Effect of Fermentation Time and Blending Ratio on Microbial Dynamics, Nutritional Quality and Sensory Acceptability of Shameta: A Traditional Cereal-Based Fermented Porridge for Lactating Mothers in Ethiopia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
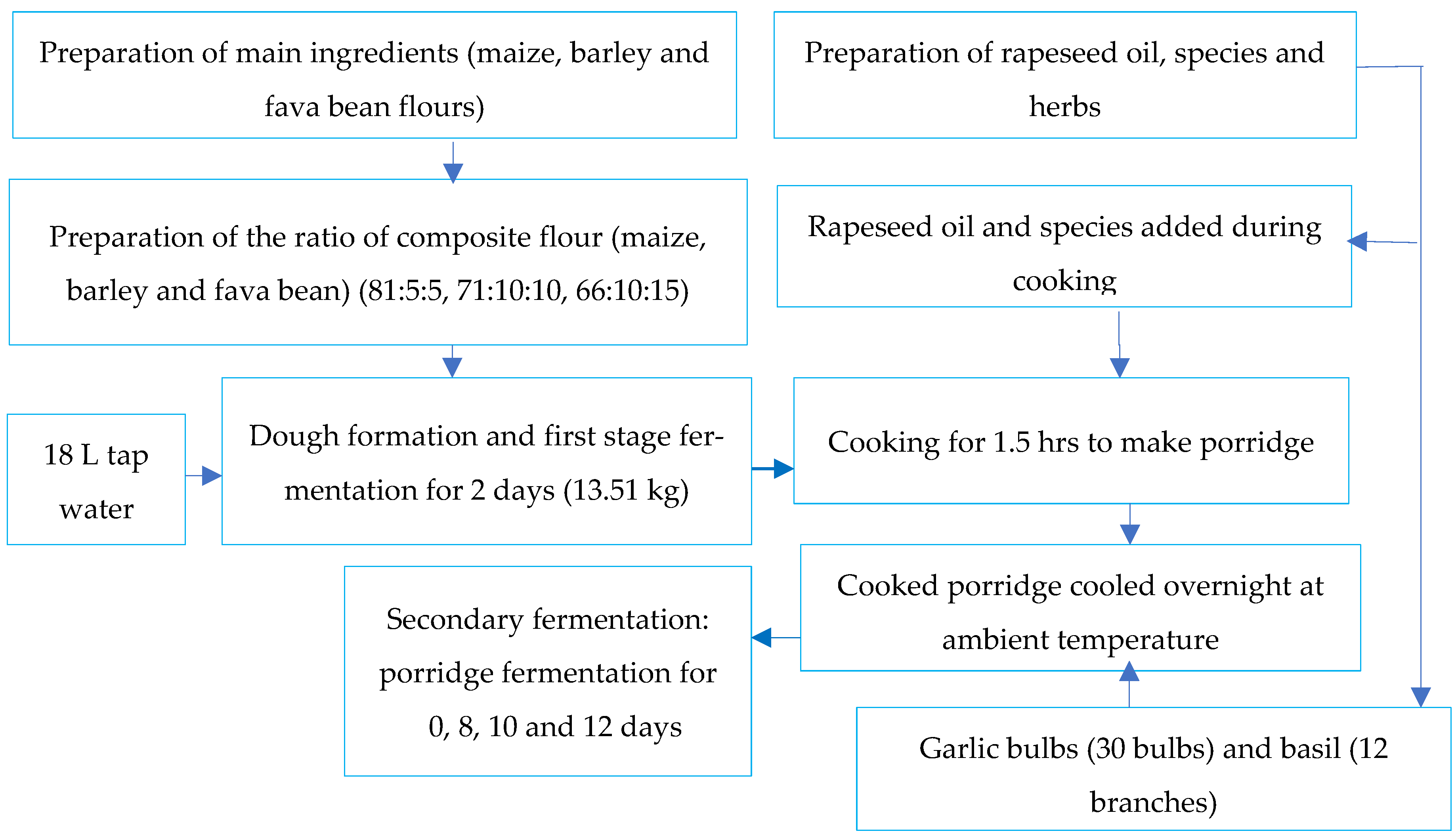
2.2. Experimental Design and Plan
2.3. Samples Preparation
2.4. Composite Flour for Shameta Preparation
2.5. Fermentation of Composite Ingredients
2.6. Determination of Microbial Dynamic in the Second Phase of Fermentation
2.7. Sample Preparation for Analytical Measurements
2.8. Determination of pH and Titratable Acidity (TA.)
2.9. Determination of Proximate Composition and Gross Energy
2.10. Determination of Minerals Content
2.11. Determination of Anti-Nutritional Factors
2.11.1. Determination of Condensed Tannin Contents
2.11.2. Determination of Phytate Contents
2.12. Determination of Total Antioxidant Activities
2.13. Sensory Evaluation
2.14. Data Analysis
3. Results and Discussion
3.1. Microbial Dynamics of Shameta Fermentation
3.2. Effect of Flour Composition and Fermentation Time on the Chemical Composition of Shameta
3.2.1. Effect of Flour Composition and Fermentation Time on pH and Titratable Acidity (TA)
3.2.2. Effect of Flour Composition and Fermentation Time on Proximate Composition
3.2.3. Effect of Flour Composition and Fermentation Time on Minerals Contents
3.2.4. Effect of Flour Composition and Fermentation Time on Anti-Nutritional Factors and Antioxidant Capacity
3.3. Sensory Properties
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose, and effecacy: A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Rollan, G.C.; Gerez, C.L.; LeBlanc, J.G. Lactic fermentation as a strategy to improve the nutritional and functional values of pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 2–14. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.G. Investigation of pickled water spinach (Ipomoea aquatic) fermentation by Lactobacillus sp. Int. J. Multidiscip. Res. Dev. 2014, 1, 71–80. [Google Scholar]
- CARE (Cooperative for Assistance and Relief Everywhere). Emergency Nutritional Assistance; CARE International: Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Ahmed, W.A.M.; Ahmed, E.A.; Arafa, K.A.O.; El-Amin, E.I.S.; Alostaz, Z.M.; Khalid, K.E. Nutritional status of mothers and its determinants in Alemtidad area, Khartoum. Food Nutr. Sci. 2014, 5, 2203–2208. [Google Scholar] [CrossRef]
- Central Statistical Agency of Ethiopia and ORC Macro. Ethiopia Demographic and Health Survey 2005; Central Statistical Agency and ORC Macro: Addis Ababa, Ethiopia; Calverton, MD, USA, 2006. [Google Scholar]
- Alemayehu, M.; Argaw, A.; Mariam, A.G. Factors associated with malnutrition among lactating women in subsistence farming households from Dedo and Seqa-Chekorsa Districts, Jimma Zone. Dev. Ctry. Stud. 2015, 5, 117–118. [Google Scholar]
- Moges, A.; Gudina, E.; Yadeta, D. Under Nutrition and Associated Factors among Adolescent Pregnant Women in Afdem District, Ethiopian Somali Region; Harmaya University: Haramaya, Ethiopia, 2018. [Google Scholar]
- Kitessa, D.A.; Bacha, K.; Tola, Y.B.; Murimi, M. Assessment of the Preparation and Consumption of Shameta: An Indigenous Cereal-based Fermented Porridge in Ethiopia. East Afr. J. Sci. 2023, 17, 55–70. [Google Scholar] [CrossRef]
- Kitessa, D.A.; Bacha, K.; Tola, Y.B.; Murimi, M.; Smith, E.; Gershe, S. Nutritional compositions and bioactive compounds of “Shameta”, a traditional home made fermented porridge provided exclusively to lactating mothers in the western part of Ethiopia. Heliyon 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Kitessa, D.A.; Bacha, K.; Tola, Y.B.; Murimi, M.; Gershe, S.; Guta, M. Microbial Quality and Growth Dynamics in Shameta: A Traditional Ethiopian Cereal-Based Fermented Porridge. Fermentation 2022, 8, 124. [Google Scholar] [CrossRef]
- Mugula, J.K.; Nnko, S.A.M.; Sorhaug, T. Changes in quality attributes during storage of togwa: A lactic acid fermented gruel. J. Food Saf. 2001, 21, 181–194. [Google Scholar] [CrossRef]
- Soda, M.E.; Ahmed, N.; Omran, N.; Osman, G.; Morsi, A. Isolation, identification and selection of lactic acid bacteria cultures for cheese making. Emir. J. Agric. Sci. 2003, 15, 51–71. [Google Scholar]
- Abegaz, K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde: An Ethiopian cereal-based beverage. Afr. J. Biotechnol. 2007, 6, 1469–1478. [Google Scholar]
- Antony, U.; Chandra, T. Microbial Population and biochemical changes in fermenting finger millet (Eleusine coracana). World J. Microbiol. Biotechnol. 1997, 13, 533–537. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, 18th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- FAO. Fermented Fruits Vegetables: AGlobal Perspective; Battcock, M., Azam-Ali, S., Eds.; FAO, Agricultural Service Bulletin No. 134; FAO: Rome, Italy, 1998. [Google Scholar]
- Nguyen, T.T.T.; Loiseau, G.; Icard-Vernière, C.; Rochette, I.; Trèche, S.; Guyot, J.-P. Effect of fermentation by amylolytic lactic acid bacteria, in process combinations, on characteristics of rice/soybean slurries: A new method for preparing high energy density complementary foods for young children. Food Chem. 2007, 100, 623–631. [Google Scholar] [CrossRef]
- AACC. Official Methods of Analysis of the AACC, 11th ed.; AACC: Washington, DC, USA, 2000. [Google Scholar]
- Maxson, E.D. Evaluation of Methods for Tannin Analysis in Sorghum Grain; American Association of Cereal Chemists: St Paul, MN, USA, 1972. [Google Scholar]
- Vaintraub, I.A.; Lapteva, N.A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 1988, 175, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Temba, M.C. Quality Evaluation of Porridges Prepared from Maize-Groundnut Composite Flour. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2016. [Google Scholar]
- Liptáková, D.; Matejčeková, Z.; Valík, Ľ. Lactic Acid Bacteria and Fermentation of Cereals and Pseudocereals. Ferment. Process. 2017, 10, 224–242. [Google Scholar]
- Nsofor, C.A.; Ume, S.C.; Uzor, B.C. Isolation and characterization of lactic acid bacteria from ogi sold in Elele, Nigeria. J. Biol. Food Sci. Res. 2014, 3, 19–22. [Google Scholar]
- Nemo, R.; Bacha, K. Microbial dynamic and growth potential of selected pathogens in Ethiopian traditional fermented beverages. Ann. Microbiol. 2021, 71, 22. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Center for Food Safety (CFS). Microbiological Guidelines for Food: For Ready-to-Eat Foods in General and Specific Food Items (Revised); Environmental Hygiene Department, Queensway: Hong Kong, China, 2014. [Google Scholar]
- Abawari, R.A. Microbiology of keribo fermentation: An Ethiopian traditional fermented beverage. Pak. J. Biol. Sci. 2013, 16, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Alba, C.; Alberto, A.; Leticia, G.; Domingo, F.; Marcos, L.; Tornadijo, E. Effect of fermentation on microbiological, physicochemical and physical characteristics of sourdough and impact of its use on bread quality. Czech J. Food Sci. 2017, 35, 496–506. [Google Scholar] [CrossRef]
- Nwokoro, O.; Chukwu, B.C. Studies on Akamu; a traditional fermented maize food. Rev. Chil. Nutr. 2012, 39, 180–184. [Google Scholar]
- Adebayo, W.A.; Ogunsina, B.S.; Gbadamosi, S.O. Some physicochemical and functional properties of kariya (Hildegardia baterii) kernel flours. IFE J. Sci. 2013, 15, 477–488. [Google Scholar]
- GFIRA (German Federal Institute for Risk Assessment). Escherichia coli in flour—Sources, risks and prevention. BfR Opin. 2020, 4, 1–28. [CrossRef]
- Delbeke, S. Prevalence, Behavior and Risk Assessment of Salmonella spp. and Shiga Toxin-Producing Escherichia coli on basil Leaves and Strawberries. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2015. [Google Scholar]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Banerjee, M.; Sarkar, P.K. Inhibitory effect of garlic on bacterial pathogens from spices. World J. Microbiol. Biotechnol. 2003, 19, 565–569. [Google Scholar] [CrossRef]
- Maffei, D.F.; de Arruda Silveira, N.F.; da Penha Longo Mortatti Catanozi, M. Microbiological quality of organic and conventional vegetables sold in Brazil. Food Control 2013, 29, 226–230. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Zannerni, R.; Inayat, A.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Kamil, M.; Kikas, T. Current progress in anaerobic digestion reactors and parameters optimization. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef]
- Cook, F.K.; Johnson, B.L. Microbiological Spoilage of Cereal Products. In Compendium of the Microbiological Spoilage of Foods and Beverages. Food Microbiology and Food Safety; Sperber, W., Doyle, M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Dias, I.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Ricardo-Rodrigues, S.; João Fraqueza, M.J.; Oliveira, M.; Elias, M. Staphylococcus spp. and Lactobacillus sakei Starters with High Level of Inoculation and an Extended Fermentation Step Improve Safety of Fermented Sausages. Fermentation 2022, 8, 49. [Google Scholar] [CrossRef]
- Heo, S.; Lee, J.H.; Jeong, D.W. Food-derived coagulase-negative Staphylococcus as starter cultures for fermented foods. Food Sci. Biotechnol. 2020, 29, 1023–1035. [Google Scholar] [CrossRef]
- FSANZ (Food Standards Australia New Zealand). Compendium of Microbiological Criteria for Food; FSANZ: Kingston, Australia; Wellington, New Zealand, 2022; pp. 1–51. [Google Scholar]
- Fredlund, E.; Druvefors, U.Ä.; Olstorpe, M.N.; Passoth, V.; Schnürer, J. Influence of ethyl acetate production and ploidy on the anti-mould activity of Pichia anomala. FEMS Microbiol. Lett. 2004, 238, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.K.; Omeje, F.I.; Obinwa, G.N. Microbial succession pattern in Ogi fermentation. Int. J. Adv. Res. Biol. Sci. 2018, 5, 247–251. [Google Scholar]
- Almeida, E.G.; Rachid, C.C.; Schwan, R.F. Microbial population present in fermented beverage ‘cauim’ produced by Brazilian Amerindians. Int. J. Food Microbiol. 2007, 120, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bacha, K. Microbial Ecology of Borde and Shamita Fermentation. Master’s Thesis, Department of Biology, Addis Ababa University, Addis Ababa, Ethiopia, 1997. [Google Scholar]
- Preetha, S.S.; Narayanan, R. Factors Influencing the Development of Microbes in Food. Shanlax Int. J. Arts Sci. Humanit. 2020, 7, 57–77. [Google Scholar] [CrossRef]
- Jespersen, L.; Halm, M.; Kpodo, K.; Jacobsen, M. Significance of yeasts and moulds occurring in maize dough fermentation for kenkey production. Int. J. Food Microbiol. 1994, 24, 239–248. [Google Scholar] [CrossRef]
- Omemu, A.M.; Oyewole, O.B.; Bankole, M.O.; Akintokun, A.K. Yeasts and moulds associated with ogi; a cereal-based weaning food during storage. Res. J. Microbiol. 2007, 2, 141–148. [Google Scholar] [CrossRef]
- Kunyanga, C.N. Microbiological Studies of Kirario, an Indigenous Kenyan Fermented Porridge Based on Green Maize and Millet. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2006. [Google Scholar]
- Omemu, A.M. Fermentation dynamics during production of ogi, a Nigerian fermented cereal porridge. Rep. Opin. 2011, 3, 8–15. [Google Scholar]
- Abegaz, K.; Beyene, F.; Thor, L.; Judith, A.N. Parameters of processing and microbial changes during fermentation of borde, a traditional Ethiopian beverage. J. Food Technol. Afr. 2002, 7, 85–92. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef]
- Ali, A.; Shehzad, A.; Khan, M.R.; Shabbir, M.A.; Amjid, M.R. Yeast, its types and role in fermentation during bread making process: A review. Pak. J. Food Sci. 2012, 22, 171–179. [Google Scholar]
- Romano, P.; Capace, A.; Jespersen, L. Taxonomic and ecological diversity of food and beverage yeasts. In The Yeast Handbook-Yeasts in Food and Beverages; Querol, A., Fleet, G.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 13–53. [Google Scholar]
- Tamang, J.P.; Fleet, G.H. Yeasts diversity in fermented foods and beverages. In Yeasts Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer: New York, NY, USA, 2009; pp. 169–198. [Google Scholar]
- Ashenafi, M.; Mehari, T. Some microbiological and nutritional properties of “borde” and “shamita”, traditional Ethiopian fermented beverages. Ethiop. J. Health Dev. 1995, 9, 105–110. [Google Scholar]
- Gebrelibanos, L. Microbiological and Physicochemical Study of Azo, A Traditional Fermented Condiment Prepared from Sorghum and Leaves of Endod (Phytolacca dodecandra) in KaftaHumera, Tigray Regional State. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Binitu, B.W.; Fekadu, H.G.; Zewdu, A.W. Nutritional and alcoholic contents of Cheka: A traditional fermented beverage in South western Ethiopia. Food Sci. Nutr. 2018, 6, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Boontim, N.; Khanongnuch, C.; Pathom-aree, W.; Niamsup, P.; Lumyong, S. Production of l-lactic acid by thermotorelant lactic acid bacteria. Chiang Mai J. Sci. 2018, 45, 68–76. [Google Scholar]
- Coelho, L.F.; de Lima, C.J.B.; Rodovalho, C.M.; Bernardo, M.P.; Contiero, J. Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses: Optimization of medium composition. Braz. J. Chem. Eng. 2011, 28, 27–36. [Google Scholar] [CrossRef]
- Skory, C.D. Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J. Ind. Microbiol. Biotechnol. 2003, 30, 22–27. [Google Scholar] [CrossRef]
- Minton, P.E. Handbook of Evaporation Technology, 1st ed.; Noyes Publications: West Wood, NJ, USA, 1986. [Google Scholar]
- Alrosan, M.; Tan, T.-C.; Koh, W.Y.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H. Overview of fermentation process: Structurefunction relationship on protein quality and nonnutritive compounds of plant-based proteins and carbohydrates. Crit. Rev. Food Sci. Nutr. 2022, 63, 7677–7691. [Google Scholar] [CrossRef]
- Mannheim, C.H.; Liu, J.X.; Gilbert, S.G. Control of water in foods during storage. J. Food Eng. 1994, 22, 509–532. [Google Scholar] [CrossRef]
- Panja, P.; Deepika; Bhattacharjee, D. An overview of the principles and effects of intermediate moisture fruits and vegetables. Int. J. Chem. Stud. 2019, 6, 848–855. [Google Scholar]
- Omenna, E.C.; Olanipekun, O.T.; Ogunwale, F.J. Nutritional and sensory properties of co-fermented maize, millet and sorghum/soybean pap-(ogi). MOJ Food Process. Technol. 2018, 6, 159–164. [Google Scholar] [CrossRef]
- Onwulata, C.; Konstance, R. Extruded corn meal and whey protein concentrate: Effect of particle size. J. Food Process. Preserv. 2006, 30, 475–487. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.K.; Park, K.I.; Hwang, I.K.; Ji, G.E. Fermentation of rice using amylo lytic Bifidobacterium. Int. J. Food Microbiol. 1999, 50, 155–161. [Google Scholar] [CrossRef]
- Svanberg, U.; Lorri, W. Fermentation and nutrient availability. Food Control 1997, 8, 319–327. [Google Scholar] [CrossRef]
- Feyera, M.; Abera, S.; Temesgen, M. Effect of fermentation time and blending ratio on nutrients and some anti-nutrient composition of complementary flour. J. Food Process. Technol. 2021, 8, 1–12. [Google Scholar]
- Ullah, I.; Ali, M.; Farooqi, A. Chemical and nutritional properties of some maize (Zea mays L.) varieties grown in NWFP, Pakistan. Pak. J. Nutr. 2010, 9, 1113–1117. [Google Scholar] [CrossRef]
- Sanjeev, P.; Chaudhary, D.P.; Sreevastava, P.; Saha, S.; Rajenderan, A.; Sekhar, J.C.; Chikkappa, G.K. Comparison of Fatty Acid Profile of Specialty Maize to Normal Maize. J. Am. Oil Chem. Soc. 2014, 91, 1001–1005. [Google Scholar] [CrossRef]
- Sagan, A.; Blicharz-Kania, A.; Szmigielski, M.; Andrejko, D.; Sobczak, P.; Zawi’slak, K.; Starek, A. Assessment of the properties of rapeseed oil enriched with oils characterized by high content of linolenic acid. Sustainability 2019, 11, 5638. [Google Scholar] [CrossRef]
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient Requirements during Pregnancy and Lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Sokoła-Wysoczanska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokoła, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders: A review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Covaciu, F.D.; Feher, I.; Molnar, C.; Floare-Avram, V.; Dehelean, A. Characterization of the Fatty Acid and Elemental Composition of Human Milk with Chemometric Processing to Determine the Nutritional Value. Anal. Lett. 2022, 56, 344–356. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Hajhoseini, L. Importance of optimal fiber consumption during pregnancy. Int. J. Women’s Health Reprod. Sci. 2013, 1, 76–79. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Çavdar, G.; Papich, T.; Ryan, E.P. Microbiome, breastfeeding and public health policy in the United States: The Case for Dietary Fiber. Nutr. Metab. Insights 2019, 12, 1178638819869597. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Oligosaccharides in raw and processed legumes. Z Leb. Unters Forsch 1998, 206, 130–133. [Google Scholar] [CrossRef]
- Oyarekua, M.A. Evaluation of the nutritional and microbiological status of co-fermented cereals/cowpea ‘ogi’. Agric. Biol. J. N. Am. 2011, 2, 61–73. [Google Scholar] [CrossRef]
- Adams, S.; Sello, T.C.; Qin, G.X.; Che, D.; Han, R. Does dietary fiber affect the levels of nutritional components after feed formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef]
- Zakari, U.M.; Hassan, A.; Abbo, E.S. Physicochemical and Sensory Properties of “Agidi” from Pearl-Millet (Pennisetum glaucum) and Bambara groundnut (Vigna subterranean) flour blends. Afr. J. Food Sci. 2010, 4, 662–667. [Google Scholar]
- Yegrem, L. Evaluation of Nutritional Composition and Sensory Acceptability of tef (Eragrostis Tef (Zucc.) Trotter) Complemented with Lupine (Lupinus spp.) Injera. Master’s Thesis, Haramaya University, Haramay, Ethiopia, 2019. [Google Scholar]
- Enyisi, I.S.; Umoh, V.J.; Whong, C.M.Z.; Abdullahi, I.O.; Alabi, O. Chemical and nutritional value of maize and maize products obtained from selected markets in Kaduna State, Nigeria. Afr. J. Food Sci. Technol. 2014, 5, 100–104. [Google Scholar]
- Nemo, R.; Bacha, K. Microbial, physico-chemical and proximate analysis of selected Ethiopian traditional fermented beverages. LWT Food Sci. Technol. 2020, 131, 109713. [Google Scholar] [CrossRef]
- Wilson, P.R.; Pugh, L.C. Promoting Nutrition in Breastfeeding Women. JOGNN-J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 120–124. [Google Scholar] [CrossRef]
- Segura, S.A.; Ansotegui, J.A.; Diaz-Gomez, N.M. The importance of maternal Nutrition during lactation, do nursing mothers need nutritional supplements? An. Pediatr. 2016, 84, 347. [Google Scholar] [CrossRef]
- Cherie, Z.; Gregory, R.Z.; Fekadu, H.G.; Zewdu, A.W. Optimization and modeling of teff-maize-rice based formulation by simplex lattice mixture design for the preparation of brighter and acceptable injera. Cogent Food Agric. 2018, 4, 1444–3381. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, M.J. Review calcium intake and health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Worku, B.B.; Woldegiorgis, A.Z.; Gemeda, H.F. Indigenous processing methods of Cheka: A traditional fermented beverage in Southwestern Ethiopia. J. Food Process. Technol. 2015, 7, 540. [Google Scholar]
- Adegbehingbe, K.T. Effect of starter cultures on the anti-nutrient contents, minerals and viscosity of ogwo; a fermented sorghum-Irish potato gruel. Int. Food Res. J. 2015, 22, 1247–1252. [Google Scholar]
- Sian, L.; Krebs, N.F.; Westcott, J.E.; Fengliang, L.; Tong, L.; Miller, L.V.; Sonko, B.; Hambidge, M. Zinc homeostasis during lactation in a population with a low zinc intake. Am. J. Clin. Nutr. 2002, 75, 99–103. [Google Scholar] [CrossRef]
- Gemede, H.F. Potential health benefits and adverse effects associated with phytate in foods: A review. Glob. J. Med. Res. 2014, 27, 2224–6088. [Google Scholar]
- Osagie, A.U.; Eka, O.U. Nutritional Quality of Plant Foods: Post-Harvest Research Unit; Department of Biochemistry, University of Benin: Benin, Nigeria, 1998; pp. 221–244. [Google Scholar]
- Roger, T.; Léopold, T.N.; Funtong, M. Nutritional properties and anti-nutritional factors of corn paste (kutukutu) fermented by different strains of lactic acid bacteria. Int. J. Food Sci. 2015, 2015, 502910. [Google Scholar] [CrossRef]
- Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc absorption is not related to dietary phytate intake in infants and young children based on modeling combined data from multiple studies. J. Nutr. 2015, 145, 1763–1769. [Google Scholar] [CrossRef]
- Ndie, E.C.; Okaka, J.C. Risk assessment of antinutrient consumption of plant foods of south eastern Nigeria. J. Food Sci. Nutr. 2018, 1, 9–12. [Google Scholar]
- Tsopmo, A. Review Phytochemicals in Human Milk and Their Potential Antioxidative Protection. Antioxidants 2018, 7, 32. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Hunter, K.J.; Fletcher, J.M. The antioxidant activity and composition of fresh, frozen, jarred and canned vegetables. Innov. Food Sci. Emerg. Technol. 2002, 3, 399–406. [Google Scholar] [CrossRef]
- Ploenkutham, R.; Sripromma, P.; Amornraksa, S.; Yasurin, P.; Soontrunnarudrungsri, A. Effect of roasting and kneading on antioxidant activity and consumer acceptance towards Asiatic pennywort tea. MATEC Web Conf. 2018, 187, 01004. [Google Scholar] [CrossRef]
- Saikia, S.; Mahanta, C.L. Effect of steaming, boiling and microwave cooking on the total phenolics, flavonoids and antioxidant properties of different vegetables of Assam, India. Int. J. Food Nutr. Sci. 2013, 2, 47–56. [Google Scholar]
- Moskowitz, H.R.; Porretta, S.; Silcher, M. Concept Research in Food Product Design and Development; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Puleo, S.; Braghieri, A.; Pacelli, C.; Bendini, A.; Toschi, T.G.; Torri, L.; Piochi, M.; Monaco, R.D. Food Neophobia, Odor and Taste Sensitivity, and Overall Flavor Perception in Food. Foods 2021, 10, 3122. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Case, T.I.; Oaten, M.J. Frequency and recency of infection and their relationship with disgust and contamination sensitivity. Evol. Hum. Behav. 2009, 30, 363–368. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem. Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the psychological impact of food colour. Flavour 2015, 4, 21. [Google Scholar] [CrossRef]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Abioye, V.F.; Aka, M.O. Proximate composition and sensory properties of moringa fortified maize-ogi. J. Nutr. Food Sci. 2015, 12, 1–4. [Google Scholar]
- Akinsola, O.T.; Alamu, E.O.; Otegbayo, B.O.; Menkir, A.; Maziya-Dixon, B. Nutritional properties of ogi powder and sensory perception of ogi porridge made from synthetic provitamin: A maize genotype. Front. Nutr. 2021, 8, 685004. [Google Scholar] [CrossRef]
- Drewnowski, A. Taste preferences and food intake. Annu. Rev. Nutr. 1997, 17, 237–253. [Google Scholar] [CrossRef]
- Licker, S.P. Understanding Food Texture Perception and Preference Based on Mouth Behavior. Ph.D. Thesis, Cornell University, New York, NY, USA, 2019. [Google Scholar]

| FC | FT (Day) | Mean Count (log cfu/g) of Different Microbial Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic Acid Bacteria | Aerobic Mesophilic Bacteria | Enterobacteriaceae | Total Coliforms | Staphylococcus spp. | ASFB | Yeast | Mold | ||
| MBF1 | 0 | 3.75 ± 0.05 d | 4.45 ± 0.02 c | 4.17 ± 0.01 a | 3.85 ± 0.02 a | 3.23 ± 0.01 a | 3.14 ± 0.01 c | 2.51 ± 0.01 d | 3.36 ± 0.02 a |
| 8 | 8.41 ± 0.01 c | 6.43 ± 0.03 a | <2 | <2 | 3.21 ± 0.01 a | 3.42 ± 0.01 a | 5.94 ± 0.02 b | 2.75 ± 0.01 b | |
| 10 | 8.88 ± 0.01 b | 5.11 ± 0.01 b | <2 | <2 | 3.14 ± 0.02 b | 3.30 ± 0.01 b | 6.65 ± 0.02 a | 2.53 ± 0.01 c | |
| 12 | 9.41 ± 0.01 a | 4.16 ± 0.02 d | <2 | <2 | 2.48 ± 0.01 c | 2.83 ± 0.02 d | 4.83 ± 0.01 c | 2.52 ± 0.02 c | |
| CV | 0.35 | 0.42 | 0.48 | 1.04 | 0.44 | 0.42 | 0.32 | 0.32 | |
| LSD | 0.06 | 0.05 | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | |
| MBF2 | 0 | 4.45 ± 0.03 d | 5.20 ± 0.2 a | 3.75 ± 0.02 a | 3.30 ± 0.01 a | 3.81 ± 0.01 a | 3.29 ± 0.01 a | 2.52 ± 0.01 d | 2.52 ± 0.02 a |
| 8 | 7.95 ± 0.03 a | 5.44 ± 0.02 a | <2 | <2 | 3.38 ± 0.01 b | 3.28 ± 0.01 a | 6.45 ± 0.03 a | <2 | |
| 10 | 7.56 ± 0.03 b | 4.61 ± 0.01 b | <2 | <2 | 2.52 ± 0.02 c | 2.58 ± 0.01 b | 5.26 ± 0.03 b | <2 | |
| 12 | 6.28 ± 0.02 c | 4.04 ± 0.04 c | <2 | <2 | 2.47 ± 0.02 d | 2.51 ± 0.01 c | 4.43 ± 0.01 c | <2 | |
| CV | 0.42 | 2.13 | 1.07 | 0.61 | 0.52 | 0.30 | 0.48 | 0.64 | |
| LSD | 0.07 | 0.26 | 0.02 | 0.01 | 0.04 | 0.02 | 0.05 | 0.01 | |
| MBF3 | 0 | 4.44 ± 0.02 d | 5.54 ± 0.01 a | 3.82 ± 0.02 a | 3.03 ± 0.01 a | 3.22 ± 0.01 a | 3.64 ± 0.02 a | 2.22 ± 0.01 d | 2.47 ± 0.01 a |
| 8 | 7.71 ± 0.01 a | 4.15 ± 0.02 b | <2 | <2 | 2.83 ± 0.02 b | 2.55 ± 0.01 b | 5.94 ± 0.02 a | <2 | |
| 10 | 7.46 ± 0.03 b | 3.95 ± 0.03 c | <2 | <2 | 2.22 ± 0.01 c | 2.51 ± 0.01 c | 4.97 ± 0.02 b | <2 | |
| 12 | 6.45 ± 0.04 c | 3.85 ± 0.01 d | <2 | <2 | 2.13 ± 0.01 d | 2.07 ± 0.01 d | 4.25 ± 0.02 c | <2 | |
| CV | 0.42 | 0.44 | 1.05 | 0.66 | 0.51 | 0.49 | 0.23 | 1.01 | |
| LSD | 0.07 | 0.05 | 0.02 | 0.01 | 0.03 | 0.03 | 0.04 | 0.01 | |
| Flour Composition | Fermentation Time (Days) | pH | Titratable Acidity (TA) |
|---|---|---|---|
| MBF1 | 0 | 5.26 ± 0.03 a | 0.37 ± 0.01 h |
| 8 | 4.42 ± 0.02 b | 0.58 ± 0.01 f | |
| 10 | 4.21 ± 0.21 c | 0.63 ± 0.03 e | |
| 12 | 4.00 ± 0.02 d | 0.66 ± 0.02 d | |
| MBF2 | 0 | 5.24 ± 0.02 a | 0.39 ± 0.01 gh |
| 8 | 4.42 ± 0.02 b | 0.61 ± 0.01 e | |
| 10 | 4.02 ± 0.02 d | 0.68 ± 0.01 cd | |
| 12 | 3.98 ± 0.01 d | 0.69 ± 0.01 bc | |
| MBF3 | 0 | 5.22 ± 0.01 a | 0.41 ± 0.01 g |
| 8 | 4.04 ± 0.04 d | 0.63 ± 0.01 e | |
| 10 | 4.01 ± 0.01 d | 0.71 ± 0.01 ab | |
| 12 | 3.87 ± 0.01 e | 0.73 ± 0.01 a | |
| CV | 1.27 | 2.22 | |
| LSD | 0.09 | 0.02 | |
| Flour Composition | Fermentation Time (Days) | Moisture Content | CP Content | CF Content | Fiber Content | Ash Content | Carbohydrate Content | Gross Energy (kcal/100g) |
|---|---|---|---|---|---|---|---|---|
| MBF1 | 0 | 61.34 ± 0.34 g | 12.22 ± 0.02 g | 12.46 ± 0.05 d | 3.61 ± 0.01 c | 2.45 ± 0.01 l | 72.20 ± 0.82 a | 449.79 ± 0.11 a |
| 8 | 65.89 ± 0.11 b | 12.82 ± 0.02 f | 12.89 ± 0.02 b | 2.43 ± 0.01 j | 2.63 ± 0.01 j | 69.23 ± 0.05 b | 444.24 ± 0.03 ab | |
| 10 | 66.00 ± 0.00 b | 13.00 ± 0.5 f | 13.11 ± 0.11 a | 2.24 ± 0.02 k | 2.64 ± 0.02 j | 69.01 ± 0.42 bc | 446.05 ± 0.47 ab | |
| 12 | 66.32 ± 0.02 a | 13.52 ± 0.02 e | 13.23 ± 0.01 a | 2.21 ± 0.01 l | 2.61 ± 0.01 k | 68.43 ± 0.06 bcd | 446.87 ± 0.02 ab | |
| MBF2 | 0 | 60.58 ± 0.09 i | 13.56 ± 0.1 e | 11.68 ± 0.03 e | 4.66 ± 0.02 b | 2.87 ± 0.01 e | 67.24 ± 0.15 bcd | 428.29 ± 0.05 cd |
| 8 | 64.77 ± 0.08 def | 13.77 ± 0.1 de | 12.41 ± 0.10 d | 3.34 ± 0.01 b | 2.85 ± 0.01 f | 67.63 ± 0.19 bcd | 437.31 ± 0.47 bc | |
| 10 | 64.79 ± 0.20 de | 13.98 ± 0.01 d | 12.81 ± 0.04 b | 3.31 ± 0.01 f | 2.84 ± 0.01 g | 67.06 ± 0.04 bcd | 439.43 ± 0.16 b | |
| 12 | 65.11 ± 0.11 c | 14.68 ± 0.1 c | 12.63 ± 0.06 c | 2.98 ± 0.01 g | 2.82 ± 0.12 h | 66.89 ± 0.14 bcd | 439.94 ± 0.30 ab | |
| MBF3 | 0 | 60.87 ± 0.13 h | 14.89 ± 0.1 c | 10.01 ± 0.21 g | 4.84 ± 0.02 a | 3.75 ± 0.01 a | 66.51 ± 0.09 cd | 415.73 ± 1.13 e |
| 8 | 64.56 ± 0.19 f | 15.88 ± 0.1 b | 10.75 ± 0.06 f | 3.36 ± 0.02 d | 3.70 ± 0.02 d | 66.31 ± 0.18 d | 425.49 ± 0.19 de | |
| 10 | 64.66 ± 0.33 ef | 16.32 ± 0.32 a | 10.80 ± 0.11 f | 2.88 ± 0.01 h | 3.72 ± 0.01 c | 66.28 ± 0.22 d | 427.57 ± 0.48 cd | |
| 12 | 64.98 ± 0.02 cd | 16.56 ± 0.1 a | 10.66 ± 0.01 f | 2.86 ± 0.01 i | 3.73 ± 0.02 b | 66.19 ± 0.11 d | 426.91 ± 0.14 d | |
| CV | 0.20 | 1.36 | 0.69 | 0.13 | 0.15 | 2.18 | 1.35 | |
| LSD | 0.22 | 0.33 | 0.14 | 0.007 | 0.008 | 2.51 | 9.97 | |
| Flour Compositions | Fermentation Time (Day) | Calcium (Ca) | Iron (Fe) | Zinc (Zn) |
|---|---|---|---|---|
| MBF1 | 0 | 25.75 ± 0.22 h | 5.66 ± 0.01 f | 6.76 ± 0.18 e |
| 8 | 27.90 ± 0.19 g | 6.81 ± 0.01 d | 7.47 ± 0.46 cd | |
| 10 | 27.98 ± 0.23 g | 6.83 ± 0.01 d | 7.50 ± 0.47 cd | |
| 12 | 28.12 ± 0.22 g | 6.84 ± 0.01 d | 7.51 ± 0.46 cd | |
| MBF2 | 0 | 35.53 ± 0.22 f | 6.32 ± 0.01 e | 7.14 ± 0.31 de |
| 8 | 36.86 ± 0.04 e | 6.87 ± 0.01 d | 7.67 ± 0.16 c | |
| 10 | 37.49 ± 0.64 d | 6.89 ± 0.01 d | 7.71 ± 0.18 c | |
| 12 | 37.93 ± 0.71 d | 6.89 ± 0.01 d | 7.73 ± 0.20 c | |
| MBF3 | 0 | 59.15 ± 0.56 c | 7.06 ± 0.05 c | 8.24 ± 0.21 b |
| 8 | 60.16 ± 0.47 b | 8.72 ± 0.13 b | 8.83 ± 0.16 a | |
| 10 | 60.66 ± 0.19 ab | 8.73 ± 1.15 b | 8.87 ± 0.14 a | |
| 12 | 61.27 ± 0.42 a | 8.83 ± 1.16 a | 8.89 ± 0.14 a | |
| CV | 0.89 | 0.84 | 3.03 | |
| LSD | 0.63 | 0.10 | 0.40 | |
| Flour Composition | Fermentation Time (Days) | Phytate | Tannin | Antioxidant Activities (IC50) (mg AAE/g) |
|---|---|---|---|---|
| MBF1 | 0 | 1.76 ± 0.03 a | 1.58 ± 0.01 a | 10.16 ± 0.23 b |
| 8 | 0.56 ± 0.02 e | 0.26 ± 0.01 h | 0.64 ± 0.02 c | |
| 10 | 0.34 ± 0.02 j | 0.20 ± 0.00 i | 0.44 ± 0.01 cde | |
| 12 | 0.28 ± 0.01 k | 0.19 ± 0.00 i | 0.31 ± 0.01 de | |
| MBF2 | 0 | 1.34 ± 0.02 b | 1.26 ± 0.02 b | 10.89 ± 0.19 a |
| 8 | 0.77 ± 0.02 d | 0.47 ± 0.01 d | 0.52 ± 0.03 cd | |
| 10 | 0.42 ± 0.02 i | 0.39 ± 0.00 e | 0.46 ± 0.03 cde | |
| 12 | 0.53 ± 0.01 fg | 0.34 ± 0.02 g | 0.19 ± 0.05 e | |
| MBF3 | 0 | 1.22 ± 0.02 c | 0.98 ± 0.01 c | 11.15 ± 0.45 a |
| 8 | 0.54 ± 0.02 f | 0.39 ± 0.00 e | 0.71 ± 0.17 c | |
| 10 | 0.52 ± 0.01 gh | 0.36 ± 0.01 f | 0.56 ± 0.11 cd | |
| 12 | 0.51 ± 0.01 h | 0.34 ± 0.01 g | 0.31 ± 0.08 de | |
| CV | 0.85 | 1.75 | 5.44 | |
| LSD | 0.01 | 0.02 | 0.28 |
| Formulation | Aroma | Color | Taste | Texture | Overall Acceptability |
|---|---|---|---|---|---|
| C1 | 4.13 ± 0.86 a | 4.82 ± 0.39 a | 4.55 ± 0.50 a | 4.55 ± 0.50 a | 4.45 ± 0.50 a |
| C2 | 4.18 ± 0.88 a | 3.91 ± 0.40 b | 4.07 ± 0.26 b | 4.36 ± 0.52 a | 4.02 ± 0.24 b |
| F1 | 4.30 ± 0.71 a | 3.41 ± 0.89 c | 3.96 ± 0.69 b | 4.05 ± 0.49 b | 4.05 ± 0.35 b |
| F2 | 4.42 ± 0.68 a | 3.40 ± 0.89 c | 3.91 ± 0.67 b | 4.09 ± 0.51 b | 4.02 ± 0.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitessa, D.A.; Bacha, K.; Tola, Y.B.; Murimi, M. Effect of Fermentation Time and Blending Ratio on Microbial Dynamics, Nutritional Quality and Sensory Acceptability of Shameta: A Traditional Cereal-Based Fermented Porridge for Lactating Mothers in Ethiopia. Fermentation 2024, 10, 118. https://doi.org/10.3390/fermentation10030118
Kitessa DA, Bacha K, Tola YB, Murimi M. Effect of Fermentation Time and Blending Ratio on Microbial Dynamics, Nutritional Quality and Sensory Acceptability of Shameta: A Traditional Cereal-Based Fermented Porridge for Lactating Mothers in Ethiopia. Fermentation. 2024; 10(3):118. https://doi.org/10.3390/fermentation10030118
Chicago/Turabian StyleKitessa, Daniel Asfaw, Ketema Bacha, Yetenayet B. Tola, and Mary Murimi. 2024. "Effect of Fermentation Time and Blending Ratio on Microbial Dynamics, Nutritional Quality and Sensory Acceptability of Shameta: A Traditional Cereal-Based Fermented Porridge for Lactating Mothers in Ethiopia" Fermentation 10, no. 3: 118. https://doi.org/10.3390/fermentation10030118
APA StyleKitessa, D. A., Bacha, K., Tola, Y. B., & Murimi, M. (2024). Effect of Fermentation Time and Blending Ratio on Microbial Dynamics, Nutritional Quality and Sensory Acceptability of Shameta: A Traditional Cereal-Based Fermented Porridge for Lactating Mothers in Ethiopia. Fermentation, 10(3), 118. https://doi.org/10.3390/fermentation10030118






