Effect of Isopropyl Ester of Hydroxy Analogue of Methionine on Rumen Microbiome, Active Enzymes, and Protein Metabolism Pathways of Yak
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
2.3. DNA Extraction, Library Construction, and Metagenomic Sequencing
2.4. Sequence Quality Control and Genome Assembly
2.5. Gene Prediction, Taxonomy, and Functional Annotation
2.6. Statistical Analysis
3. Results
3.1. Microbial Metagenomic Sequence Data
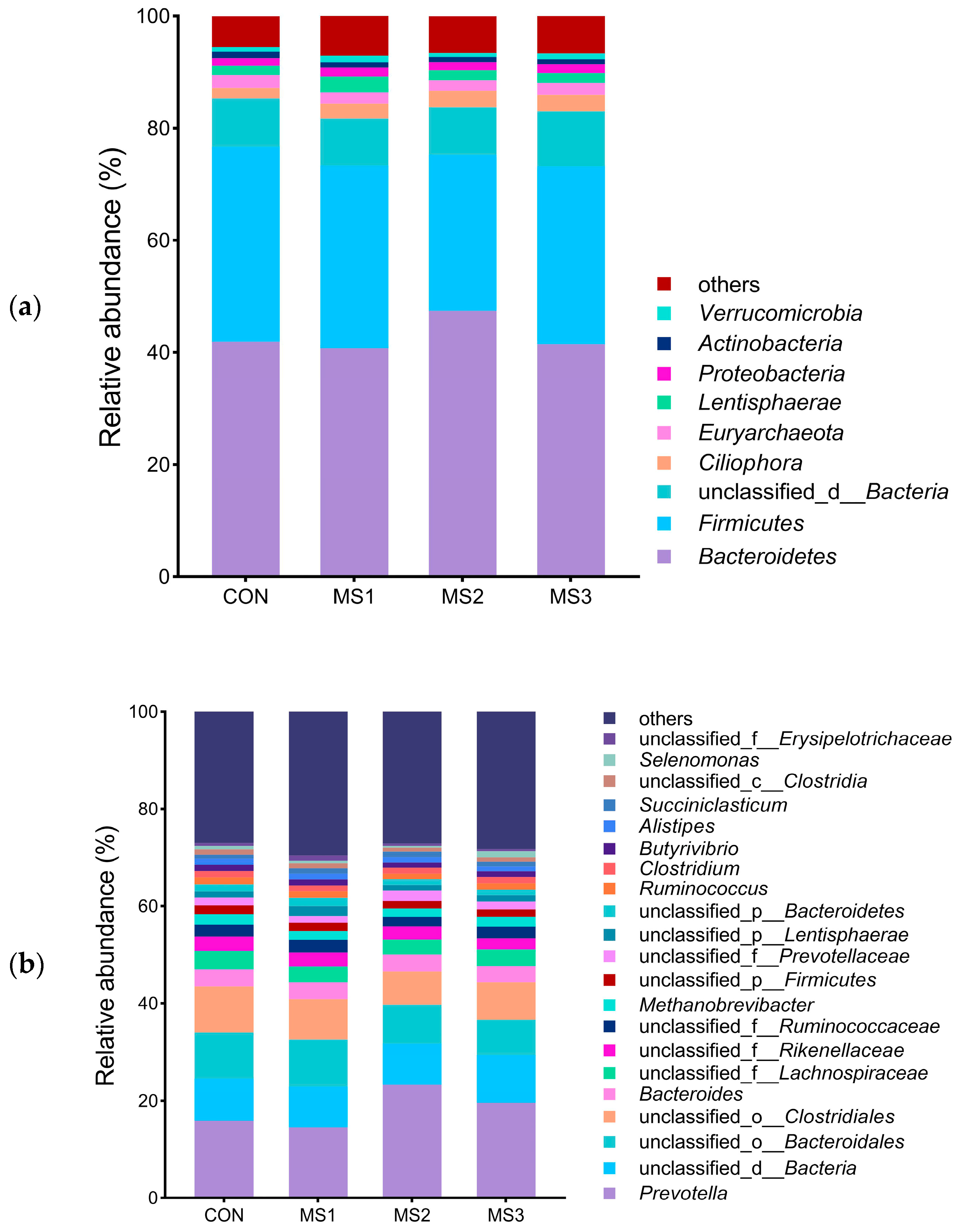
3.2. Analysis of Rumen Microbial Community Composition
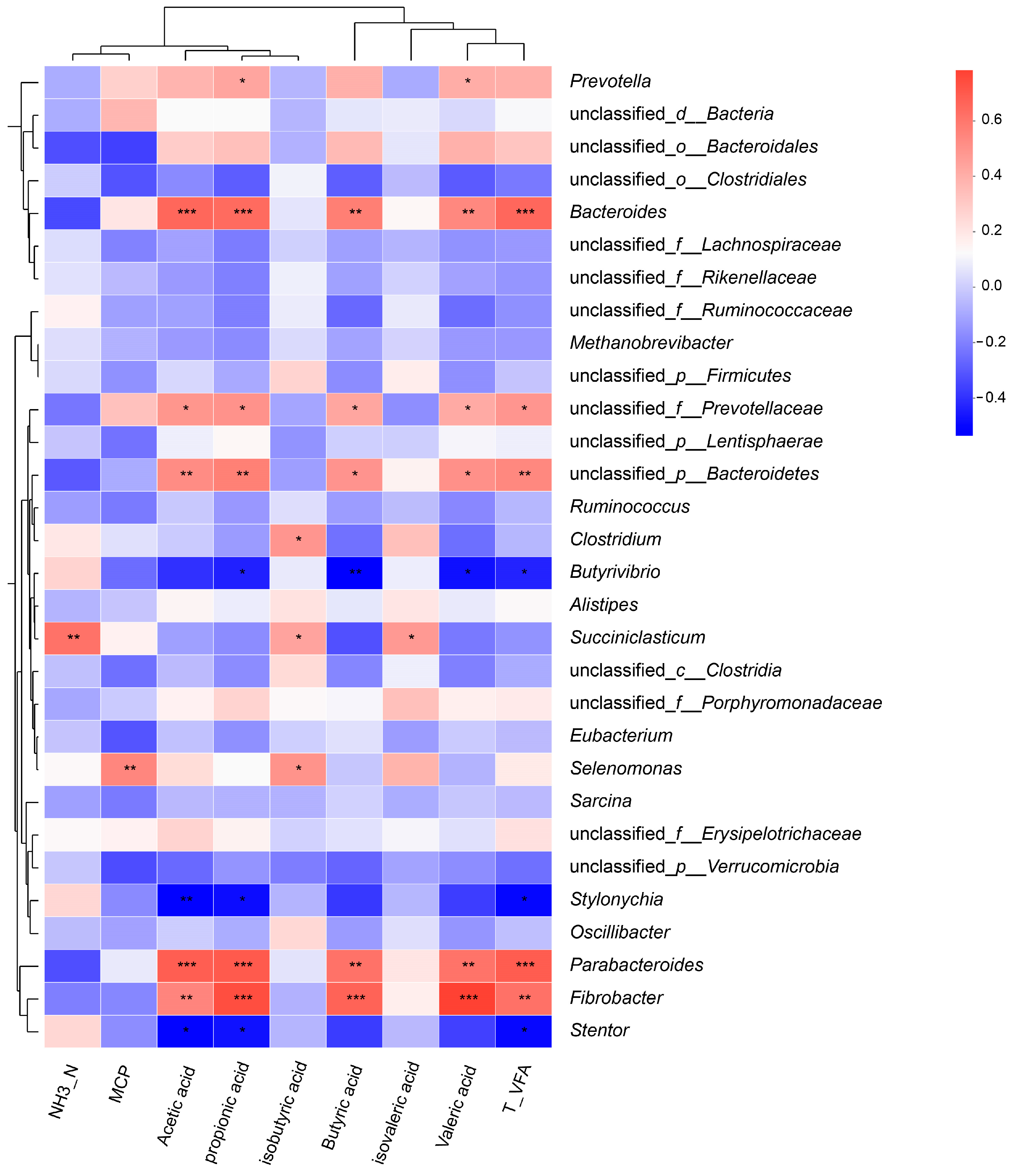
3.3. Relationship between Rumen Bacteria and Fermentation Parameters
3.4. CAZy Functional Annotation
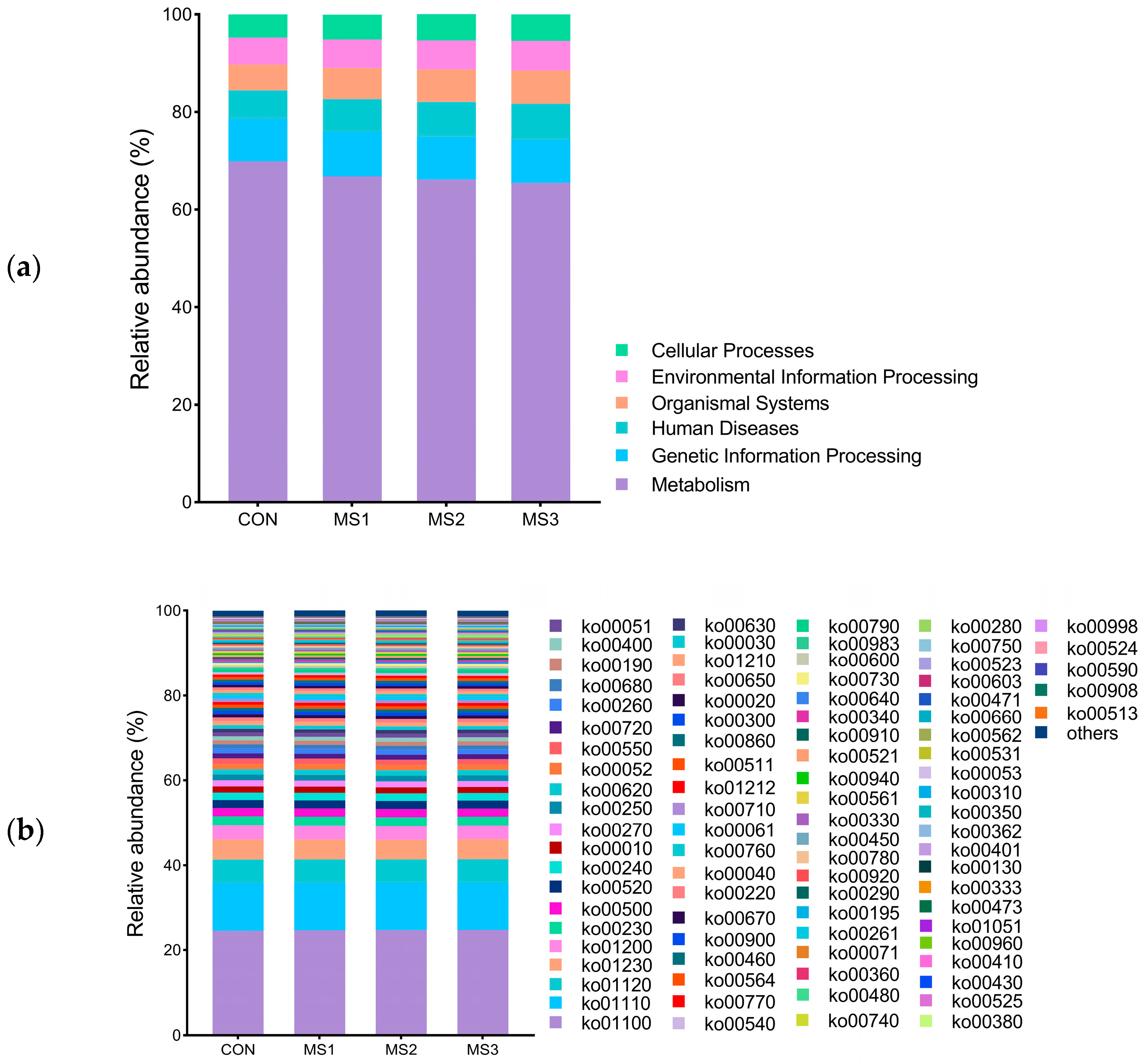
3.5. The eggNOG Functional Annotation
3.6. KEGG Functional Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, W.; Li, Y.; Wang, L.; Wang, J.; Xu, Q.; Yan, T.; Xue, B. Evaluation of composition and individual variability of rumen microbiota in yaks by 16S rRNA high-throughput sequencing technology. Anaerobe 2015, 34, 74–79. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, M.; Ma, T.; Bi, S.; Wang, W.; Zhang, Y.; Huang, X.; Guan, L.L.; Long, R. Survey of rumen microbiota of domestic grazing yak during different growth stages revealed novel maturation patterns of four key microbial groups and their dynamic interactions. Anim. Microbiome 2020, 2, 23. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Z.; Liu, Y.; Wang, C.; Peng, Z.; Zhong, J.; Zhang, M.; Wang, H. Effect of Methionine Analogues on Growth Performance, Serum Biochemical Parameters, Serum Free Amino Acids and Rumen Fermentation of Yaks. Animals 2022, 12, 3175. [Google Scholar] [CrossRef]
- Hao, L.; Xiang, Y.; Degen, A.; Huang, Y.; Niu, J.; Sun, L.; Chai, S.; Zhou, J.; Ding, L.; Long, R.; et al. Adding heat-treated rapeseed to the diet of yak improves growth performance and tenderness and nutritional quality of the meat. Anim. Sci. J. 2019, 90, 1177–1184. [Google Scholar] [CrossRef]
- Liu, E.; VandeHaar, M. Relationship of residual feed intake and protein efficiency in lactating cows fed high- or low-protein diets. J. Dairy Sci. 2020, 103, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, K.A.; Ostaszewski, P.; Fuller, J.C., Jr.; Ciecierska, A.; Sadkowski, T. Dietary supplementation of β-hydroxy-β-methylbutyrate in animals–a review. J. Anim. Physiol. Anim. Nutr. 2015, 99, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, S.; Zhu, X.; Zhou, C.; Lage, C.; Fetter, M.; Silvestre, T.; Stefenoni, H.; Wasson, D.; Cueva, S.; Eun, J.-S.; et al. Production effects and bioavailability of N-acetyl-l-methionine in lactating dairy cows. J. Dairy Sci. 2022, 105, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Ji, P.; Drackley, J.; Luchini, D.; Loor, J. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, B.; Zuo, Z.; Qi, Y.; Zhao, S.; Zhang, X.; Lan, L.; Shi, Y.; Liu, X.; Li, S.; et al. Effects of Two Different Straw Pellets on Yak Growth Performance and Ruminal Microbiota during Cold Season. Animals 2023, 13, 335. [Google Scholar] [CrossRef]
- Zhu, Y.; Cidan, Y.; Sun, G.; Li, X.; Shahid, M.A.; Luosang, Z.; Suolang, Z.; Suo, L.; Basang, W. Comparative analysis of gut fungal composition and structure of the yaks under different feeding models. Front. Veter.-Sci. 2023, 10, 1193558. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Tang, H.; Doak, T.G.; Ye, Y. Comparing bacterial communities inferred from 16S rRNA gene sequencing and shotgun metagenomics. Pac. Symp. Biocomput. 2011, 2011, 165–176. [Google Scholar]
- Graulet, B.; Richard, C.; Robert, J. Methionine Availability in Plasma of Dairy Cows Supplemented with Methionine Hydroxy Analog Isopropyl Ester. J. Dairy Sci. 2005, 88, 3640–3649. [Google Scholar] [CrossRef]
- Fowler, C.; Plank, J.; Devillard, E.; Bequette, B.; Firkins, J. Assessing the ruminal action of the isopropyl ester of 2-hydroxy-4-(methylthio) butanoic acid in continuous and batch cultures of mixed ruminal microbes. J. Dairy Sci. 2015, 98, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Li, H.; Wu, F.; Qiu, Q.; Niu, W.; Gao, Z.; Su, H.; Cao, B. Rumen fermentation, intramuscular fat fatty acid profiles and related rumen bacterial populations of Holstein bulls fed diets with different energy levels. Appl. Microbiol. Biotechnol. 2019, 103, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Huang, T.; Liao, W.; Xu, Y.; Li, Z.; Gu, J. Gencore: An efficient tool to generate consensus reads for error suppressing and duplicate removing of NGS data. BMC Bioinform. 2019, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Shi, W.; Moon, C.D.; Leahy, S.C.; Kang, D.; Froula, J.; Kittelmann, S.; Fan, C.; Deutsch, S.; Gagic, D.; Seedorf, H.; et al. Methane yield phenotypes linked to differential gene expression in the sheep rumen microbiome. Genome Res. 2014, 24, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.A.; Shirley, R.L.; Moore, J.E. Effect of Methionine Hydroxy Analog on Bacterial Protein Synthesis from Urea and Glucose, Starch or Cellulose by Rumen Microbes, In vitro. J. Anim. Sci. 1973, 37, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Salsbury, R.; Marvil, D.; Woodmansee, C.; Haenlein, G. Utilization of Methionine and Methionine Hydroxy Analog by Rumen Microorganisms in Vitro. J. Dairy Sci. 1971, 54, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, H.; Hao, L.; Cao, X.; Degen, A.; Zhou, J.; Zhang, C. Rumen Bacterial Community of Grazing Lactating Yaks (Poephagus grunniens) Supplemented with Concentrate Feed and/or Rumen-Protected Lysine and Methionine. Animals 2021, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Grover, M. Role of gut pathogens in development of irritable bowel syndrome. Indian J. Med. Res. 2014, 139, 11–18. [Google Scholar] [PubMed]
- Gruninger, R.J.; Ribeiro, G.O.; Cameron, A.; McAllister, T.A. Invited review: Application of meta-omics to understand the dynamic nature of the rumen microbiome and how it responds to diet in ruminants. Animal 2019, 13, 1843–1854. [Google Scholar] [CrossRef]
- Hu, R.; Zou, H.; Wang, H.; Wang, Z.; Wang, X.; Ma, J.; Shah, A.M.; Peng, Q.; Xue, B.; Wang, L.; et al. Dietary Energy Levels Affect Rumen Bacterial Populations that Influence the Intramuscular Fat Fatty Acids of Fattening Yaks (Bos grunniens). Animals 2020, 10, 1474. [Google Scholar] [CrossRef]
- Xue, M.-Y.; Sun, H.-Z.; Wu, X.-H.; Liu, J.-X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Li, H.; Li, R.; Chen, H.; Gao, J.; Wang, Y.; Zhang, Y.; Qi, Z. Effect of different seasons (spring vs summer) on the microbiota diversity in the feces of dairy cows. Int. J. Biometeorol. 2019, 64, 345–354. [Google Scholar] [CrossRef]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, R.; Liu, R.; Li, R.; Sun, C. Cultivation and Functional Characterization of a Deep-Sea Lentisphaerae Representative Reveals Its Unique Physiology and Ecology. Front. Mar. Sci. 2022, 9, 848136. [Google Scholar] [CrossRef]
- Gohain, A.; Manpoong, C.; Saikia, R.; De Mandal, S. Actinobacteria: Diversity and biotechnological applications. In Recent Advancements in Microbial Diversity; Academic Press: Cambridge, MA, USA, 2020; pp. 217–231. [Google Scholar]
- Purushe, J.; the North American Consortium for Rumen Bacteria; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative Genome Analysis of Prevotella ruminicola and Prevotella bryantii: Insights into Their Environmental Niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Kumar, S.; Sharma, R.; Banakar, P.S.; Deb, R.; Tyagi, A.K. Rumen Microbial Diversity, Enteric Methane Emission and Nutrient Utilization of Crossbred Karan-Fries Cattle (Bos Taurus) and Murrah Buffalo (Bubalus Bubalis) Consuming Varied Roughage Concentrate Ratio. Anim. Biotechnol. 2023, 34, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Murillo, C.L.; Aguilar-Marín, S.B.; Jovel, J. Prevotella: A Key Player in Ruminal Metabolism. Microorganisms 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Grilli, D.J.; Cerón, M.E.; Paez, S.; Egea, V.; Schnittger, L.; Cravero, S.; Escudero, M.S.; Allegretti, L.; Arenas, G.N. Isolation of Pseudobutyrivibrio ruminis and Pseudobutyrivibrio xylanivorans from rumen of Creole goats fed native forage diet. Folia Microbiol. 2013, 58, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Huang, S.; Ji, S.; Suen, G.; Wang, F.; Li, S. The Rumen Bacterial Community in Dairy Cows Is Correlated to Production Traits During Freshening Period. Front. Microbiol. 2021, 12, 630605. [Google Scholar] [CrossRef]
- Bi, Y.; Zeng, S.; Zhang, R.; Diao, Q.; Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018, 18, 69. [Google Scholar] [CrossRef]
- Chiquette, J.; Allison, M.; Rasmussen, M. Prevotella bryantii 25A Used as a Probiotic in Early-Lactation Dairy Cows: Effect on Ruminal Fermentation Characteristics, Milk Production, and Milk Composition. J. Dairy Sci. 2008, 91, 3536–3543. [Google Scholar] [CrossRef]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; et al. Gut Microbiota of Healthy and Malnourished Children in Bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef]
- Varghese, V.K.; Poddar, B.J.; Shah, M.P.; Purohit, H.J.; Khardenavis, A.A. A comprehensive review on current status and future perspectives of microbial volatile fatty acids production as platform chemicals. Sci. Total. Environ. 2022, 815, 152500. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A.M.; Lee, S.S.; Mamuad, L.L.; Choi, Y.J.; Jeong, C.D.; Son, A.; Cho, K.K.; Kim, E.T.; Kim, S.B.; Lee, S.S. Enhancing Butyrate Production, Ruminal Fermentation and Microbial Population through Supplementation with Clostridium saccharobutylicum. J. Microbiol. Biotechnol. 2019, 29, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.T., 2nd; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Goad, D.W.; Goad, C.L.; Nagaraja, T.G. Ruminal microbial and fermentative changes associated with experimentally induced subacute acidosis in steers. J. Anim. Sci. 1998, 76, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Mrázek, J.; Tepšič, K.; Avguštin, G.; Kopečný, J. Diet-dependent shifts in ruminal butyrate-producing bacteria. Folia Microbiol. 2006, 51, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Abu-Doleh, A.; Plank, J.; Catalyurek, U.V.; Firkins, J.L.; Yu, Z. The transcriptome of the rumen ciliate Entodinium caudatum reveals some of its metabolic features. BMC Genom. 2019, 20, 1008. [Google Scholar] [CrossRef] [PubMed]
- Saborío-Montero, A.; López-García, A.; Gutiérrez-Rivas, M.; Atxaerandio, R.; Goiri, I.; García-Rodriguez, A.; Jiménez-Montero, J.A.; González, C.; Tamames, J.; Puente-Sánchez, F.; et al. A dimensional reduction approach to modulate the core ruminal microbiome associated with methane emissions via selective breeding. J. Dairy Sci. 2021, 104, 8135–8151. [Google Scholar] [CrossRef]
- Pope, P.B.; Smith, W.; Denman, S.E.; Tringe, S.G.; Barry, K.; Hugenholtz, P.; McSweeney, C.S.; McHardy, A.C.; Morrison, M. Isolation of Succinivibrionaceae Implicated in Low Methane Emissions from Tammar Wallabies. Science 2011, 333, 646–648. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, Y.; Xu, X.L.; Yang, Y.X. Effects of Branched-chain Amino Acids on In vitro Ruminal Fermentation of Wheat Straw. Asian-Australas. J. Anim. Sci. 2013, 26, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.H.; Chen, Y.X.; Cheng, Z.H.; Duan, Q.H.; Meng, Q.H.; Tao, X.P.; Shang, B.; Dong, H.M. Age-Related Response of Rumen Microbiota to Mineral Salt and Effects of Their Interactions on Enteric Methane Emissions in Cattle. Microb. Ecol. 2017, 73, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhou, S.; Luo, R.; Gesang, Z.; Suolang, S. Metagenomic insights into the diversity of carbohydrate-degrading enzymes in the yak fecal microbial community. BMC Microbiol. 2020, 20, 302. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Cao, H.-W.; Chai, Z.-X.; Chen, X.-Y.; Zhang, C.-F.; Zhu, Y.; Xin, J.-W. Dynamic alterations in yak (Bos grunniens) rumen microbiome in response to seasonal variations in diet. Physiol. Genom. 2022, 54, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Kabel, M.A.; Yeoman, C.J.; Han, Y.; Dodd, D.; Abbas, C.A.; de Bont, J.A.M.; Morrison, M.; Cann, I.K.O.; Mackie, R.I. Biochemical Characterization and Relative Expression Levels of Multiple Carbohydrate Esterases of the Xylanolytic Rumen Bacterium Prevotella ruminicola 23 Grown on an Ester-Enriched Substrate. Appl. Environ. Microbiol. 2011, 77, 5671–5681. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Grondin, J.M.; Tamura, K.; Déjean, G.; Abbott, D.W.; Brumer, H. Polysaccharide Utilization Loci: Fueling Microbial Communities. J. Bacteriol. 2017, 199, e00860-16. [Google Scholar] [CrossRef]
- Singh, K.M.; Reddy, B.; Patel, D.; Parmar, N.; Patel, A.; Patel, J.B.; Joshi, C.G. High Potential Source for Biomass Degradation Enzyme Discovery and Environmental Aspects Revealed through Metagenomics of Indian Buffalo Rumen. BioMed. Res. Int. 2014, 2014, 267189. [Google Scholar] [CrossRef]
- Sha, Y.; Hu, J.; Shi, B.; Dingkao, R.; Wang, J.; Li, S.; Zhang, W.; Luo, Y.; Liu, X. Characteristics and Functions of the Rumen Microbial Community of Cattle-Yak at Different Ages. BioMed. Res. Int. 2020, 2020, 3482692. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Zeng, T.; Chen, L.; Huang, T.; Cai, Y.-D. Determining protein–protein functional associations by functional rules based on gene ontology and KEGG pathway. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2021, 1869, 140621. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG Bioinformatics Resource for Plant Genomics and Metabolomics. Methods Mol. Biol. 2016, 1374, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Wagg, J.; Green, M.L.; Kaiser, D.; Krummenacker, M.; Karp, P.D. Computational prediction of human metabolic pathways from the complete human genome. Genome Biol. 2005, 6, R2. [Google Scholar] [CrossRef] [PubMed]
- Wrzodek, C.; Büchel, F.; Ruff, M.; Dräger, A.; Zell, A. Precise generation of systems biology models from KEGG pathways. BMC Syst. Biol. 2013, 7, 15. [Google Scholar] [CrossRef] [PubMed]
| Items | Content |
|---|---|
| Ingredients | [g kg−1] |
| Corn straw silage | 550 |
| Corn meal | 157.5 |
| Soybean meal | 76.5 |
| Sprayed corn bran | 45 |
| Soybean | 45 |
| Corn germ meal | 36 |
| Soybean hull | 31.5 |
| Rapeseed meal | 22.5 |
| Molasses | 22.5 |
| Premix 1 | 13.5 |
| Nutrition composition 2 | [g kg−1] |
| OM | 907.5 |
| CP | 131.3 |
| NDF | 439.2 |
| ADF | 156.3 |
| NEm (MJ/kg) | 3.52 |
| NEg (MJ/kg) | 5.58 |
| Items | Treatments 1 | SEM | p-Value 2 | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MS1 | MS2 | MS3 | Treat | Linear | Quadratic | ||
| Bacteroidetes | 41.99 | 40.59 | 47.25 | 41.15 | 3.018 | 0.372 | 0.103 | 0.247 |
| Firmicutes | 34.70 | 32.58 | 27.46 | 31.90 | 2.505 | 0.251 | 0.197 | 0.799 |
| Cillophora | 1.85 | 2.73 | 3.23 | 3.08 | 1.231 | 0.859 | 0.939 | 0.278 |
| Euryarchaeota | 2.34 | 1.99 | 1.95 | 2.28 | 0.493 | 0.918 | 0.909 | 0.212 |
| Lentisphaerae | 1.65 a | 2.85 b | 1.73 a | 1.47 a | 0.258 | 0.005 | 0.467 | 0.022 |
| Proteobacteria | 1.35 | 1.62 | 1.48 | 1.54 | 0.107 | 0.350 | 0.393 | 0.206 |
| Actinobacteria | 0.98 | 0.94 | 0.82 | 0.79 | 0.076 | 0.251 | 0.029 | 0.308 |
| verrucomicrobia | 0.84 | 1.14 | 0.68 | 0.87 | 0.161 | 0.244 | 0.484 | 0.578 |
| Spirochaetes | 0.71 | 0.79 | 0.57 | 0.49 | 0.119 | 0.331 | 0.138 | 0.893 |
| planctomycetes | 0.54 | 0.53 | 0.43 | 0.56 | 0.095 | 0.759 | 0.937 | 0.527 |
| Fibrobacteres | 0.36 | 0.45 | 0.56 | 0.43 | 0.066 | 0.158 | 0.216 | 0.212 |
| Chytridiomycota | 0.27 | 0.38 | 0.47 | 0.45 | 0.174 | 0.845 | 0.747 | 0.277 |
| Items | Treatments 1 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MS1 | MS2 | MS3 | Treat | Linear | Quadratic | ||
| Prevotella | 16.00 | 14.52 | 23.26 | 21.22 | 2.518 | 0.068 | 0.013 | 0.770 |
| Clostridium | 1.29 | 1.38 | 1.27 | 1.29 | 0.125 | 0.919 | 0.818 | 0.800 |
| Bacteroides | 3.52 | 3.41 | 3.52 | 3.33 | 0.243 | 0.937 | 0.434 | 0.972 |
| Butyrivibrio | 1.30 | 1.15 | 0.99 | 1.19 | 0.140 | 0.501 | 0.014 | 0.450 |
| Fibrobacter | 0.35 | 0.45 | 0.57 | 0.42 | 0.060 | 0.114 | 0.227 | 0.213 |
| Alistipes | 1.27 | 1.15 | 1.12 | 0.97 | 0.093 | 0.187 | 0.084 | 0.999 |
| Succiniclasticum | 0.77 | 1.11 | 1.08 | 0.98 | 0.220 | 0.709 | 0.127 | 0.118 |
| Ruminococcus | 1.43 | 1.30 | 1.12 | 1.32 | 0.085 | 0.104 | 0.555 | 0.944 |
| Methanobrevibacter | 1.84 | 1.80 | 1.76 | 1.42 | 0.320 | 0.790 | 0.436 | 0.786 |
| Items | Treatments 1 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MS1 | MS2 | MS3 | Treat | Linear | Quadratic | ||
| Glycoside Hydrolases (GH) | 49.56 | 49.61 | 49.54 | 48.29 | 0.828 | 0.623 | 0.304 | 0.507 |
| Glycosyl Transferases (GT) | 25.06 | 25.18 | 26.14 | 27.00 | 1.033 | 0.519 | 0.144 | 0.698 |
| Carbohydrate Esterases (CE) | 15.21 | 14.58 | 14.23 | 14.51 | 0.331 | 0.235 | 0.040 | 0.911 |
| Carbohydrate-Binding Modules (CBM) | 5.07 | 5.52 | 5.06 | 5.04 | 0.209 | 0.312 | 0.473 | 0.944 |
| Auxiliary Activities (AA) | 2.76 | 2.59 | 2.52 | 2.64 | 0.081 | 0.249 | 0.822 | 0.653 |
| Polysaccharide Lyases (PL) | 2.36 | 2.38 | 2.51 | 2.52 | 0.190 | 0.890 | 0.434 | 0.076 |
| Items | Treatments 1 | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | MS1 | MS2 | MS3 | Treat | Linear | Quadratic | ||
| L | 7.33 | 7.39 | 7.05 | 7.47 | 0.242 | 0.643 | 0.429 | 0.673 |
| G | 7.10 | 6.84 | 6.87 | 6.60 | 0.171 | 0.258 | 0.080 | 0.232 |
| M | 6.67 | 6.48 | 6.84 | 6.64 | 0.286 | 0.952 | 0.521 | 0.356 |
| E | 6.15 | 5.95 | 5.73 | 5.74 | 0.173 | 0.300 | 0.006 | 0.194 |
| C | 4.53 | 4.36 | 4.27 | 4.27 | 0.115 | 0.347 | 0.017 | 0.501 |
| T | 3.82 | 4.08 | 4.37 | 4.34 | 0.413 | 0.762 | 0.956 | 0.244 |
| O | 3.72 | 3.88 | 3.98 | 3.96 | 0.205 | 0.801 | 0.810 | 0.191 |
| K | 3.92 | 3.86 | 3.71 | 3.82 | 0.085 | 0.354 | 0.835 | 0.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Zuo, Z.; Wang, C.; Peng, Z.; Zhong, J.; Wang, H. Effect of Isopropyl Ester of Hydroxy Analogue of Methionine on Rumen Microbiome, Active Enzymes, and Protein Metabolism Pathways of Yak. Fermentation 2024, 10, 94. https://doi.org/10.3390/fermentation10020094
Zhang X, Liu Y, Zuo Z, Wang C, Peng Z, Zhong J, Wang H. Effect of Isopropyl Ester of Hydroxy Analogue of Methionine on Rumen Microbiome, Active Enzymes, and Protein Metabolism Pathways of Yak. Fermentation. 2024; 10(2):94. https://doi.org/10.3390/fermentation10020094
Chicago/Turabian StyleZhang, Xirui, Yao Liu, Zizhen Zuo, Chenxi Wang, Zhongli Peng, Jincheng Zhong, and Haibo Wang. 2024. "Effect of Isopropyl Ester of Hydroxy Analogue of Methionine on Rumen Microbiome, Active Enzymes, and Protein Metabolism Pathways of Yak" Fermentation 10, no. 2: 94. https://doi.org/10.3390/fermentation10020094
APA StyleZhang, X., Liu, Y., Zuo, Z., Wang, C., Peng, Z., Zhong, J., & Wang, H. (2024). Effect of Isopropyl Ester of Hydroxy Analogue of Methionine on Rumen Microbiome, Active Enzymes, and Protein Metabolism Pathways of Yak. Fermentation, 10(2), 94. https://doi.org/10.3390/fermentation10020094






