Extraction and Chemical Composition Analyses of Intracellular and Extracellular Polysaccharides from Trametes lactinea Liquid Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Body Collection
2.2. Fungal Isolation, Purification, and Identification
2.3. Liquid Fermentation
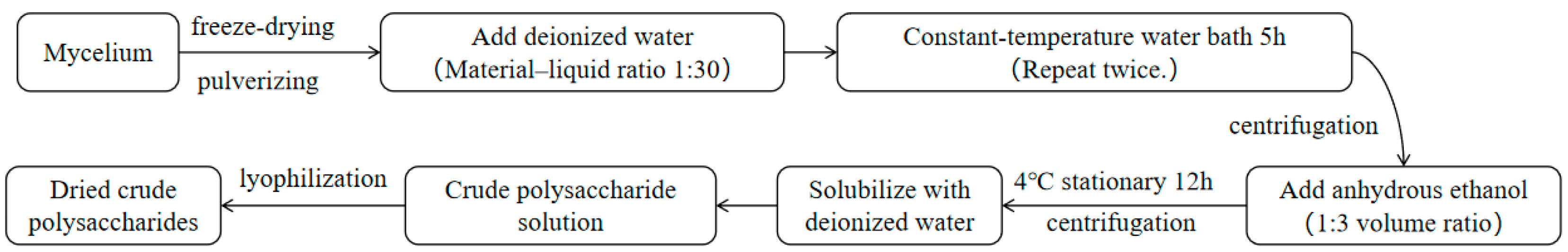
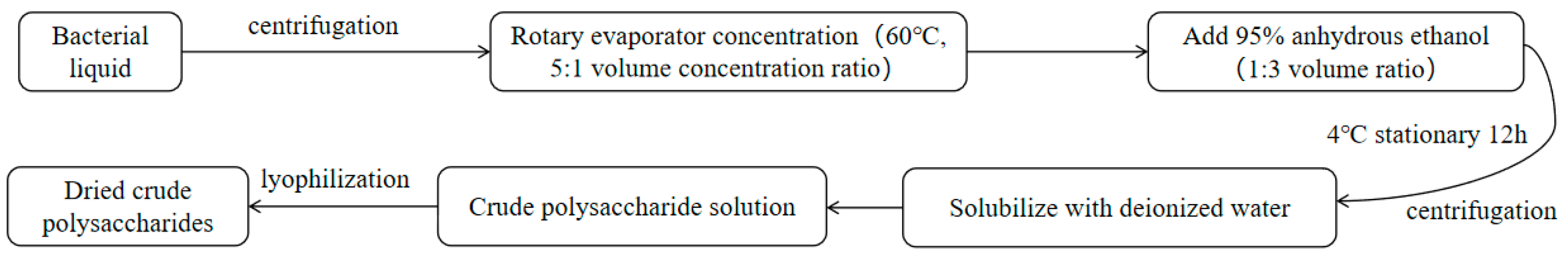
2.4. Polysaccharide Extraction Process
2.4.1. Intracellular Polysaccharide Extraction
2.4.2. Extracellular Polysaccharide Extraction
2.4.3. Determination of Extraction Rate (Amount)
2.4.4. One-Way Test of Variance
- (1)
- Intracellular polysaccharides
- (2)
- Extracellular polysaccharides
2.4.5. Orthogonal Test
- (1)
- Intracellular polysaccharides
- (2)
- Extracellular polysaccharides
- (3)
- Verification test
2.5. Determination of Chemical Composition
2.6. Data Analysis
3. Results
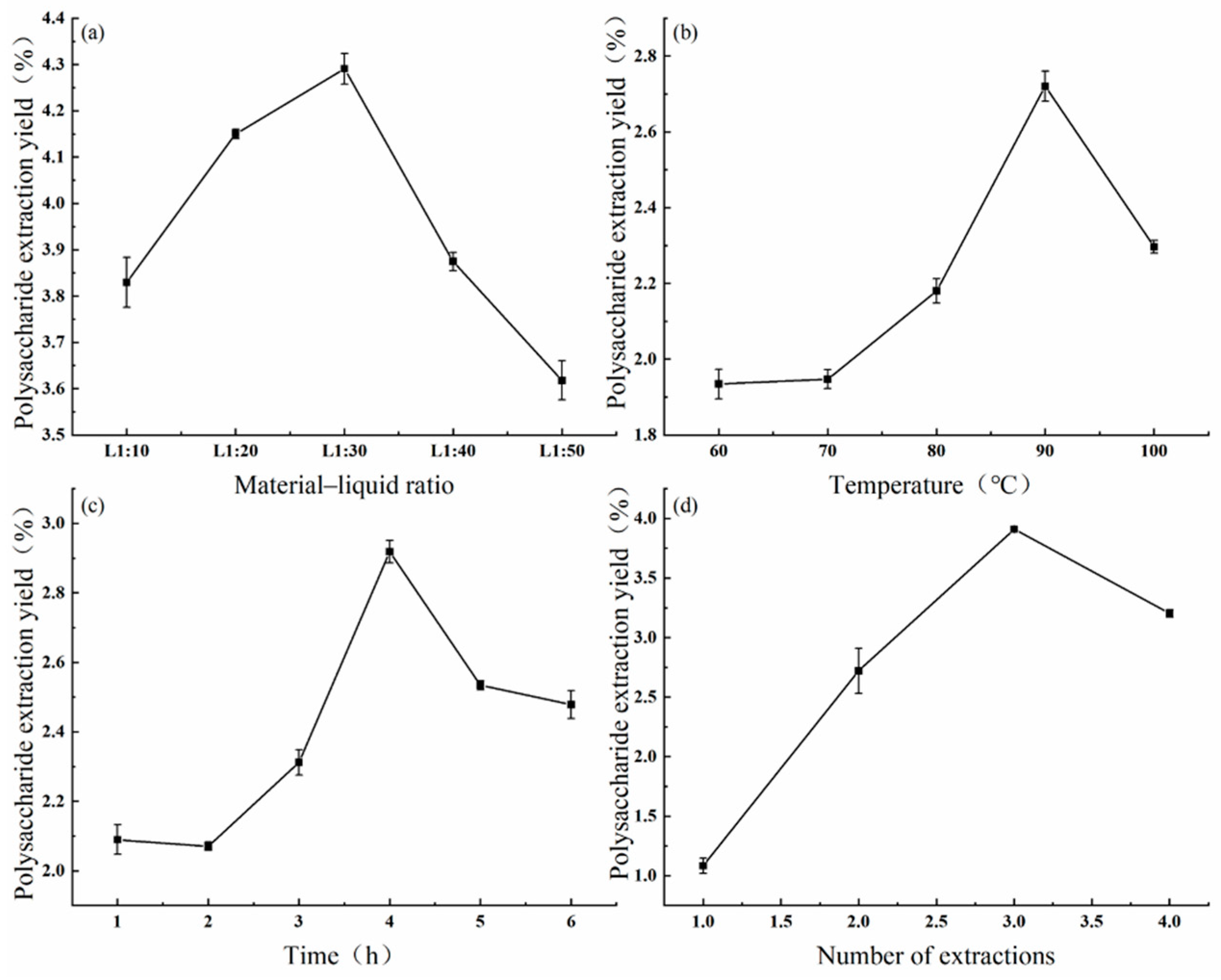
3.1. One-Way Test of Variance for the Intracellular Polysaccharide Extraction Process
3.2. Orthogonal Optimization of Intracellular Polysaccharide Extraction
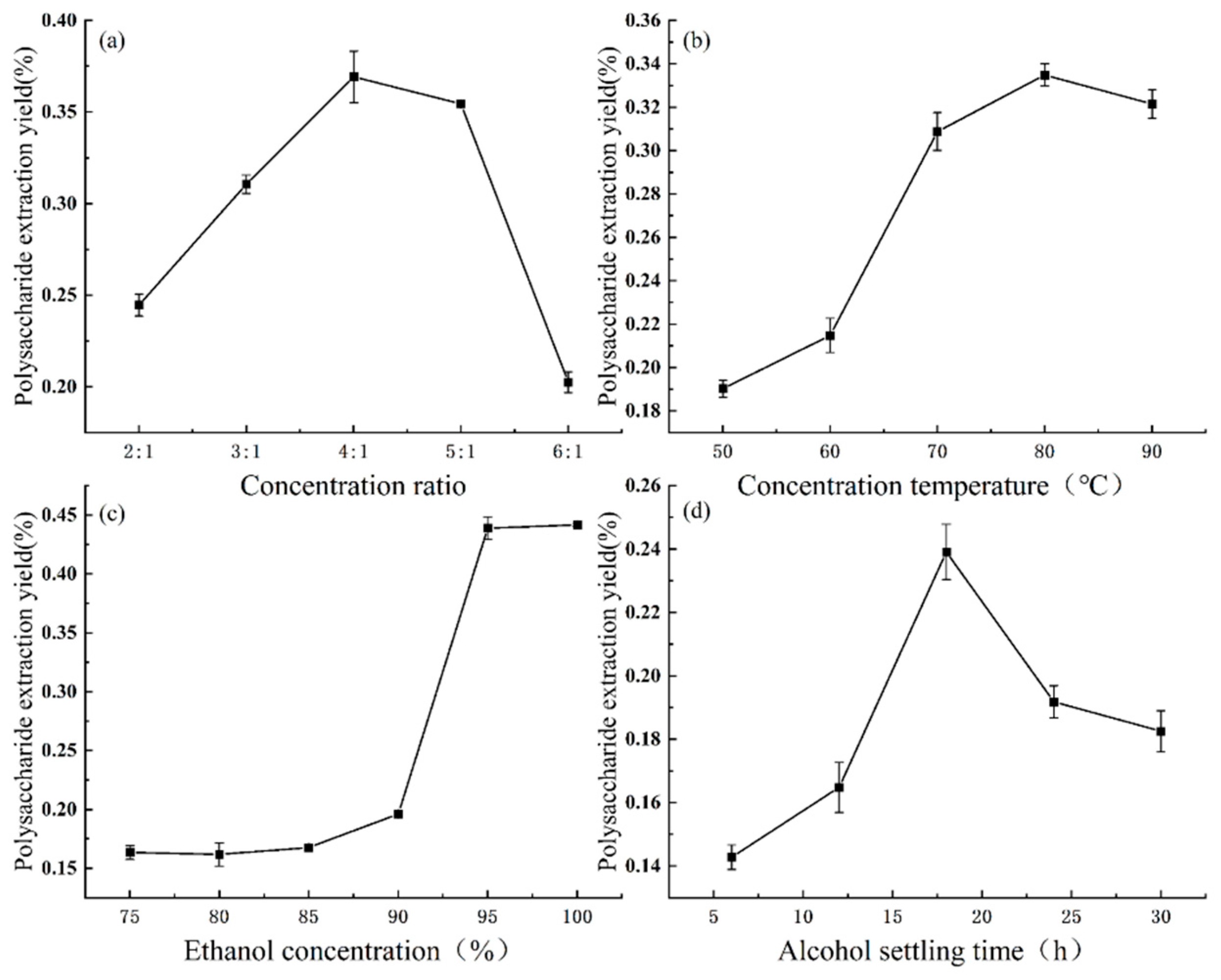
3.3. One-Way Test of Variance for the Extracellular Polysaccharide Extraction Process
3.4. Orthogonal Optimization of Extracellular Polysaccharide Extraction
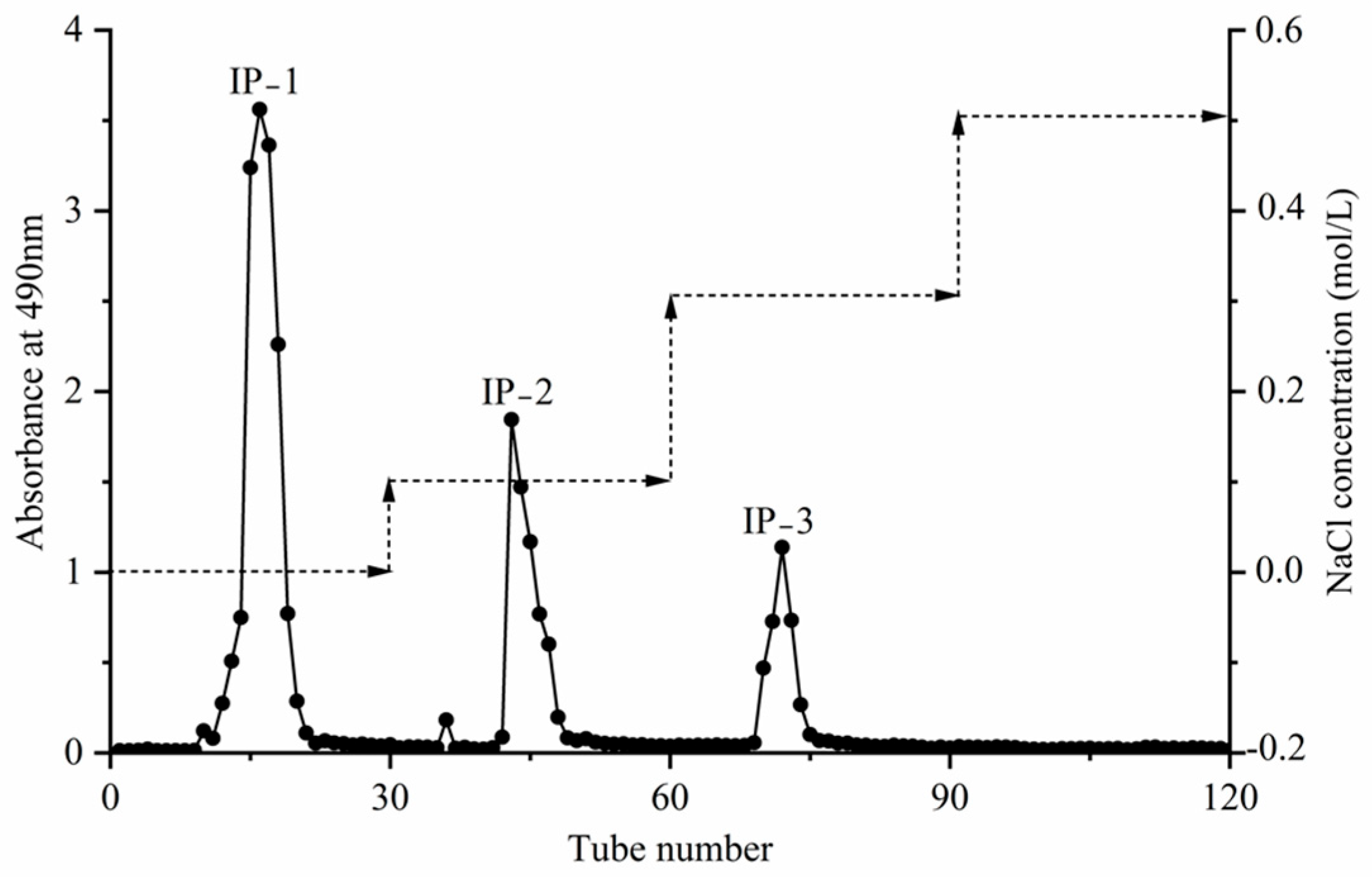
3.5. Isolation and Purification of Intracellular Polysaccharides
3.5.1. Results of the Isolation and Purification of Intracellular Crude Polysaccharides
3.5.2. Results of the Separation and Purification of Intracellular Polysaccharides
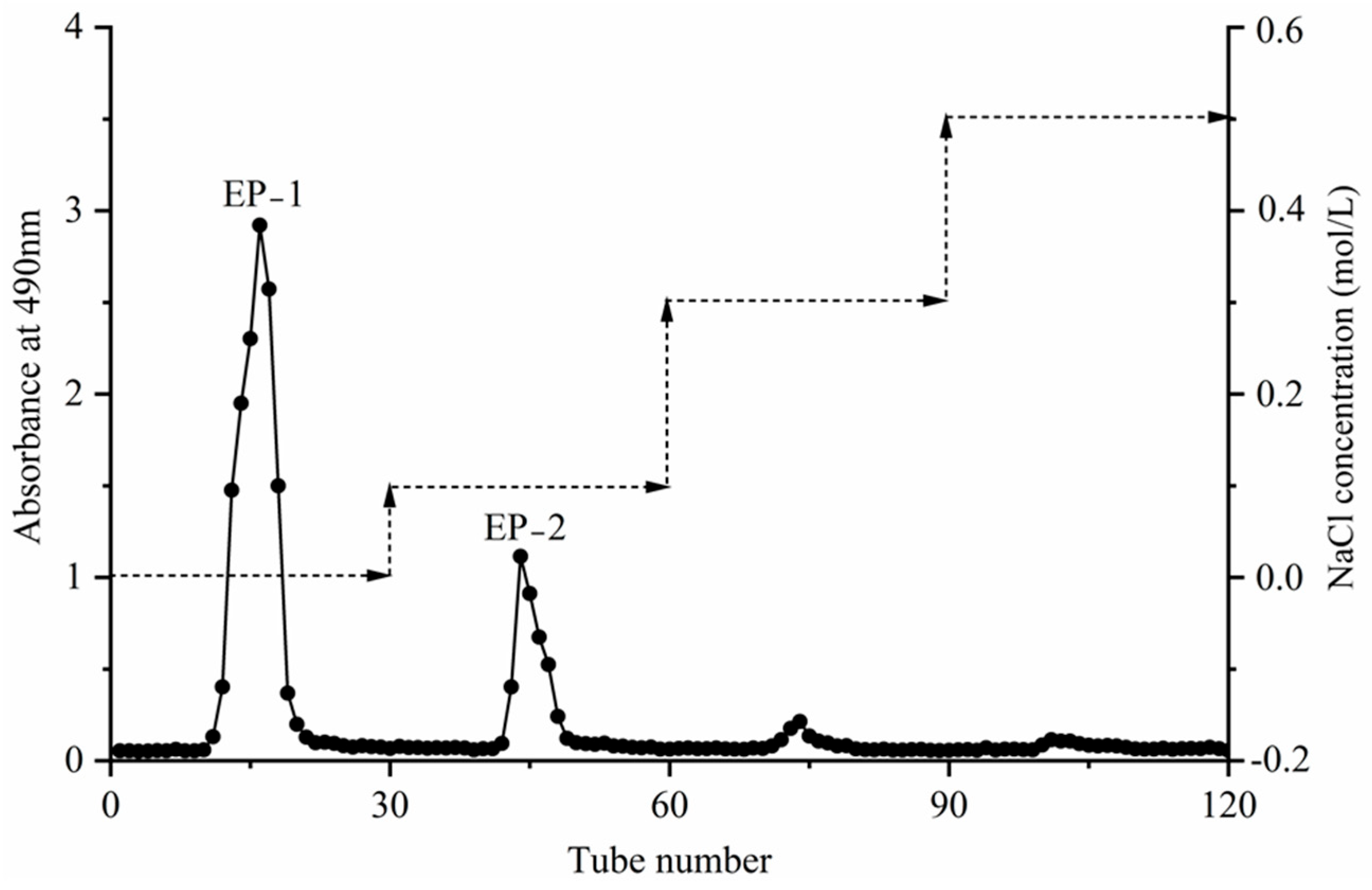
3.6. Isolation and Purification of Extracellular Polysaccharides
3.6.1. Results of the Isolation and Purification of Extracellular Crude Polysaccharides
3.6.2. Results of the Separation and Purification of Extracellular Polysaccharides
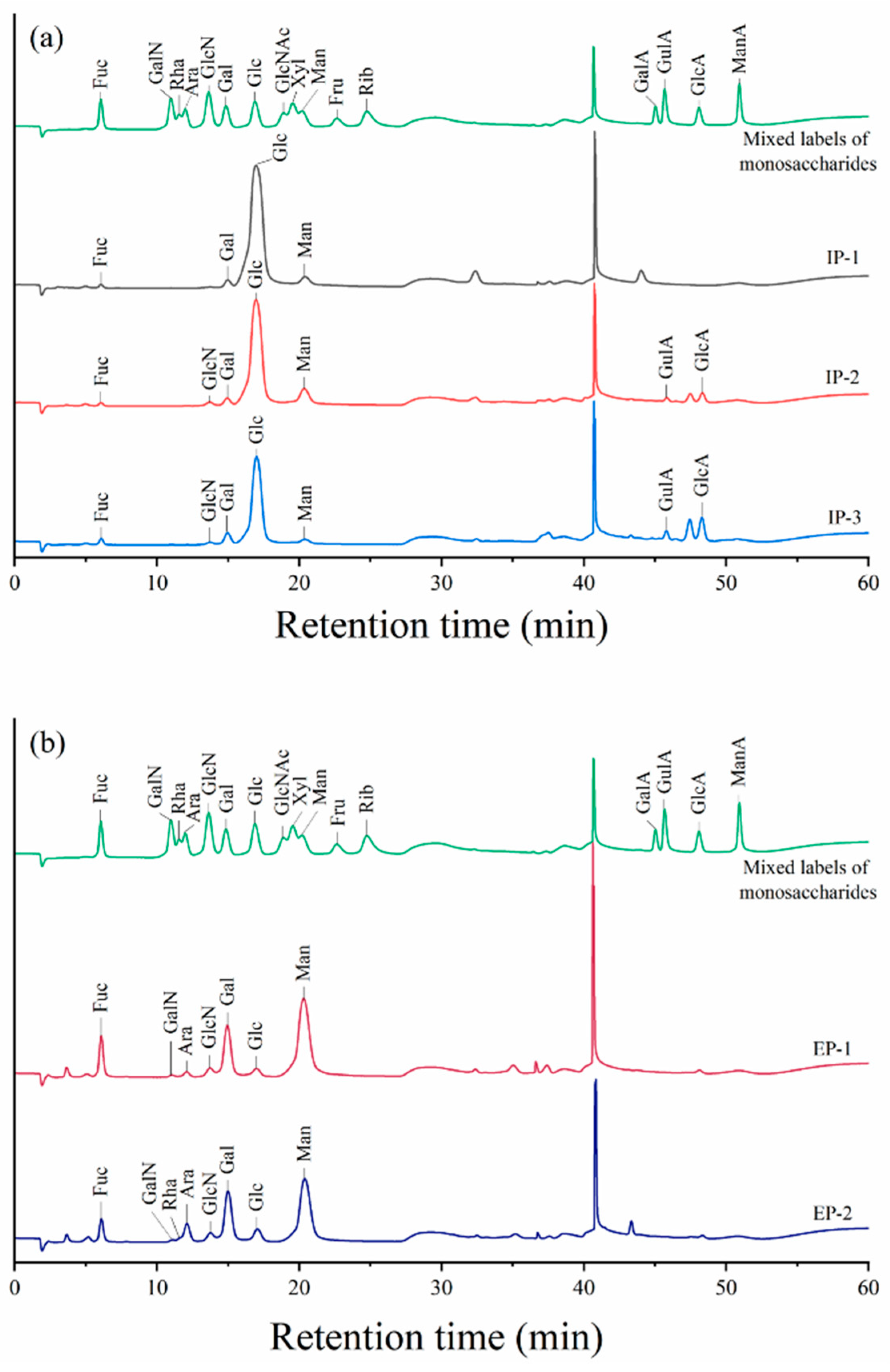
3.7. Chemical Compositional Analysis
3.7.1. Polysaccharide Chemical Composition
3.7.2. Monosaccharide Composition
4. Discussion
4.1. Polysaccharide Extraction
4.2. Polysaccharide Purification
4.3. Chemical Composition of Polysaccharide Components
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Q.; Pang, L.; Li, J.; Zhang, C.; Li, J.; Zhang, X.; Mao, T.; Wu, D.; Ma, X.; Geng, F.; et al. Characterization and diabetic wound healing benefits of protein-polysaccharide complexes isolated from an animal ethno-medicine Periplaneta americana L. Int. J. Biol. Macromol. 2022, 195, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Wang, Z.; Shi, J. Extraction optimization, structural characterization, and anticoagulant activity of acidic polysaccharides from Auricularia auricula—judae. Molecules 2020, 25, 710. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, T.; Wang, F.; Zhang, J.; Li, C.; Chen, X.; Li, Q.; Sun, S. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohyd. Polym. 2013, 92, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Studies on the pharmacological effects of Poria cocos polysaccharides. Chin. J. Mod. Drug Appl. 2013, 7, 217–218. [Google Scholar]
- Ma, Y.; Zhao, X. Clinical observation of Lentinan injection combined with chemotherapy in treatment of colorectal cancer. Chin. Arch. Tradit. Chin. Med. 2013, 31, 691–694. [Google Scholar]
- Zhang, B.; Wu, Y.; Liu, W.; Qiu, H.; Sun, P.; Zhang, A. Research progress on preparation and pharmacological activities of polysaccharide from Grifola frondosa. Edible Med. Mushrooms 2019, 27, 99–105. [Google Scholar]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Zhang, Y.; Zhang, Z. Bioactivity of the crude polysaccharides from fermented soybean curd residue by Flammulina velutipes. Carbohyd. Polym. 2012, 89, 1268–1276. [Google Scholar] [CrossRef]
- Zhai, X.; Zhao, A.; Geng, L.; Xu, C. Fermentation characteristics and hypoglycemic activity of an exopolysaccharide produced by submerged culture of Stropharia rugosoannulata #2. Ann. Microbiol. 2013, 63, 1013–1020. [Google Scholar]
- Wang, J.C.; Hu, S.H.; Wang, J.T.; Chen, K.S.; Chia, Y.C. Hypoglycemic effect of extract of Hericium erinaceus. J. Sci. Food Agric. 2005, 85, 641–646. [Google Scholar] [CrossRef]
- Mao, Y.; Mao, J.; Meng, X. Extraction optimization and bioactivity of exopolysaccharides from Agaricus bisporus. Carbohyd. Polym. 2013, 92, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N.; Ramu Ganesan, A.; Velmurugan, K.; Sathishkumar, P.; Jayakumar, R.; Seedevi, P. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits—A comprehensive review. Carbohyd. Polym. 2020, 238, 116185. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.T.; Abas, F.; Abdullah, J.O.; Mohd Tohit, E.R.; Hamid, M.; Shi-Biao, W.; Wu, S. Anticoagulant activity of polyphenolic-polysaccharides isolated from Melastoma malabathricum L. Evid.-Based Complement. Altern. Med. 2014, 2014, 614273. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Carralero, C.; Mansukhani, D.; Ruiz-Matute, A.I.; Martínez-Castro, I.; Ramos, L.; Sanz, M.L. Extraction and characterization of low molecular weight bioactive carbohydrates from mung bean (Vigna radiata). Food Chem. 2018, 266, 146–154. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Wang, S.; Guoying, Q.; Qiuhua, Z.; Yuting, L.; Wei, W. Optimization of ultrasonic-assisted extraction technology of Sargassum fusiforme polysaccharides and evaluation of their antioxidant activity. Food Sci. Technol. Int. Tokyo 2013, 19, 157–162. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Li, X.; Qiu, L.; Nie, F.; Qiao, B.; Liang, C.; Zhao, C.; Li, C. Study on ultrasonic-boiling water extraction of polysaccharide Fromficus carica L. leaves. Heilongjiang Agric. Sci. 2021, 73–76. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Zhang, J.; Yang, Q.; Ren, Z.; Zhang, C.; Liu, M.; Gao, Z.; Zhao, H.; Jia, L. Anti-inflammatory and hepatoprotective effects of exopolysaccharides isolated from Pleurotus geesteranus on alcohol-induced liver injury. Sci. Rep. 2018, 8, 10413–10493. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Cui, S.W.; Xu, M.; Ding, H.; Xie, M. Characterization of a bioactive polysaccharide from Ganoderma atrum: Re-elucidation of the fine structure. Carbohyd. Polym. 2017, 158, 58–67. [Google Scholar] [CrossRef]
- Zeng, X.; Li, P.; Chen, X.; Kang, Y.; Xie, Y.; Li, X.; Xie, T.; Zhang, Y. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides. Int. J. Biol. Macromol. 2019, 126, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yuan, M.; Zou, S.; Guo, L. Extraction, separation, purification and structure identification of polysaccharides from Truffles. Food Ferment. Ind. 2020, 46, 196–200. [Google Scholar]
- Cui, B.; Li, H.; Ji, X.; Zhou, J.; Song, J.; Si, J.; Yang, Z.; Dai, Y. Species diversity, taxonomy and phylogeny of polyporaceae (Basidiomycota) in china. Fungal Divers. 2019, 97, 137–392. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, N.; Wang, J.; Luo, H.; He, H.; Ding, M.; Deng, W.; Zou, K. The h+/k+-atpase inhibitory activities of trametenolic acid b from Trametes lactinea pat, and its effects on gastric cancer cells. Fitoterapia 2013, 89, 210. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; He, H.; Liu, H.; Yan, X.; Zou, K. Trametenolic acid b reverses multidrug resistance in breast cancer cells through regulating the expression level of p-glycoprotein: Trametenolic acid b reverses multidrug resistance through regulating p-glycoprotein. Phytother. Res. 2013, 28, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Meng, G.; Yang, J.; Wu, L.; Ma, S.; Ye, L.; Liu, G.; Fu, J.; Wu, X. Physicochemical properties, antioxidant activities and liver protective effects of polysaccharides from fruiting bodies of Trametes lactinea. Mycosystema 2020, 39, 2355–2368. [Google Scholar]
- Hao, J.; Ye, L.; Meng, G.; Song, Y.; Fu, J.; Wu, X. The protective effect and crucial biological pathways analysis of Trametes lactinea mycelium polysaccharides on acute alcoholic liver injury in mice based on transcriptomics and metabonomics. Food Sci. Hum. Wellness 2021, 10, 480–489. [Google Scholar] [CrossRef]
- Yan, A. Advancement in extraction methods of fungal intracellular polysaccharides. J. Microbiol. 2011, 31, 82–86. [Google Scholar]
- Li, M.; Sun, Y.; Zeng, Y.; Lei, Y.; Bao, M. Extraction process of polysaccharide content of mycelium and extracellular polysaccharide from Morchella crassipes. J. Fungal Res. 2017, 15, 195–200. [Google Scholar]
- Hou, X.; Lin, F.; Chang, M.; Liu, J.; Meng, J.; Wei, Y. Effects of ultrasound and deproteinization treatments on the extraction of polysaccharides from fruit-bodies of Hericium erinaceus. Mycosystema 2019, 38, 895–906. [Google Scholar]
- Zhang, C.; Yu, Y.; Liang, Y.; Chen, X. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Xu, J. Determination of momordica charantia polysaccharide by improved phenol-sulfuric acid method. Food Res. Dev. 2015, 36, 82–85. [Google Scholar]
- Liu, Y.; Qian, T.; Jiiang, D.; He, Y.; Shen, X.; Jiang, S. The comparison with kjeldah and coomassie brilliant blue method on testing protein content of the polysaccharide from Sipunculus nudus. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 96–98. [Google Scholar]
- Wang, W.; Guo, S.; Li, L.; Wang, M.; Liang, J. Determination of uronic acids in polysaccarides from Stanuntonia chinensis. Food Sci. Technol. 2007, 32, 84–86. [Google Scholar]
- Qiu, F.; Zhang, L.; Yu, J. Determination of sulfate groups in antler velvet pilose antler polysaccharides by barium sulfate turbidimetric method. J. Chang. Univ. Techonol. (Nat. Sci. Ed.) 2005, 268–270. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Deng, J.; Wu, Y.; Liu, L.; Tu, Y.; Zhou, Y. Extraction and antioxidant activity in vitro of okra flavonoids. Food Sci. 2014, 35, 121–125. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Yu, Q.; Shang, S.; Feng, Y.; Wang, Y. Determination of monosaccharide composition of polysaccharide in Ganoderma lucidum spore by ion chromatography. Chin. Pharm. J. 2014, 49, 344–347. [Google Scholar]
- Alliouche Kerboua, K.; Benosmane, L.; Namoune, S.; Ouled-Diaf, K.; Ghaliaoui, N.; Bendjeddou, D. Anti-inflammatory and antioxidant activity of the hot water-soluble polysaccharides from Anacyclus pyrethrum (L.) Lag. Roots. J. Ethnopharmacol. 2021, 281, 114491. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zhang, L.; Nakamura, Y.; Norisuye, T. Chemical structure of the water-insoluble polysaccharide isolated from the fruiting body of Ganoderma lucidum. Polym. J. 1998, 30, 838–842. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, Y.; Liu, Y.; Liu, S. Extraction process optimization, structural characterization and antioxidant activities of polysaccharide from Morchella sextelata. Mycosystema 2019, 38, 1548–1558. [Google Scholar]
- Qiao, Y.; Chen, W.; Xie, X.; Deng, B.; Peng, H.; Wang, Y. Optimization of selected parameters affecting extracellular polysaccharide extraction yields from Lentinula edodes spent culture fluid using response surface methodology. Acta Edulis Fungi 2015, 22, 69–73. [Google Scholar]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohyd. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, Y.; Jia, J.; Ren, X.; Wang, Y. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int. J. Biol. Macromol. 2019, 128, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, A.; Liu, L.; Tian, G.; Xu, F. Extraction of polysaccharides under vacuum condition from Lentinus edodes stipe and their antioxidant activities in vitro. Food Sci. Biotechnol. 2019, 28, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Tang, X.; Xu, L.; Liu, J. Optimization of the extraction process for polysaccharide from Terminalia chebula retz. by orthogonal test combined with response surface method. J. Mol. Sci. 2017, 33, 291–296. [Google Scholar]
- Xiao, Y.; Zhou, X.; Xu, Q.; Wang, R.; Zhao, C. Optimization of the extraction process of polysaccharides from wild boletus edulis by orthogonal method. J. Chengde Med. Coll. 2018, 35, 147–149. [Google Scholar]
- Hao, J.; Fu, M.; Wang, L.; Wu, X. Optimization of extraction technology, structure characterization and antioxidant activities of polysaccharides from mycelium of Trametes lactinea. Mycosystema 2021, 40, 2461–2479. [Google Scholar]
- Du, P.; Wang, L.; Liu, D.; Zhang, Y. Response surface methodology for optimizing extraction of polysaccharides from Ganoderma applanatum. J. Anhui Agric. Sci. 2018, 46, 148–151, 168. [Google Scholar]
- Ma, S.; Yu, N.; Liu, C.; Zhang, L. Research on extracting technique of excellularpolysaccharide from Pleurotus nebrodensis. J. Shenyang Agric. Univ. 2008, 39, 374–376. [Google Scholar]
- Li, Z.; Wang, S.; Sheng, W. Extraction conditions optimization of extracellular polysaccharides of Ganoderma applanatum. J. Anhui Sci. Technol. Univ. 2013, 27, 22–25. [Google Scholar]
- Zhang, G.; Xin, X.; Liang, J.; Liu, X.; Wang, S.; Kong, D. Selection of the method for determining extracelluar polysaccharides and optimization of extracting technology in Pleurotus citrinopileatus. Jiangsu J. Agric. Sci. 2005, 21, 230–233. [Google Scholar]
- Lu, T.; Li, D.; Xie, W.; Yu, C.; Chen, H.; Shang, H.; Yang, L.; Liu, Y.; Xie, Z. Optimization of extraction process of Perenniporia fraxinea polysaccharide and its antioxidant activity. China Brew. 2021, 40, 188–194. [Google Scholar]
- Chen, C.; Liu, J. Extraction conditions optimization of extracellular polysaccharides in tricholoma matsutake. Food Nutr. China 2012, 18, 60–62. [Google Scholar]
- Wei, Y.; Hou, X.; Du, X.; Chang, M.; Meng, J.; Liu, J. Optimization of extraction process of exopolysaccharides from Cordyceps militaris and its anti-colon cancer activity. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2020, 40, 121–128. [Google Scholar]
- Sichert, A.; Le Gall, S.; Klau, L.J.; Laillet, B.; Rogniaux, H.; Aachmann, F.L.; Hehemann, J. Ion-exchange purification and structural characterization of five sulfated fucoidans from brown algae. Glycobiology 2021, 31, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ma, C.; Song, H.; Li, T. Study on deproteinization and decoloration in extraction of Armillariella polysaccharide. Pharm. Clin. Res. 2007, 45–46. [Google Scholar] [CrossRef]
- Ji, X.; Peng, B.; Ding, H.; Cui, B.; Nie, H.; Yan, Y. Purification, structure and biological activity of pumpkin polysaccharides: A review. Food Rev. Int. 2023, 39, 307–319. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Extraction, structural analysis, and activities of rice bran polysaccharide. Chem. Biol. Drug Des. 2021, 98, 631–638. [Google Scholar] [CrossRef]
- He, N.; Tian, L.; Zhai, X.; Zhang, X.; Zhao, Y. Composition characterization, antioxidant capacities and anti-proliferative effects of the polysaccharides isolated from Trametes lactinea (berk.) Pat. Int. J. Biol. Macromol. 2018, 115, 114–123. [Google Scholar] [CrossRef]
- Bao, Z.; Yao, L.; Zhang, X.; Lin, S. Isolation, purification, characterization, and immunomodulatory effects of polysaccharide from Auricularia auricula on RAW264.7 macrophages. J. Food Biochem. 2020, 44, e13516. [Google Scholar] [CrossRef]
- Shi, K.; Yang, G.; He, L.; Yang, B.; Li, Q.; Yi, S. Purification, characterization, antioxidant, and antitumor activity of polysaccharides isolated from silkworm cordyceps. J. Food Biochem. 2020, 44, e13482. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, L.; Hong, H.; Gou, D.; Liu, D. Isolation, purification, structure analysis and comparison of antioxidant activity of the polysaccharides from Ostericum sieboldii. Mod. Food Sci. Technol. 2022, 38, 124–132. [Google Scholar]
- Zhang, H.; Li, H.; Netala, V.R.; Hou, T.; Zhang, Z. Optimization of complex enzyme-ultrasonic synergistic extraction of water-soluble polysaccharides from Perilla frutescens seed meal: Purification, characterization and in vitro antioxidant activity. J. Food Process. Preserv. 2022, 46, e16201. [Google Scholar] [CrossRef]
- Xu, T.; Zuo, Y.; Lin, W.; Liu, P. Isolation, purification, chemical composition and antioxidant activities of polysaccharides from Dictyosphaerium sp. 1A10. Food Res. Dev. 2023, 44, 57–65. [Google Scholar]
- De Jesus, L.I.; Smiderle, F.R.; Cordeiro, L.M.C.; de Freitas, R.A.; Van Griensven, L.J.L.D.; Iacomini, M. Simple and effective purification approach to dissociate mixed water-insoluble α- and β-d-glucans and its application on the medicinal mushroom Fomitopsis betulina. Carbohyd. Polym. 2018, 200, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ma, S.; Guo, H.; Cui, X.; Wang, S.; Zhong, X.; Wu, Y.; Zheng, W.; Wang, H.; Yu, J.; et al. Comparative study on the monosaccharide compositions, antioxidant and hypoglycemic activities in vitro of intracellular and extracellular polysaccharides of liquid fermented Coprinus comatus. Int. J. Biol. Macromol. 2019, 139, 543–549. [Google Scholar] [CrossRef] [PubMed]



| Level | Factor | |||
|---|---|---|---|---|
| A | B | C | D | |
| Material–Liquid Ratio | Extraction Temperature (°C) | Extraction Time (h) | Number of Extractions | |
| 1 | 1:20 | 80 | 3 | 2 |
| 2 | 1:30 | 90 | 4 | 3 |
| 3 | 1:40 | 100 | 5 | 4 |
| Level | Factor | |||
|---|---|---|---|---|
| A | B | C | D | |
| Concentration Ratio | Concentration Temperature (°C) | Ethanol Concentration (%) | Alcohol Settling Time (h) | |
| 1 | 3:1 | 70 | 90.0 | 12 |
| 2 | 4:1 | 80 | 95.0 | 18 |
| 3 | 5:1 | 90 | 100.0 | 24 |
| Test Number | A | B | C | D | Polysaccharide Extraction Yield (%) |
|---|---|---|---|---|---|
| Material–Liquid Ratio | Extraction Temperature (°C) | Extraction Time (h) | Number of Extractions | ||
| 1 | 1 | 1 | 1 | 1 | 2.5 ± 0.1 |
| 2 | 2 | 1 | 2 | 2 | 2.9 ± 0.0 |
| 3 | 3 | 1 | 3 | 3 | 4.0 ± 0.3 |
| 4 | 3 | 2 | 1 | 2 | 3.2 ± 0.0 |
| 5 | 1 | 2 | 2 | 3 | 4.1 ± 0.0 |
| 6 | 2 | 2 | 3 | 1 | 3.4 ± 0.4 |
| 7 | 2 | 3 | 1 | 3 | 4.3 ± 0.1 |
| 8 | 3 | 3 | 2 | 1 | 3.9 ± 0.4 |
| 9 | 1 | 3 | 3 | 2 | 3.9 ± 0.1 |
| K1 | 3.4962 | 3.1134 | 3.3455 | 3.2530 | |
| K2 | 3.5214 | 3.5809 | 3.6335 | 3.3216 | |
| K3 | 3.7067 | 4.0301 | 3.7454 | 4.1498 | |
| R | 0.2105 | 0.9167 | 0.3999 | 0.8967 |
| Source of Variance | Degrees of Freedom | Square Sum | Mean Square | F | p |
|---|---|---|---|---|---|
| Material–liquid ratio | 2 | 0.238 | 0.119 | 49.88 | <0.01 |
| Extraction temperature (°C) | 2 | 3.782 | 1.891 | 792.96 | <0.01 |
| Extraction time (h) | 2 | 0.766 | 0.383 | 160.67 | <0.01 |
| Number of extractions | 2 | 4.484 | 2.242 | 940.30 | <0.01 |
| Inaccuracies | 18 | 0.043 | 0.026 | ||
| Aggregates | 27 | 9.313 |
| Test Number | A | B | C | D | Polysaccharide Extraction Yield (mg/mL) |
|---|---|---|---|---|---|
| Concentration Ratio | Concentration Temperature (°C) | Ethanol Concentration (%) | Alcohol Settling Time (h) | ||
| 1 | 1 | 1 | 1 | 1 | 0.217 ± 0.081 |
| 2 | 1 | 2 | 2 | 2 | 0.198 ± 0.005 |
| 3 | 1 | 3 | 3 | 3 | 0.189 ± 0.058 |
| 4 | 2 | 2 | 1 | 3 | 0.201 ± 0.027 |
| 5 | 2 | 3 | 2 | 1 | 0.197 ± 0.006 |
| 6 | 2 | 1 | 3 | 2 | 0.333 ± 0.016 |
| 7 | 3 | 3 | 1 | 2 | 0.243 ± 0.055 |
| 8 | 3 | 1 | 2 | 3 | 0.300 ± 0.010 |
| 9 | 3 | 2 | 3 | 1 | 0.391 ± 0.005 |
| K1 | 0.1969 | 0.2829 | 0.218 | 0.2738 | |
| K2 | 0.2540 | 0.2632 | 0.243 | 0.2552 | |
| K3 | 0.3098 | 0.2145 | 0.300 | 0.2317 | |
| R | 0.1129 | 0.0684 | 0.083 | 0.0421 |
| Source of Variance | Degrees of Freedom | Square Sum | Mean Square | F | p |
|---|---|---|---|---|---|
| Concentration ratio | 2 | 0.057 | 0.029 | 239.916 | <0.01 |
| Concentration temperature (°C) | 2 | 0.022 | 0.011 | 93.458 | <0.01 |
| Ethanol concentration (%) | 2 | 0.032 | 0.016 | 135.184 | <0.01 |
| Alcohol settling time (h) | 2 | 0.008 | 0.004 | 33.615 | <0.01 |
| Inaccuracies | 18 | 0.002 | 0.002 | ||
| Aggregates | 27 | 0.122 |
| Ingredient | Concentration (%) | ||||
|---|---|---|---|---|---|
| IP-1 | IP-2 | IP-3 | EP-1 | EP-2 | |
| Total sugars | 52.9 ± 0.1 | 35.4 ± 0.1 | 28.0 ± 0.5 | 55.7 ± 0.9 | 13.9 ± 0.2 |
| Proteins | 0.4 ± 0.0 | — | — | 0.1 ± 0.0 | — |
| Glucuronides | 0.2 ± 0.0 | 6.4 ± 0.1 | 10.1 ± 0.3 | 0.4 ± 0.0 | 1.2 ± 0.2 |
| Sulfate group | 10.8 ± 3.0 | 11.2 ± 5.4 | 12.2 ± 3.8 | 12.8 ± 2.7 | 10.1 ± 1.2 |
| Flavonoids | — | 0.2 ± 0.1 | 1.2 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.2 |
| Total phenols | 0.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.1 | 0.5 ± 0.0 | 0.0 ± 0.0 |
| Monosaccharide | Intracellular | Extracellular | |||
|---|---|---|---|---|---|
| IP-1 | IP-2 | IP-3 | EP-1 | EP-2 | |
| Fuc | 1.3 | 1.3 | 2.9 | 11.5 | 7.0 |
| GalN | — | — | — | 0.2 | 0.2 |
| Rha | — | — | — | — | 2.3 |
| Ara | — | — | — | 2.0 | 8.0 |
| GlcN | — | 0.9 | 0.5 | 1.6 | 1.8 |
| Gal | 3.5 | 3.7 | 7.1 | 22.7 | 25.4 |
| Glc | 90.2 | 74.9 | 63.3 | 2.0 | 3.7 |
| Man | 5.0 | 11.3 | 3.1 | 55.9 | 51.6 |
| GulA | — | 1.8 | 4.7 | — | — |
| GlcA | — | 6.1 | 18.5 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shen, L.; Yang, M.; Yang, K.; Cheng, F. Extraction and Chemical Composition Analyses of Intracellular and Extracellular Polysaccharides from Trametes lactinea Liquid Fermentation. Fermentation 2024, 10, 76. https://doi.org/10.3390/fermentation10020076
Liu Y, Shen L, Yang M, Yang K, Cheng F. Extraction and Chemical Composition Analyses of Intracellular and Extracellular Polysaccharides from Trametes lactinea Liquid Fermentation. Fermentation. 2024; 10(2):76. https://doi.org/10.3390/fermentation10020076
Chicago/Turabian StyleLiu, Yijun, Lu Shen, Mei Yang, Kaitai Yang, and Fei Cheng. 2024. "Extraction and Chemical Composition Analyses of Intracellular and Extracellular Polysaccharides from Trametes lactinea Liquid Fermentation" Fermentation 10, no. 2: 76. https://doi.org/10.3390/fermentation10020076
APA StyleLiu, Y., Shen, L., Yang, M., Yang, K., & Cheng, F. (2024). Extraction and Chemical Composition Analyses of Intracellular and Extracellular Polysaccharides from Trametes lactinea Liquid Fermentation. Fermentation, 10(2), 76. https://doi.org/10.3390/fermentation10020076





