 18.5 d ± 0.3 | 10 (1;2;3) |
| Ketone | ||||||||||||
| 2-heptanone | <LOD | <LOD | 0.04 e ± 0.01 | <LOD | <LOD | <LOD | <LOD | 1.38 b ± 0.04 | 5.4 a ± 0.2 | 0.31 d ± 0.01 | 0.44 c ± 0.01 | 0.0082 (4) |
| 2-octanone | <LOD | 0.06 ± 0.01 | <LOD | 0.02 ± 0.01 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.15 (7) |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleutério dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT- Food Sci. Tech. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of Yeasts for Apple Juice Fermentation and Production of Cider Volatile Compounds. LWT- Food Sci. Tech. 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Cerri, M.L.; Gomes, T.A.; Carraro, M.D.M.; Wojeicchowski, J.P.; Demiate, I.M.; Lacerda, L.G.; Alberti, A.; Nogueira, A. Assessing the Impact of Simultaneous Co-Fermentation on Malolactic Bioconversion and the Quality of Cider Made with Low-Acidity Apples. Fermentation 2023, 9, 1017. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Yuan, Y.; Yue, T. Chemical Composition, Sensorial Properties, and Aroma-Active Compounds of Ciders Fermented with Hanseniaspora osmophila and Torulaspora quercuum in Co- and Sequential Fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef]
- Cabranes, C.; Mangas, J.J.; Blanco, D. Selection and Biochemical Characterisation of Saccharomyces cerevisiae and Kloeckera apiculata Strains Isolated From Spanish Cider. J. Inst. Brew. 1997, 103, 165–169. [Google Scholar] [CrossRef]
- Liu, S.Q.; Aung, M.T.; Lee, P.R.; Yu, B. Yeast and Volatile Evolution in Cider Co-Fermentation with Saccharomyces cerevisiae and Williopsis saturnus. Ann. Microbiol. 2016, 66, 307–315. [Google Scholar] [CrossRef]
- Aung, M.T.; Lee, P.R.; Yu, B.; Liu, S.Q. Cider Fermentation with Three Williopsis saturnus Yeast Strains and Volatile Changes. Ann. Microbiol. 2015, 65, 921–928. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Yuan, Y.; Yue, T. Assessment of Chemical Composition and Sensorial Properties of Ciders Fermented with Different Non-Saccharomyces Yeasts in Pure and Mixed Fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Daccache, M.A.L.; Salameh, D.; Chamy, L.E.L.; Koubaa, M.; Maroun, R.G.; Vorobiev, E.; Louka, N. Evaluation of the Fermentative Capacity of an Indigenous Hanseniaspora sp. Strain Isolated from Lebanese Apples for Cider Production. FEMS Microbiol. Lett. 2021, 367. [Google Scholar] [CrossRef]
- Mančić, S.; Stojanović, S.S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological Characterization of Native Hanseniaspora uvarum Strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- De Arruda Moura Pietrowski, G.; Dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of Fermentation with Hanseniaspora sp. Yeast on the Volatile Profile of Fermented Apple. J. Agric. Food Chem. 2012, 60, 9815–9821. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.D.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Pietrowski, G.A.M.; Bittencourt, J.V.M.; Brandão, L.R.; Rosa, C.A.; Alberti, A.; Nogueira, A. Identification and Selection of Non-Saccharomyces Strains Isolate from Brazilian Apple Must. Cienc. Rural. 2018, 48, e20170886. [Google Scholar] [CrossRef]
- Reid, M.S.; Padfield, C.A.S.; Watkins, C.B.; Harman, J.E. Starch Iodine Pattern as a Maturity Index for Granny Smith Apples. N. Zeal. J. Agric. Res. 1982, 25, 229–237. [Google Scholar] [CrossRef]
- Libkind, D.; Brizzio, S.; Ruffini, A.; Gadanho, M.; van Broock, M.; Sampaio, J.P. Molecular Characterization of Carotenogenic Yeasts from Aquatic Environments in Patagonia, Argentina. Anton. Leeuw. Int. J. G 2003, 84, 313–322. [Google Scholar] [CrossRef]
- Brandão, L.R.; Libkind, D.; Vaz, A.B.M.; Espírito Santo, L.C.; Moliné, M.; de García, V.; van Broock, M.; Rosa, C.A. Yeasts from an Oligotrophic Lake in Patagonia (Argentina): Diversity, Distribution and Synthesis of Photoprotective Compounds and Extracellular Enzymes. FEMS Microbiol. Ecol. 2011, 76, 1–13. [Google Scholar] [CrossRef]
- Eleutério dos Santos, C.M.; Pietrowski, G.D.A.M.; Braga, C.M.; Rossi, M.J.; Ninow, J.; dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar] [CrossRef]
- Schmidell, W.; Lima, U.A.; Aquarone, E.; Borzani, W. Biotecnologia Industrial: Engenharia Bioquímica; Blucher: São Paulo, Brazil, 2001; Volume 2. [Google Scholar]
- Crous, P.W.; Verkley, G.J.M.; Groenewald, J.Z.; Samson, R.A. Fungal Biodiversity. CBS Laboratory Manual Series; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2009; Volume 1. [Google Scholar]
- Roger, J.M.; Sablayrolles, J.M.; Steyer, J.P.; Bellon-Maurel, V. Pattern Analysis Techniques to Process Fermentation Curves: Application to Discrimination of Enological Alcoholic Fermentations. Biotechnol. Bioeng. 2002, 79, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.; Brunner, H.R. Getränke Anlytik: Untersuchungsmethode Für Dia Labor Und Betriebspraxis; Verlag Helles: Wädesnwill, Switzerland, 1985. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., Ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy Sulphur Compounds, Higher Alcohols and Esters Production Profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii Grown as Pure and Mixed Cultures in Grape Must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.Y.; Huang, P.H.; Lai, Y.T.; Lin, S.P.; Liou, B.K.; Lin, H.W.; Hsieh, C.W.; Cheng, K.C. Screening and Identification of Yeasts from Fruits and Their Coculture for Cider Production. Fermentation 2022, 8, 1. [Google Scholar] [CrossRef]
- Way, M.L.; Jones, J.E.; Longo, R.; Dambergs, R.G.; Swarts, N.D. A Preliminary Study of Yeast Strain Influence on Chemical and Sensory Characteristics of Apple Cider. Fermentation 2022, 8, 455. [Google Scholar] [CrossRef]
- Rollero, S.; Mouret, J.R.; Bloem, A.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Camarasa, C. Quantitative 13C-Isotope Labelling-Based Analysis to Elucidate the Influence of Environmental Parameters on the Production of Fermentative Aromas during Wine Fermentation. Microb. Biotechnol. 2017, 10, 1649–1662. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, Y.; Zhu, R.; Bai, J.; Qiu, J.; Wu, Y.; Zhong, K.; Gao, H. Insight into the Characteristics of Cider Fermented by Single and Co-Culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe Based on Metabolomic and Transcriptomic Approaches. LWT- Food Sci. Tech. 2022, 163, 113538. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X. Sen The Effects of Simultaneous and Sequential Inoculation of Yeast and Autochthonous Oenococcus Oeni on the Chemical Composition of Red-Fleshed Apple Cider. LWT- Food Sci. Tech. 2020, 124, 109184. [Google Scholar] [CrossRef]
- Karl, A.D.; Zakalik, D.L.; Cook, B.S.; Krishna Kumar, S.; Peck, G.M. The Biochemical and Physiological Basis for Hard Cider Apple Fruit Quality. Plants People Planet 2023, 5, 178–189. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile Composition of Merlot Red Wine and Its Contribution to the Aroma: Optimization and Validation of Analytical Method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Fiore, A.; la Gatta, B.; Gerardi, C.; Grieco, F.; Tufariello, M. A Chemometric Approach to the Evaluation of Sparkling Ciders Produced by Champenoise and Charmat Methods. Food Biosci. 2023, 55, 102917. [Google Scholar] [CrossRef]
- Wang, J.; Yan, J.; Zhang, W.; Zhang, Y.; Dong, Z.; Luo, H.; Liu, M.; Su, J. Comparison of Potential Wickerhamomyces anomalus to Improve the Quality of Cabernet Sauvignon Wines by Mixed Fermentation with Saccharomyces cerevisiae. LWT- Food Sci. Tech. 2023, 173, 114285. [Google Scholar] [CrossRef]
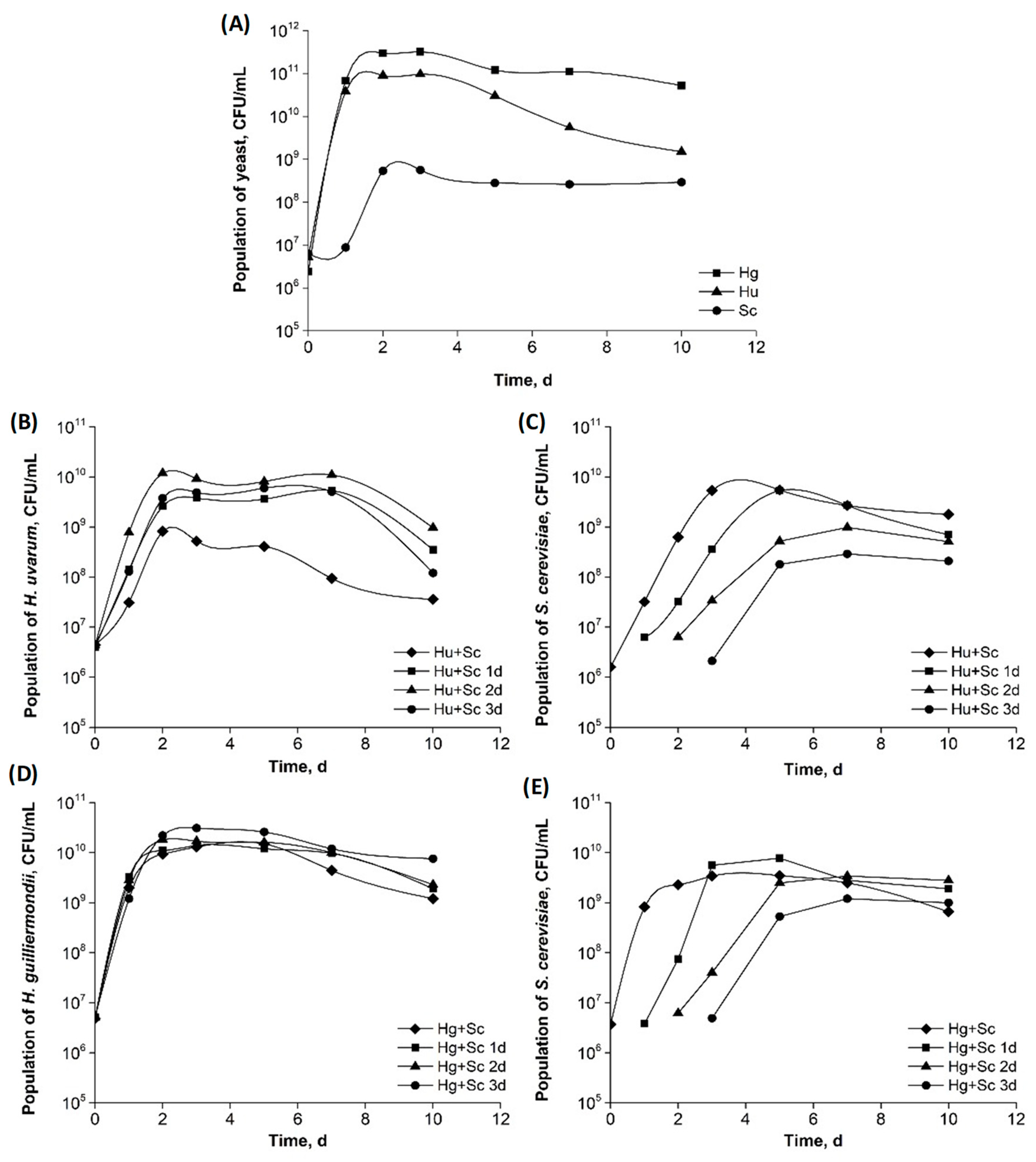
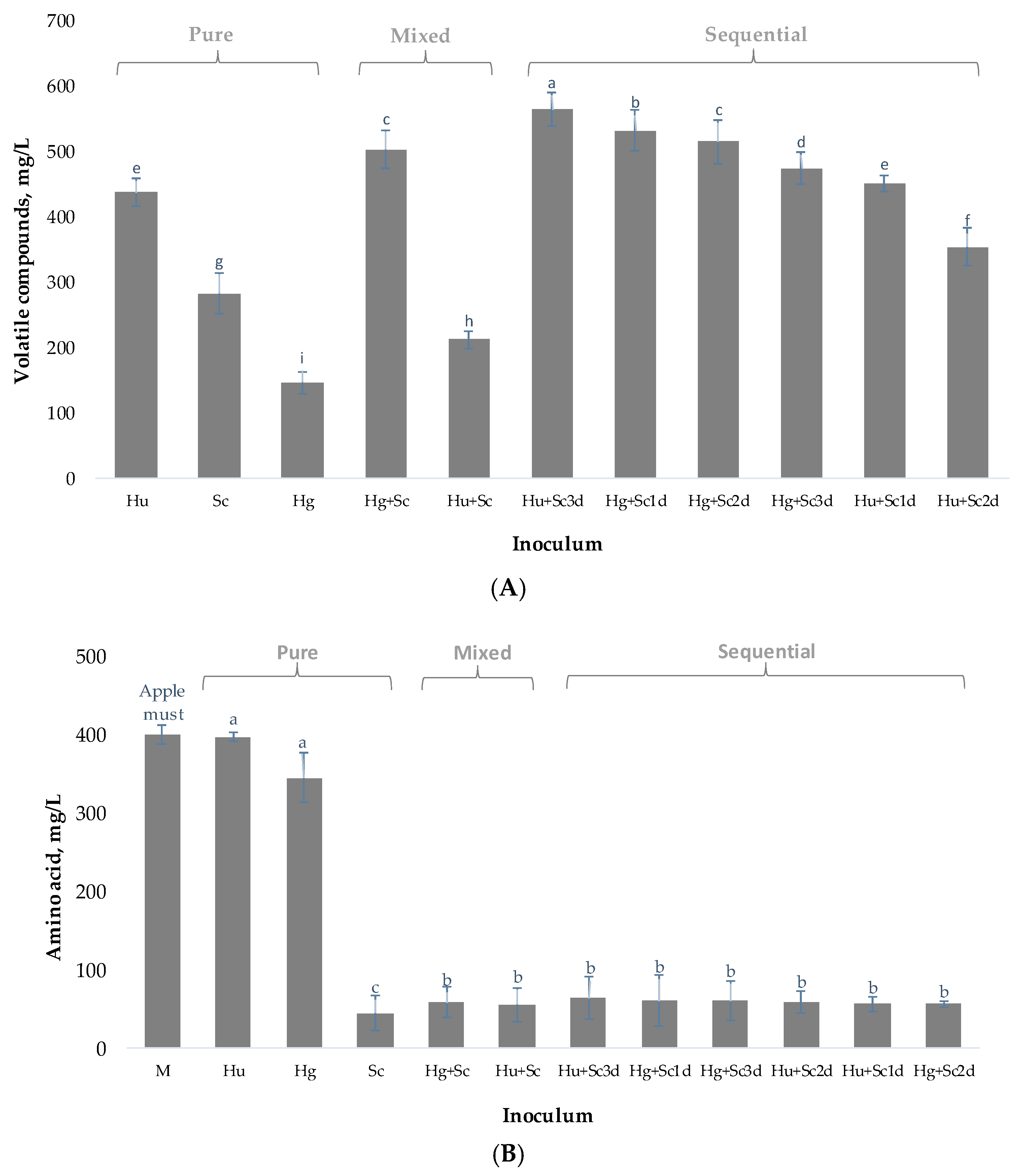
| Analytical Parameters | Apple Must | Ciders | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure | Mixed | Sequential | Mixed | Sequential | ||||||||
| Sc | Hu | Hg | Hu + Sc | Hu + Sc 1d | Hu + Sc 2d | Hu + Sc 3d | Hg + Sc | Hg + Sc 1d | Hg + Sc 2d | Hg + Sc 3d | ||
| N mg/L | 153.47 ± 0.02 | 66 b ± 7 | 106 a ± 6 | 118 a ± 3 | 69 b ± 6 | 69 b ± 6 | 67 b ± 4 | 64 b ± 4 | 65 b ± 3 | 74 b ± 3 | 68 b ± 3 | 72 b ± 2 |
| TA g/100 mL | 0.34 ± 0.01 | 0.30 b ± 0.01 | 0.41 a ± 0.01 | 0.36 a ± 0.01 | 0.36 a ± 0.00 | 0.38 a ± 0.02 | 0.39 a ± 0.01 | 0.32 b ± 0.01 | 0.36 a ± 0.01 | 0.38 a ± 0.02 | 0.39 a ± 0.01 | 0.39 a ± 0.01 |
| VA g/100 mL | 0.02 ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.01 | 0.03 b ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.01 | 0.07 a ± 0.01 | 0.06 a ± 0.01 | 0.05 a ± 0.01 | 0.05 a ± 0.01 |
| RS g/100 mL | 9.23 ± 0.09 | 0.25 g ± 0.01 | 3.86 a ± 0.04 | 2.47 b ± 0.11 | 0.78 e ± 0.03 | 0.79 e ± 0.01 | 1.23 d ± 0.06 | 1.51 c ± 0.01 | 0.18 g ± 0.01 | 0.40 f ± 0.01 | 0.59 f ± 0.01 | 1.12 d ± 0.01 |
| TS g/100 mL | 11.78 ± 0.05 | 1.38 f ± 0.04 | 3.70 b ± 0.07 | 2.82 d ± 0.02 | 2.11 e ± 0.08 | 2.27 e ± 0.02 | 2.90 a ± 0.02 | 4.04 d ± 0.02 | 1.66 f ± 0.01 | 1.57 f ± 0.04 | 2.40 e ± 0.03 | 3.15 c ± 0.07 |
| Ethanol % vol. | - | 5.4 a ± 0.1 | 1.9 f ± 0.1 | 1.3 e ± 0.1 | 5.1 b ± 0.1 | 5.0 b ± 0.1 | 4.9 c ± 0.1 | 4.2 d ± 0.1 | 5.5 a ± 0.1 | 5.4 a ± 0.1 | 5.2 b ± 0.1 | 4.9 c ± 0.1 |
| Vmax | - | 11.5 | 3.8 | 2.3 | 11.9 | 9.3 | 8.0 | 7.5 | 14.5 | 10.1 | 8.5 | 6.5 |
| Time Vmax (d) | - | 1.7 | 4.2 | 4.7 | 2.7 | 3.5 | 4.2 | 7.0 | 1.7 | 3.0 | 5.0 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sola, I.M.M.S.; Evers, L.D.; Wojeicchowski, J.P.; Assis, T.M.d.; Marinho, M.T.; Demiate, I.M.; Alberti, A.; Nogueira, A. Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders. Fermentation 2024, 10, 177. https://doi.org/10.3390/fermentation10040177
Sola IMMS, Evers LD, Wojeicchowski JP, Assis TMd, Marinho MT, Demiate IM, Alberti A, Nogueira A. Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders. Fermentation. 2024; 10(4):177. https://doi.org/10.3390/fermentation10040177
Chicago/Turabian StyleSola, Isabela Maria Macedo Simon, Larissa Deckij Evers, José Pedro Wojeicchowski, Tatiane Martins de Assis, Marina Tolentino Marinho, Ivo Mottin Demiate, Aline Alberti, and Alessandro Nogueira. 2024. "Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders" Fermentation 10, no. 4: 177. https://doi.org/10.3390/fermentation10040177
APA StyleSola, I. M. M. S., Evers, L. D., Wojeicchowski, J. P., Assis, T. M. d., Marinho, M. T., Demiate, I. M., Alberti, A., & Nogueira, A. (2024). Impact of Pure, Co-, and Sequential Fermentations with Hanseniaspora sp. and Saccharomyces cerevisiae on the Volatile Compounds of Ciders. Fermentation, 10(4), 177. https://doi.org/10.3390/fermentation10040177







