Fermentation Techniques and Biotechnological Applications of Modified Bacterial Cellulose: An Up-to-Date Overview
Abstract
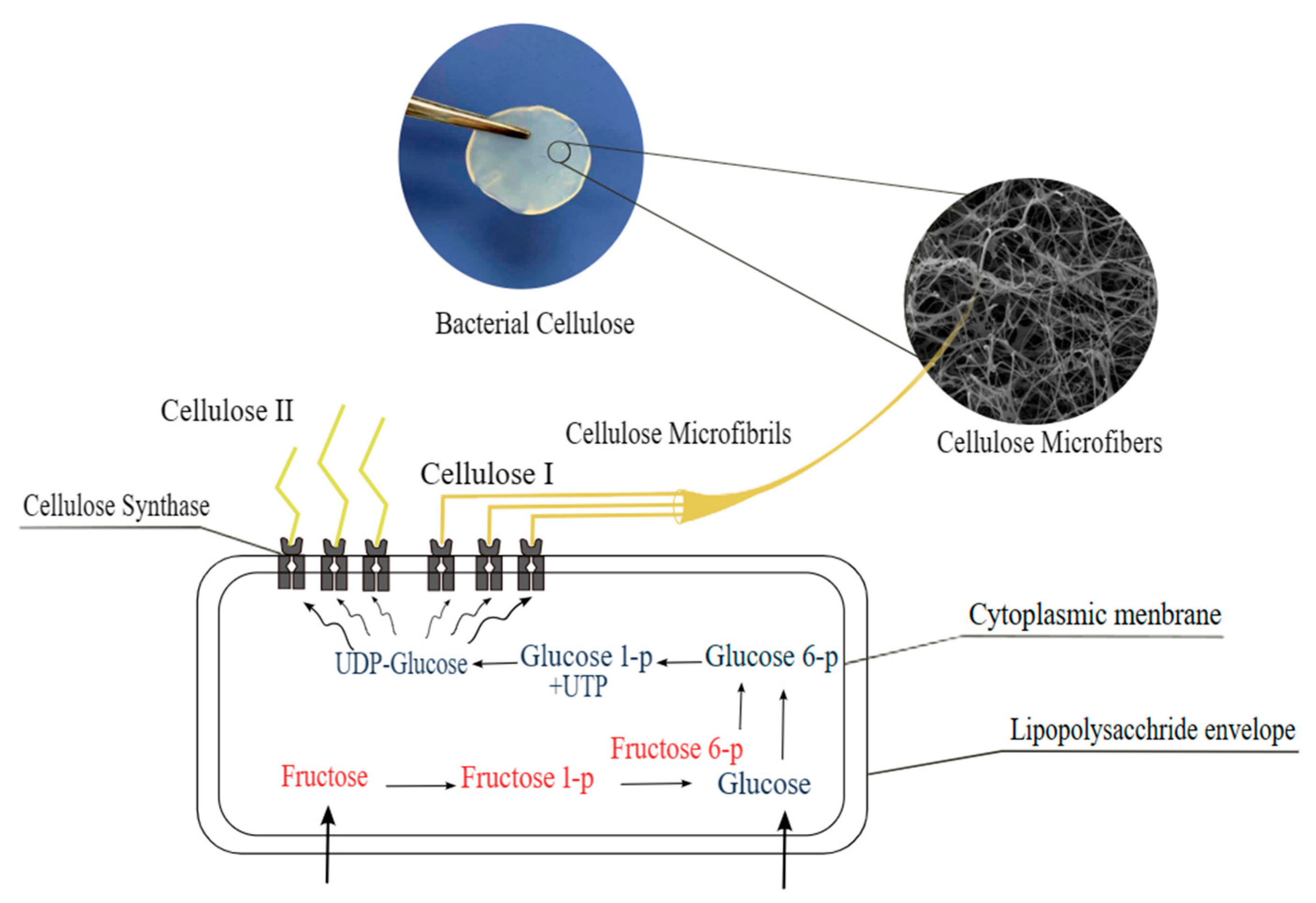
1. Introduction
2. BC Production Methods
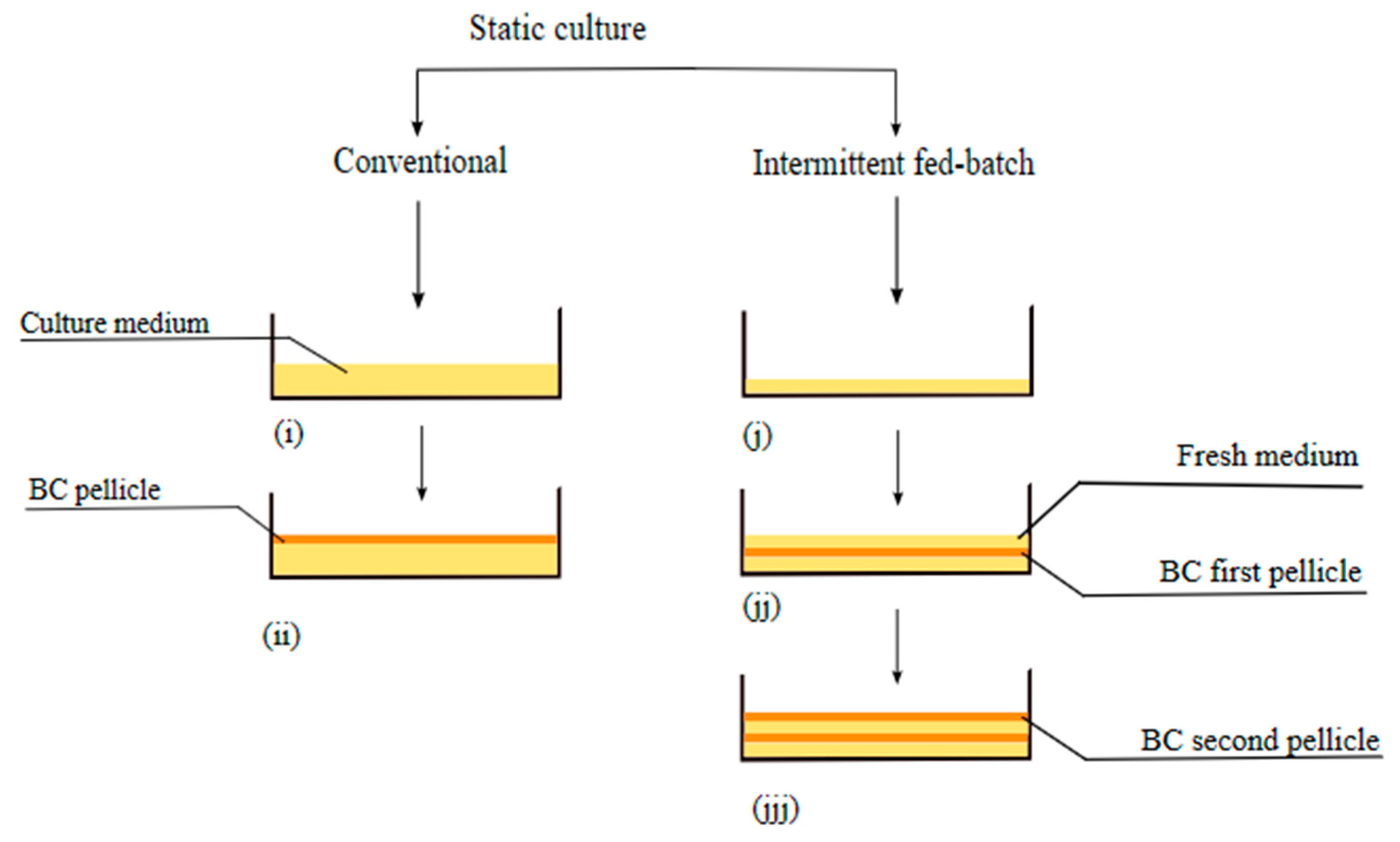
2.1. Static Culture Method
2.2. Submerged Fermentation
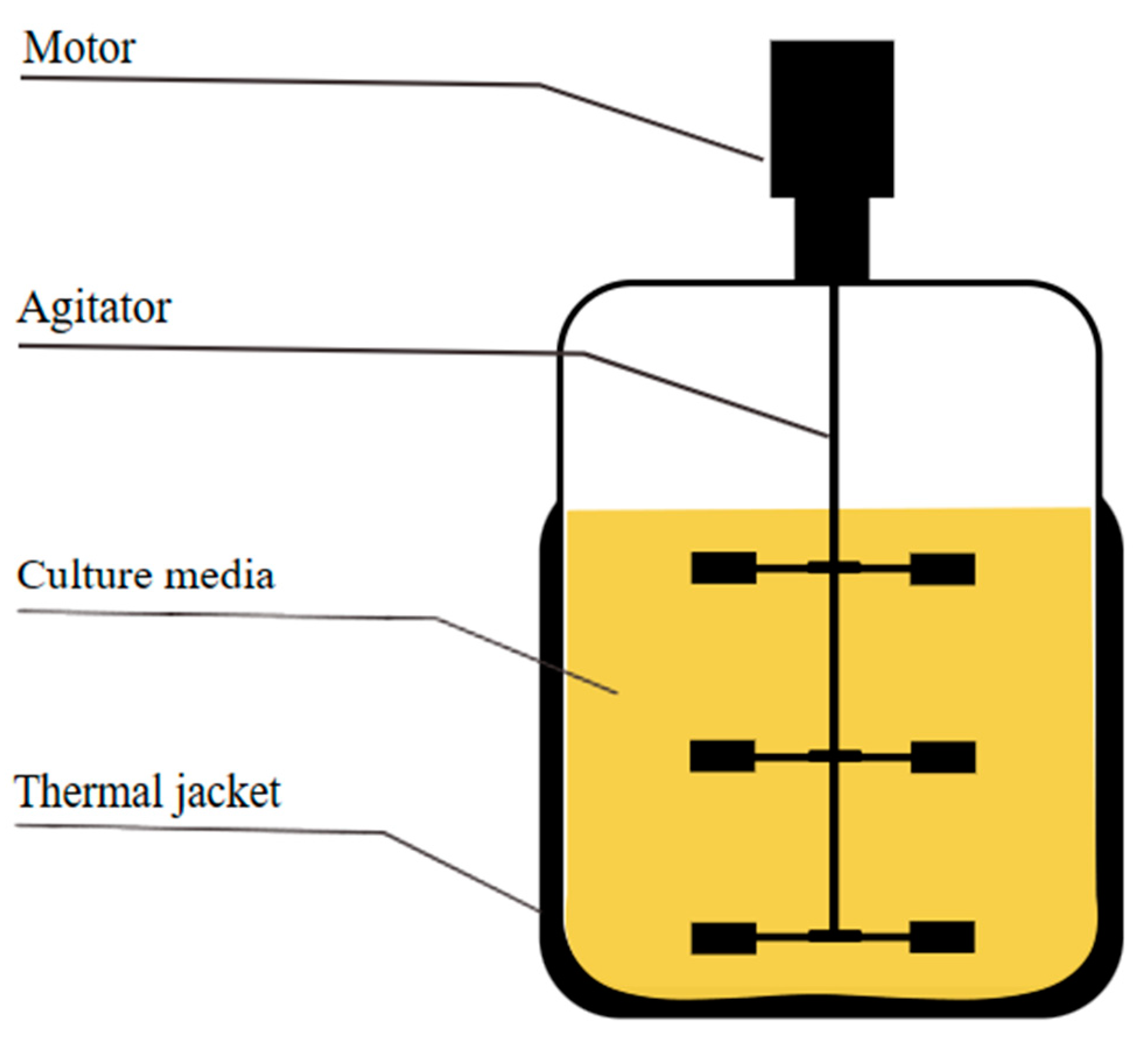
2.2.1. Stirred Tank Bioreactor
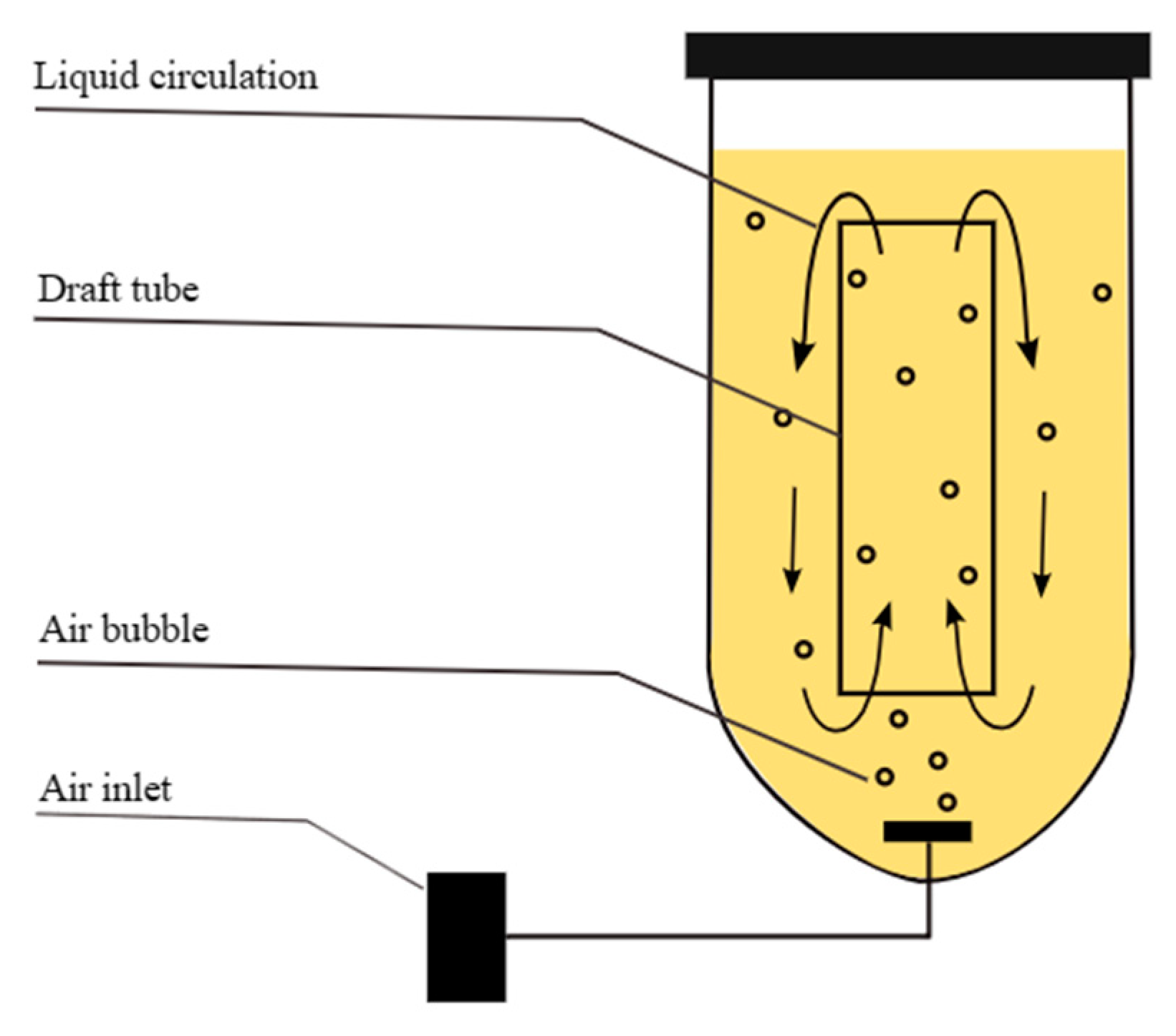
2.2.2. Airlift Bioreactor
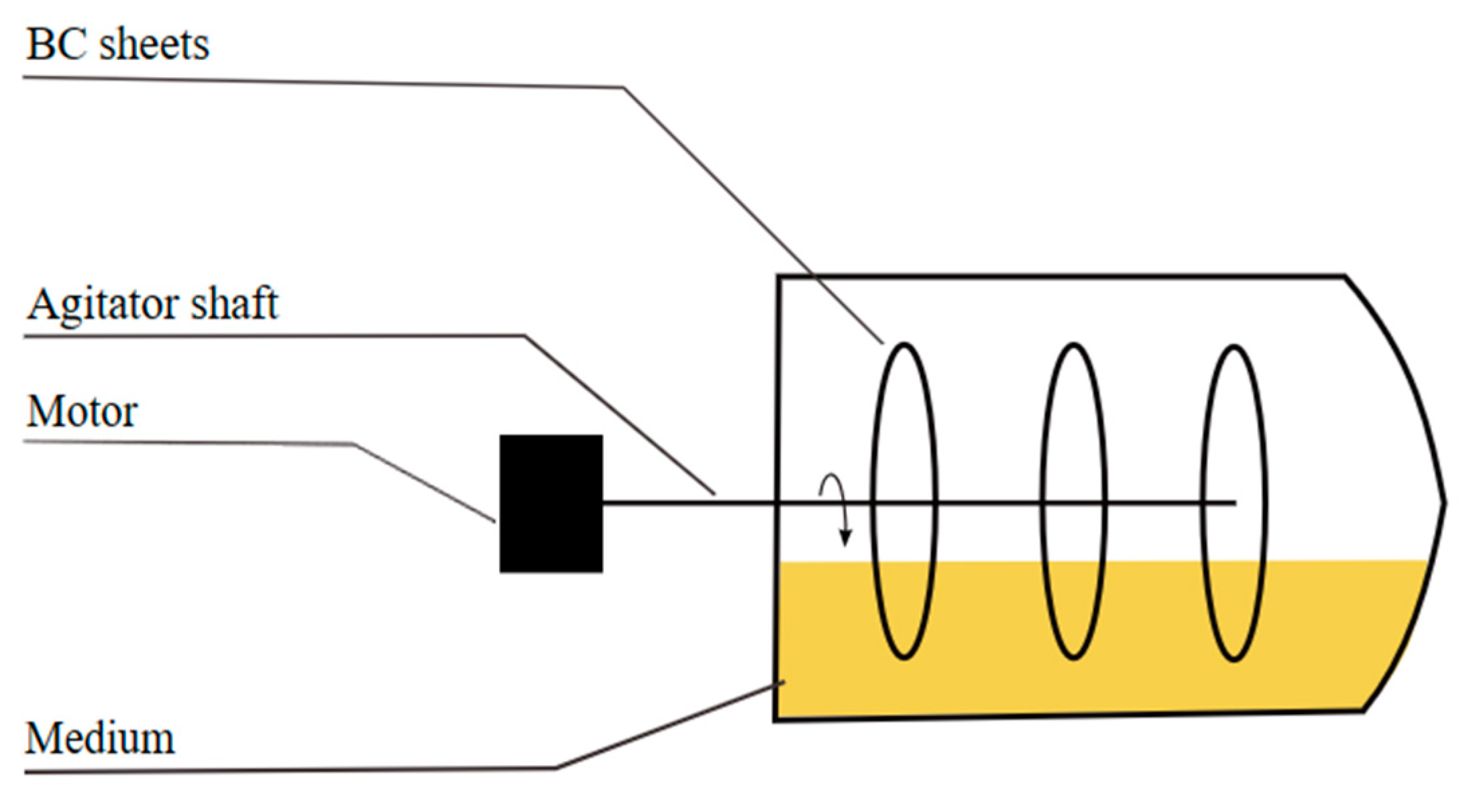
2.2.3. Rotating Disc Bioreactor
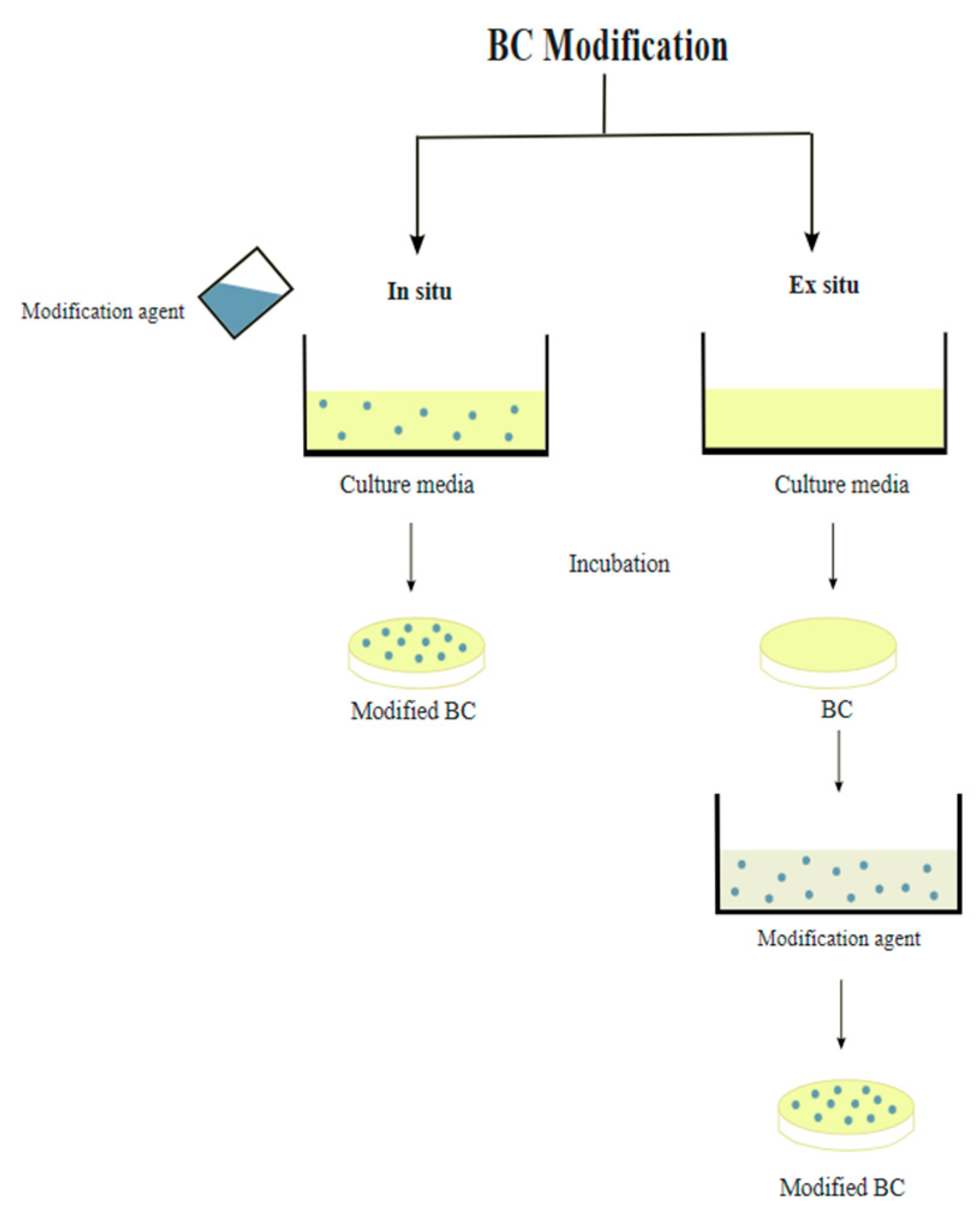
3. BC Modification and Functionalization
3.1. BC In Situ Modification
3.2. BC Ex Situ Modification
4. Biotechnological Application of Modified Bacterial Cellulose
4.1. BC and Food Industry
4.1.1. BC as Food Component
4.1.2. BC and Food Packaging
4.2. BC and Biomedical Applications
4.2.1. BC and Wound Dressings
4.2.2. BC as Drug Delivery Systems
4.2.3. BC in Skin and Bone Tissue Engineering Applications
4.2.4. BC as Artificial Blood Vessels
5. Evaluation of the Environmental Impacts of Bacterial Cellulose Production
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Brown, A.J. XIX.—The Chemical Action of Pure Cultivations of Bacterium Aceti. J. Chem. Soc. Trans. 1886, 49, 172–187. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an Acetic Ferment Which Forms Cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Lin, S.-P.; Hsieh, S.-C.; Chen, K.-I.; Demirci, A.; Cheng, K.-C. Semi-Continuous Bacterial Cellulose Production in a Rotating Disk Bioreactor and Its Materials Properties Analysis. Cellulose 2014, 21, 835–844. [Google Scholar] [CrossRef]
- Bimmer, M.; Mientus, M.; Klingl, A.; Ehrenreich, A.; Liebl, W. The Roles of the Various Cellulose Biosynthesis Operons in Komagataeibacter hansenii ATCC 23769. Appl. Environ. Microbiol. 2022, 88, e0246021. [Google Scholar] [CrossRef]
- Singh, A.; Walker, K.T.; Ledesma-Amaro, R.; Ellis, T. Engineering Bacterial Cellulose by Synthetic Biology. Int. J. Mol. Sci. 2020, 21, 9185. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; La China, S.; Falcone, P.M.; Giudici, P. Biotechnological Production of Cellulose by Acetic Acid Bacteria: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 6885–6898. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial Cellulose Biosynthesis: Diversity of Operons, Subunits, Products, and Functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A Natural in Situ Fabrication Method of Functional Bacterial Cellulose Using a Microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Buldum, G.; Mantalaris, A. Systematic Understanding of Recent Developments in Bacterial Cellulose Biosynthesis at Genetic, Bioprocess and Product Levels. Int. J. Mol. Sci. 2021, 22, 7192. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Deng, Y.; Wei, Q. Research Progress of the Biosynthetic Strains and Pathways of Bacterial Cellulose. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab071. [Google Scholar] [CrossRef]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Yu, X.; Atalla, R.H. Production of Cellulose II by Acetobacter Xylinum in the Presence of 2,6-Dichlorobenzonitrile. Int. J. Biol. Macromol. 1996, 19, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial Cellulose Composites: Synthetic Strategies and Multiple Applications in Bio-medical and Electro-conductive Fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, A. Optimization of Culture Conditions for Bacterial Cellulose Production from Gluconacetobacter hansenii UAC09. Ann. Microbiol. 2011, 61, 781–787. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More Than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Erbas Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of Bacterial Cellulose Using Hot Water Extracted Wood Sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Urbina, L.; Algar, I.; García-Astrain, C.; Gabilondo, N.; González, A.; Corcuera, M.; Eceiza, A.; Retegi, A. Biodegradable Composites with Improved Barrier Properties and Transparency from the Impregnation of PLA to Bacterial Cellulose Membranes. J. Appl. Polym. Sci. 2016, 133, 43669. [Google Scholar] [CrossRef]
- Urbina, L.; Eceiza, A.; Gabilondo, N.; Corcuera, M.Á.; Retegi, A. Valorization of Apple Waste for Active Packaging: Multicomponent Polyhydroxyalkanoate Coated Nanopapers with Improved Hydrophobicity and Antioxidant Capacity. Food Packag. Shelf Life 2019, 21, 100356. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of Reusable Novel Membranes Based on Bacterial Cellulose and Chitosan for the Filtration of Copper in Wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The Growing Merits and Dwindling Limitations of Bacterial Cellulose-Based Tissue Engineering Scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Gromovykh, T.I.; Pigaleva, M.A.; Gallyamov, M.O.; Ivanenko, I.P.; Ozerova, K.E.; Kharitonova, E.P.; Bahman, M.; Feldman, N.B.; Lutsenko, S.V.; Kiselyova, O.I. Structural Organization of Bacterial Cellulose: The Origin of Anisotropy and Layered Structures. Carbohydr. Polym. 2020, 237, 116140. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current Progress on the Production, Modification, and Applications of Bacterial Cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A Novel Static Cultivation of Bacterial Cellulose Production by Intermittent Feeding Strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Bae, S.; Shoda, M. Bacterial Cellulose Production by Fed-Batch Fermentation in Molasses Medium. Biotechnol. Prog. 2004, 20, 1366–1371. [Google Scholar] [CrossRef]
- Shezad, O.; Khan, S.; Khan, T.; Park, J.K. Production of Bacterial Cellulose in Static Conditions by a Simple Fed-Batch Cultivation Strategy. Korean J. Chem. Eng. 2009, 26, 1689–1692. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, J.; Singh, R.P. Biotransformation of Sweet Lime Pulp Waste into High-Quality Nanocellulose with an Excellent Productivity Using Komagataeibacter Europaeus SGP37 under Static Intermittent Fed-Batch Cultivation. Bioresour. Technol. 2018, 247, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Matsutani, M.; Ito, K.; Azuma, Y.; Ogino, H.; Shirai, M.; Yakushi, T.; Matsushita, K. Adaptive Mutation Related to Cellulose Producibility in Komagataeibacter Medellinensis (Gluconacetobacter xylinus) NBRC 3288. Appl. Microbiol. Biotechnol. 2015, 99, 7229–7240. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent Advances in Bacterial Cellulose: A Low-Cost Effective Production Media, Optimization Strategies and Applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, C.; Wu, X.-Y.; Wei, Y.-Q.; Bo, T.; Han, P.-P.; Jia, S.-R. Metabolomic Profiling Coupled with Metabolic Network Reveals Differences in Gluconacetobacter xylinus from Static and Agitated Cultures. Biochem. Eng. J. 2015, 101, 85–98. [Google Scholar] [CrossRef]
- Zeng, M. Bacterial Cellulose: Fabrication, Characterization and Biocompatibility Studies; Universitat Autònoma de Barcelona, Ed.; Universitat Autònoma de Barcelona: Barcelona, Spain, 2014. [Google Scholar]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of Cell Growth and Crystalline Nanocellulose Production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Wu, S.-C.; Li, M.-H. Production of Bacterial Cellulose Membranes in a Modified Airlift Bioreactor by Gluconacetobacter xylinus. J. Biosci. Bioeng. 2015, 120, 444–449. [Google Scholar] [CrossRef]
- Chao, Y.; Ishida, T.; Sugano, Y.; Shoda, M. Bacterial Cellulose Production byAcetobacter Xylinum in a 50-L Internal-Loop Airlift Reactor. Biotechnol. Bioeng. 2000, 68, 345–352. [Google Scholar] [CrossRef]
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of Bacterial Cellulose Produced in a Pilot-Scale Spherical Type Bubble Column Bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140. [Google Scholar] [CrossRef]
- Song, H.-J.; Li, H.; Seo, J.-H.; Kim, M.-J.; Kim, S.-J. Pilot-Scale Production of Bacterial Cellulose by a Spherical Type Bubble Column Bioreactor Using Saccharified Food Wastes. Korean J. Chem. Eng. 2009, 26, 141–146. [Google Scholar] [CrossRef]
- Bungay, H.R., III; Serafica, G.C. Production of Microbial Cellulose Using a Rotating Disk Film Bioreactor. U.S. Patent No. 5,955,326, 21 September 1999. [Google Scholar]
- Kuure-Kinsey, M.; Weber, D.; Bungay, H.R.; Plawsky, J.L.; Bequette, B.Y. Modeling and Predictive Control of a Rotating Disk Bioreactor. In Proceedings of the 2005 American Control Conference, Portland, OR, USA, 8–10 June 2005; pp. 3259–3264. [Google Scholar]
- Mormino, R.; Bungay, H. Composites of Bacterial Cellulose and Paper Made with a Rotating Disk Bioreactor. Appl. Microbiol. Biotechnol. 2003, 62, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A review of water interactions, applications in composites, and water treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Barud, H.S.; Gutierrez, J.; Lustri, W.R.; Peres, M.F.S.; Ribeiro, S.J.L.; Saska, S.; Tercjak, A. Bacterial Cellulose. In Biomaterials from Nature for Advanced Devices and Therapies; Wiley: New York, NY, USA, 2016; pp. 384–399. [Google Scholar]
- Shi, Z.; Zang, S.; Jiang, F.; Huang, L.; Lu, D.; Ma, Y.; Yang, G. In Situ Nano-Assembly of Bacterial Cellulose–Polyaniline Composites. RSC Adv. 2012, 2, 1040–1046. [Google Scholar] [CrossRef]
- Gao, C.; Wan, Y.; Yang, C.; Dai, K.; Tang, T.; Luo, H.; Wang, J. Preparation and Characterization of Bacterial Cellulose Sponge with Hierarchical Pore Structure as Tissue Engineering Scaffold. J. Porous Mater. 2011, 18, 139–145. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Wang, S.; Huang, L.; Zhang, Y.; Tian, M.; Li, X.; Zhang, J. Isolation and Characterization of Bacterial Cellulose Produced from Soybean Whey and Soybean Hydrolyzate. Sci. Rep. 2023, 13, 16024. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of Bacterial Cellulose Composites: A Multipurpose Advanced Material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Donini, Í.A.N.; De Salvi, D.T.B.; Fukumoto, F.K.; Lustri, W.R.; Barud, H.d.S.; Marchetto, R.; Messaddeq, Y.; Ribeiro, S.J.L. Biossíntese e Recentes Avanços Na Produção de Celulose Bacteriana. Eclet. Quim. 2010, 35, 165–178. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M. Improving Bacterial Cellulose Films by Ex-Situ and in-Situ Modifications: A Review. Food Hydrocoll. 2021, 113, 106514. [Google Scholar] [CrossRef]
- Samyn, P.; Meftahi, A.; Geravand, S.A.; Heravi, M.E.M.; Najarzadeh, H.; Sabery, M.S.K.; Barhoum, A. Opportunities for bacterial nanocellulose in biomedical applications: Review on biosynthesis, modification and challenges. Int. J. Biol. Macromol. 2023, 231, 123316. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in Bacterial Cellulose Matrices for Biotechnological Applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Morales, L.O.; Johansson, L.-S.; Hoeger, I.C.; Filpponen, I.; Castro, C.; Rojas, O.J.; Laine, J. Affibody Conjugation onto Bacterial Cellulose Tubes and Bioseparation of Human Serum Albumin. RSC Adv. 2014, 4, 51440–51450. [Google Scholar] [CrossRef]
- Butchosa, N.; Brown, C.; Larsson, P.T.; Berglund, L.A.; Bulone, V.; Zhou, Q. Nanocomposites of Bacterial Cellulose Nanofibers and Chitin Nanocrystals: Fabrication, Characterization and Bactericidal Activity. Green Chem. 2013, 15, 3404. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous Bacterial Cellulose as a Potential Scaffold for Bone Regeneration. Acta. Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Barud, H.S.; Gaspar, A.M.M.; Marchetto, R.; Ribeiro, S.J.L.; Messaddeq, Y. Bacterial Cellulose-Hydroxyapatite Nanocomposites for Bone Regeneration. Int. J. Biomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Lv, X.; Chen, S.; Li, Z.; Feng, C.; Wang, H.; Xu, Y. In Situ Fabrication of a Microporous Bacterial Cellulose/Potato Starch Composite Scaffold with Enhanced Cell Compatibility. Cellulose 2014, 21, 1823–1835. [Google Scholar] [CrossRef]
- Meftahi, A.; Khajavi, R.; Rashidi, A.; Sattari, M.; Yazdanshenas, M.E.; Torabi, M. The Effects of Cotton Gauze Coating with Microbial Cellulose. Cellulose 2010, 17, 199–204. [Google Scholar] [CrossRef]
- Saibuatong, O.; Phisalaphong, M. Novo Aloe Vera–Bacterial Cellulose Composite Film from Biosynthesis. Carbohydr. Polym. 2010, 79, 455–460. [Google Scholar] [CrossRef]
- Gea, S.; Bilotti, E.; Reynolds, C.T.; Soykeabkeaw, N.; Peijs, T. Bacterial Cellulose–Poly(Vinyl Alcohol) Nanocomposites Prepared by an in-Situ Process. Mater. Lett. 2010, 64, 901–904. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A. In Situ Produced Bacterial Cellulose Nanofiber-Based Hybrids for Nanocomposites. Fibers 2017, 5, 31. [Google Scholar] [CrossRef]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. In Situ Modifications to Bacterial Cellulose with the Water Insoluble Polymer Poly-3-Hydroxybutyrate. Carbohydr. Polym. 2013, 92, 1717–1723. [Google Scholar] [CrossRef]
- Figueiredo, A.R.P.; Silvestre, A.J.D.; Neto, C.P.; Freire, C.S.R. In Situ Synthesis of Bacterial Cellulose/Polycaprolactone Blends for Hot Pressing Nanocomposite Films Production. Carbohydr. Polym. 2015, 132, 400–408. [Google Scholar] [CrossRef]
- Higashi, K.; Miki, N. Hydrogel Fiber Cultivation Method for Forming Bacterial Cellulose Microspheres. Micromachines 2018, 9, 36. [Google Scholar] [CrossRef]
- Sano, M.B.; Rojas, A.D.; Gatenholm, P.; Davalos, R.V. Electromagnetically Controlled Biological Assembly of Aligned Bacterial Cellulose Nanofibers. Ann. Biomed. Eng. 2010, 38, 2475–2484. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, C.; Zheng, X.; Ye, L.; Wan, T.; Jia, S.R. Oriented Bacterial Cellulose-Glass Fiber Nanocomposites with Enhanced Tensile Strength through Electric Field. Fibers Polym. 2017, 18, 1408–1412. [Google Scholar] [CrossRef]
- Rahman, M.M.; Netravali, A.N. High-Performance Green Nanocomposites Using Aligned Bacterial Cellulose and Soy Protein. Compos. Sci. Technol. 2017, 146, 183–190. [Google Scholar] [CrossRef]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D Printing of Bacteria into Functional Complex Materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of Nano Bacterial Cellulose from Waste Water of Candied Jujube-Processing Industry Using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired Antimicrobial and Biocompatible Bacterial Cellulose Membranes Obtained by Surface Functionalization with Aminoalkyl Groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Huang, L.-H.; Zhao, X.-Q.; Li, W.-C.; Wang, Y.-Y.; Jia, S.-R.; Zhong, C. Bacterial Cellulose and Its Potential for Biomedical Applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of Bacterial Cellulose as Precursor of Carbon and Composites with Metal Oxide, Metal Sulfide and Metal Nanoparticles: A Review of Recent Advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Bacterial Cellulose Nanocrystals (BCNC) Preparation and Characterization from Three Bacterial Cellulose Sources and Development of Functionalized BCNCs as Nucleic Acid Delivery Systems. ACS Appl. Nano Mater. 2018, 1, 209–221. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Handa, K.; Nakatsubo, F.; Yano, H. Surface Modification of Bacterial Cellulose Nanofibers for Property Enhancement of Optically Transparent Composites: Dependence on Acetyl-Group DS. Biomacromolecules 2007, 8, 1973–1978. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Q.; Peng, B.; Pei, C. A Novel Thermotropic Liquid Crystalline–Benzoylated Bacterial Cellulose. Carbohydr. Polym. 2008, 74, 875–879. [Google Scholar] [CrossRef]
- Yin, X.; Yu, C.; Zhang, X.; Yang, J.; Lin, Q.; Wang, J.; Zhu, Q. Comparison of Succinylation Methods for Bacterial Cellulose and Adsorption Capacities of Bacterial Cellulose Derivatives for Cu2+ Ion. Polym. Bull. 2011, 67, 401–412. [Google Scholar] [CrossRef]
- Oshima, T.; Kondo, K.; Ohto, K.; Inoue, K.; Baba, Y. Preparation of Phosphorylated Bacterial Cellulose as an Adsorbent for Metal Ions. React. Funct. Polym. 2008, 68, 376–383. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Sen, S.; Martin, J.D.; Argyropoulos, D.S. Review of Cellulose Non-Derivatizing Solvent Interactions with Emphasis on Activity in Inorganic Molten Salt Hydrates. ACS Sustain. Chem. Eng. 2013, 1, 858–870. [Google Scholar] [CrossRef]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.A.; Koch, W. Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The Nanofication and Functionalization of Bacterial Cellulose and Its Applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. Nanocellulose and Sustainability; Sustainability Contributions through Science and Technology Series; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351262927. [Google Scholar]
- Cazón, P.; Vázquez, M.; Velazquez, G. Composite Films with UV-Barrier Properties of Bacterial Cellulose with Glycerol and Poly(Vinyl Alcohol): Puncture Properties, Solubility, and Swelling Degree. Biomacromolecules 2019, 20, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kim, J. Bacterial Cellulose/Poly(Ethylene Glycol) Composite: Characterization and First Evaluation of Biocompatibility. Cellulose 2010, 17, 83–91. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, C.; Zheng, Y.; Xie, Y.; He, W.; Wang, Y.; Qiao, K.; Yue, L. The Effects of Two Biocompatible Plasticizers on the Performance of Dry Bacterial Cellulose Membrane: A Comparative Study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X.L. Mechanical, Moisture Absorption, and Biodegradation Behaviours of Bacterial Cellulose Fibre-Reinforced Starch Biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Barud, H.S.; Ribeiro, S.J.L.; Carone, C.L.P.; Ligabue, R.; Einloft, S.; Queiroz, P.V.S.; Borges, A.P.B.; Jahno, V.D. Optically Transparent Membrane Based on Bacterial Cellulose/Polycaprolactone. Polímeros 2013, 23, 135–142. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial Cellulose/Poly(3-Hydroxybutyrate) Composite Membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y. Optical Nanocomposites Prepared by Incorporating Bacterial Cellulose Nanofibrils into Poly(3-Hydroxybutyrate). Mater. Lett. 2011, 65, 182–184. [Google Scholar] [CrossRef]
- Oliveira Barud, H.G.; Barud, H.d.S.; Cavicchioli, M.; do Amaral, T.S.; Junior, O.B.d.O.; Santos, D.M.; Petersen, A.L.d.O.A.; Celes, F.; Borges, V.M.; de Oliveira, C.I.; et al. Preparation and Characterization of a Bacterial Cellulose/Silk Fibroin Sponge Scaffold for Tissue Regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.; Hong, F. Preparation and Evaluation of a Kind of Bacterial Cellulose Dry Films with Antibacterial Properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Mihaela Jipa, I.; Dobre, L.; Stroescu, M.; Stoica-Guzun, A.; Jinga, S.; Dobre, T. Preparation and Characterization of Bacterial Cellulose-Poly(Vinyl Alcohol) Films with Antimicrobial Properties. Mater. Lett. 2012, 66, 125–127. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Yang, H.; Tang, W.; Jia, Y.; Tan, Z. Characterization of Bacteriostatic Sausage Casing: A Composite of Bacterial Cellulose Embedded with ɛ-Polylysine. Food Sci. Biotechnol. 2010, 19, 1479–1484. [Google Scholar] [CrossRef]
- Gao, C.; Yan, T.; Du, J.; He, F.; Luo, H.; Wan, Y. Introduction of Broad Spectrum Antibacterial Properties to Bacterial Cellulose Nanofibers via Immobilising ε-Polylysine Nanocoatings. Food Hydrocoll. 2014, 36, 204–211. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of Silver Sulfadiazine Loaded Bacterial Cellulose/Sodium Alginate Composite Films with Enhanced Antibacterial Property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Yang, G.; Yao, Y.; Wang, C. Green Synthesis of Silver Nanoparticles Impregnated Bacterial Cellulose-Alginate Composite Film with Improved Properties. Mater. Lett. 2017, 209, 11–14. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Chau, C.-F.; Yang, P.; Yu, C.-M.; Yen, G.-C. Investigation on the Lipid- and Cholesterol-Lowering Abilities of Biocellulose. J. Agric. Food Chem. 2008, 56, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Dourado, F.; Gama, M.; Rodrigues, A.C. A Review on the Toxicology and Dietetic Role of Bacterial Cellulose. Toxicol. Rep. 2017, 4, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Effects of Dietary Fiber Supplementation on Fatty Acid Metabolism and Intestinal Microbiota Diversity in C57BL/6J Mice Fed with a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 12706–12718. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Enhanced Anti-Obesity Effects of Bacterial Cellulose Combined with Konjac Glucomannan in High-Fat Diet-Fed C57BL/6J Mice. Food Funct. 2018, 9, 5260–5272. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, D.; Zhao, Y.; Yang, X. Bacterial Cellulose Relieves Diphenoxylate-Induced Constipation in Rats. J. Agric. Food Chem. 2018, 66, 4106–4117. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, A.M.A.; Herrera Carrera, S.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. Bioresources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Phisalaphong, M.; Chiaoprakobkij, N. Applications and Products—Nata de Coco. In Bacterial NanoCellulose; CRC Press: Boca Raton, FL, USA, 2016; pp. 143–155. [Google Scholar]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of Physicochemical Properties and Antioxidant Activities of Kombucha “Tea Fungus” during Extended Periods of Fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Okiyama, A.; Motoki, M.; Yamanaka, S. Bacterial Cellulose IV. Application to Processed Foods. Food Hydrocoll. 1993, 6, 503–511. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent Advances in Bacterial Cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Gray, D.G. Bactericidal Paper Impregnated with Silver Nanoparticles for Point-of-Use Water Treatment. Environ. Sci. Technol. 2011, 45, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Medronho, B.; Alves, L.; Norgren, M.; Nordenskiöld, L. Hydrophobic Interactions Control the Self-Assembly of DNA and Cellulose. Q. Rev. Biophys. 2021, 54, e3. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of Bacterial Cellulose and Bacterial Cellulose Nanocrystals for Their Applications in the Stabilization of Olive Oil Pickering Emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Swazey, J.M. Surfactant Thickened Systems Comprising Microfibrous Cellulose and Methods of Making Same. U.S. Patent No. 9,045,716, 2 June 2014. [Google Scholar]
- Płoska, J.; Garbowska, M.; Pluta, A.; Stasiak-Różańska, L. Bacterial cellulose-innovative biopolymer and possibilities of its applications in dairy industry. Int. Dairy J. 2023, 140, 105586. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of Bacterial Cellulose Addition on Physical Properties of WPI Emulsions. Comparison with Common Thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-Bacterial Cellulose/Soy Protein Isolate Complex Gel as Fat Substitutes in Ice Cream Model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Leger, A.; Barey, P.; Langendorff, V.; Cansell, M. Influence of Formulation on the Structural Networks in Ice Cream. Int. Dairy J. 2005, 15, 255–262. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Lin, K.W.; Lin, H.Y. Quality Characteristics of Chinese-style Meatball Containing Bacterial Cellulose (Nata). J. Food Sci. 2004, 69, SNQ107–SNQ111. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Peitler, D.; Rakoczy, R.; Żywicka, A. Survival of Probiotic Lactic Acid Bacteria Immobilized in Different Forms of Bacterial Cellulose in Simulated Gastric Juices and Bile Salt Solution. LWT-Food Sci. Technol. 2016, 68, 322–328. [Google Scholar] [CrossRef]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; Gama, F.M.P.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M.C. Bacterial Cellulose/Cashew Gum Films as Probiotic Carriers. LWT 2020, 130, 109699. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated Synthesis of Gold Nanoparticle–Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Zhang, D.; Jiang, J.; Cui, Y.; Qiu, S.; Zhou, Y.; Zhang, X. Fabrication of Bienzymatic Glucose Biosensor Based on Novel Gold Nanoparticles-Bacteria Cellulose Nanofibers Nanocomposite. Electroanalysis 2010, 22, 2543–2550. [Google Scholar] [CrossRef]
- Lv, P.; Feng, Q.; Wang, Q.; Li, G.; Li, D.; Wei, Q. Biosynthesis of Bacterial Cellulose/Carboxylic Multi-Walled Carbon Nanotubes for Enzymatic Biofuel Cell Application. Materials 2016, 9, 183. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Ton, N.M.N.; Le, V.V.M. Optimization of Saccharomyces Cerevisiae immobilization in Bacterial Cellulose by ‘Adsorption-Incubation’ Method. Int. Food Res. J. 2009, 16, 59–64. [Google Scholar]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial Cellulose in Food Industry: Current Research and Future Prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Patanè, G.T.; Corrado, I.; Giosafatto, C.V.L.; Ginestra, G.; Nostro, A.; Foti, A.; Gucciardi, P.G.; Mandalari, G.; Barreca, D.; et al. Functionalization of Polyhydroxyalkanoates (PHA)-Based Bioplastic with Phloretin for Active Food Packaging: Characterization of Its Mechanical, Antioxidant, and Antimicrobial Activities. Int. J. Mol. Sci. 2023, 24, 11628. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Mariniello, L.; Di Pierro, P.; Sorrentino, A.; Giosafatto, C.V.L. Transglutaminase Crosslinked Pectin- and Chitosan-Based Edible Films: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 223–238. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, S.; Su, J.; Zhu, M.; Zhou, M.; Chen, T.; Han, Y. Recent Advances in Carrageenan-Based Films for Food Packaging Applications. Front. Nutr. 2022, 9, 1004588. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent Trends in the Use of Pectin from Agro-Waste Residues as a Natural-Based Biopolymer for Food Packaging Applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Wang, X.; Guo, C.; Hao, W.; Ullah, N.; Chen, L.; Li, Z.; Feng, X. Development and Characterization of Agar-Based Edible Films Reinforced with Nano-Bacterial Cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef]
- Tomé, L.C.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and Characterization of Bacterial Cellulose Membranes with Tailored Surface and Barrier Properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Dobre, L.-M.; Stoica-Guzun, A.; Stroescu, M.; Jipa, I.; Dobre, T.; Ferdeş, M.; Ciumpiliac, Ş. Modelling of Sorbic Acid Diffusion through Bacterial Cellulose-Based Antimicrobial Films. Chem. Pap. 2012, 66, 144–151. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Li, W.; Yang, X. Improved Characterization of Nanofibers from Bacterial Cellulose and Its Potential Application in Fresh-Cut Apples. Int. J. Biol. Macromol. 2020, 149, 178–186. [Google Scholar] [CrossRef]
- Shahmohammadi Jebel, F.; Almasi, H. Morphological, Physical, Antimicrobial and Release Properties of ZnO Nanoparticles-Loaded Bacterial Cellulose Films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Jozala, A.F.; Pessoa, A., Jr.; Vila, M.M.D.C.; Balcão, V.M.; Franco, B.D.G.M. Immobilization of Antimicrobial Peptides from Lactobacillus Sakei Subsp. Sakei 2a in Bacterial Cellulose: Structural and Functional Stabilization. Food Packag. Shelf Life 2018, 17, 25–29. [Google Scholar] [CrossRef]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; Rebelo, M.d.A.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial Nanocellulose Membranes Combined with Nisin: A Strategy to Prevent Microbial Growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug Release and Antioxidant/Antibacterial Activities of Silymarin-Zein Nanoparticle/Bacterial Cellulose Nanofiber Composite Films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef]
- Moradian, S.; Almasi, H.; Moini, S. Development of Bacterial Cellulose-Based Active Membranes Containing Herbal Extracts for Shelf Life Extension of Button Mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2018, 42, e13537. [Google Scholar] [CrossRef]
- Chen, L.; Zou, M.; Hong, F.F. Evaluation of Fungal Laccase Immobilized on Natural Nanostructured Bacterial Cellulose. Front. Microbiol. 2015, 6, 1245. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Nanoreinforced Bacterial Cellulose–Montmorillonite Composites for Biomedical Applications. Carbohydr. Polym. 2012, 89, 1189–1197. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Saha, P. Bacterial Cellulose Based Greener Packaging Material: A Bioadhesive Polymeric Film. Mater. Res. Express 2018, 5, 115405. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Mouro, C.; Riool, M.; Gouveia, I.C. Antimicrobial Food Packaging Based on Prodigiosin-Incorporated Double-Layered Bacterial Cellulose and Chitosan Composites. Polymers 2022, 14, 315. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and Evaluation of Chitosan Based Active Nanocomposite Films Containing Bacterial Cellulose Nanocrystals and Silver Nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the Barrier Properties of Thermoplastic Corn Starch-Based Films Containing Bacterial Cellulose Nanowhiskers by Means of PHA Electrospun Coatings of Interest in Food Packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef]
- George, J.; Ramana, K.V.; Bawa, A.S. Siddaramaiah Bacterial Cellulose Nanocrystals Exhibiting High Thermal Stability and Their Polymer Nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Dayal, M.S.; Catchmark, J.M. Mechanical and Structural Property Analysis of Bacterial Cellulose Composites. Carbohydr. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef] [PubMed]
- de Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; dos Santos, A.M.; Ribeiro, S.J.L.; Barud, H.S. Effect of in Situ Modification of Bacterial Cellulose with Carboxymethylcellulose on Its Nano/Microstructure and Methotrexate Release Properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and Properties of Nanofibrous Bacterial Cellulose-Containing Polymer Composites: A Review of Recent Advances for Biomedical Applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in Biomedical and Pharmaceutical Applications of Functional Bacterial Cellulose-Based Nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Jadczak, K.; Ochędzan-Siodłak, W. Bacterial cellulose: Biopolymer with novel medical applications. J. Biomater. Appl. 2023, 38, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Ahmed, J.; Gultekinoglu, M.; Edirisinghe, M. Bacterial Cellulose Micro-Nano Fibres for Wound Healing Applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Wicher, R.; Paleczny, J.; Brożyna, M.; Junka, A.; Bartoszewicz, M. Modifications of Bacterial Cellulose in Wound Care. Polym. Med. 2021, 51, 77–84. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter Cellulose Pellicle as a Temporary Skin Substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A Multipurpose Natural and Renewable Polymer in Medical Applications: Bacterial Cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.Z.; Luo, H.L.; Gao, C.; Huang, Y. Immobilization of Gelatin on Bacterial Cellulose Nanofibers Surface via Crosslinking Technique. Mater. Sci. Eng. C 2012, 32, 536–541. [Google Scholar] [CrossRef]
- Moraes, P.R.F.d.S.; Saska, S.; Barud, H.; Lima, L.R.d.; Martins, V.d.C.A.; Plepis, A.M.d.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef]
- Wanna, D.; Alam, C.; Toivola, D.M.; Alam, P. Bacterial Cellulose–Kaolin Nanocomposites for Application as Biomedical Wound Healing Materials. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045002. [Google Scholar] [CrossRef]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In Vivo Evaluation of Bacterial Cellulose/Acrylic Acid Wound Dressing Hydrogel Containing Keratinocytes and Fibroblasts for Burn Wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wang, S.; Wu, J.; Huang, M.; Min, H.; Liu, X. Controlled Release and Antibacterial Activity of Tetracycline Hydrochloride-Loaded Bacterial Cellulose Composite Membranes. Carbohydr. Polym. 2016, 145, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Laçin, N.T. Development of Biodegradable Antibacterial Cellulose Based Hydrogel Membranes for Wound Healing. Int. J. Biol. Macromol. 2014, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of Gelatin/Bacterial Cellulose Composite Sponges as Potential Natural Wound Dressings. Int. J. Biol. Macromol. 2019, 133, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Duan, Y.-X.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Cui, J.-D.; Zhong, C. A Facile Construction of Bacterial Cellulose/ZnO Nanocomposite Films and Their Photocatalytic and Antibacterial Properties. Int. J. Biol. Macromol. 2019, 132, 692–700. [Google Scholar] [CrossRef]
- Wahid, F.; Yin, J.-J.; Xue, D.-D.; Xue, H.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Synthesis and Characterization of Antibacterial Carboxymethyl Chitosan/ZnO Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2016, 88, 273–279. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable Synthesis of Robust and Stretchable Composite Wound Dressings by Dispersing Silver Nanowires in Continuous Bacterial Cellulose. Compos. B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In Situ Synthesis of Silver-Nanoparticles/Bacterial Cellulose Composites for Slow-Released Antimicrobial Wound Dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Wahid, F.; Wang, H.-S.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Preparation, Characterization and Antibacterial Applications of Carboxymethyl Chitosan/CuO Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2017, 101, 690–695. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial Cellulose-Zinc Oxide Nanocomposites as a Novel Dressing System for Burn Wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef]
- Jalili Tabaii, M.; Emtiazi, G. Transparent Nontoxic Antibacterial Wound Dressing Based on Silver Nano Particle/Bacterial Cellulose Nano Composite Synthesized in the Presence of Tripolyphosphate. J. Drug Deliv. Sci. Technol. 2018, 44, 244–253. [Google Scholar] [CrossRef]
- Bogdanović, U.; Lazić, V.; Vodnik, V.; Budimir, M.; Marković, Z.; Dimitrijević, S. Copper Nanoparticles with High Antimicrobial Activity. Mater. Lett. 2014, 128, 75–78. [Google Scholar] [CrossRef]
- He, W.; Huang, X.; Zheng, Y.; Sun, Y.; Xie, Y.; Wang, Y.; Yue, L. In Situ Synthesis of Bacterial Cellulose/Copper Nanoparticles Composite Membranes with Long-Term Antibacterial Property. J. Biomater. Sci. Polym. Ed. 2018, 29, 2137–2153. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol Enriched Bacterial Cellulose Hydrogel as Effective Material for Third Degree Burn Wound Repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical Antimicrobial Therapy for Treating Chronic Wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, H.; Liu, X.; Sun, H.; Wang, S.; Zhang, R. pH-Responsive Release Behavior and Anti-Bacterial Activity of Bacterial Cellulose-Silver Nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of Bacterial Cellulose Experimental Dressings Saturated with Gentamycin for Management of Bone Biofilm in Vitro and Ex Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhijiang, C.; Guang, Y. Bacterial Cellulose/Collagen Composite: Characterization and First Evaluation of Cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar] [CrossRef]
- Zheng, Y.; Wen, X.; Wu, J.; Wang, L.-N.; Yuan, Z.; Peng, J.; Meng, H. Immobilization of Collagen Peptide on Dialdehyde Bacterial Cellulose Nanofibers via Covalent Bonds for Tissue Engineering and Regeneration. Int. J. Nanomed. 2015, ume 10, 4623. [Google Scholar] [CrossRef]
- Kirdponpattara, S.; Phisalaphong, M.; Kongruang, S. Gelatin-Bacterial Cellulose Composite Sponges Thermally Cross-Linked with Glucose for Tissue Engineering Applications. Carbohydr. Polym. 2017, 177, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Amelia, S.T.W.; Adiningsih, S.N.; Widiyastuti, W.; Nurtono, T.; Setyawan, H.; Panatarani, C.; Praseptiangga, D.; Nazir, N.; Syamani, F.A. Novel cross-linking of toxic-free biopolymers for cellulose-gelatin films from avocado seed waste. Bioresour. Technol. Rep. 2024, 25, 101725. [Google Scholar] [CrossRef]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of Antibacterial Bacterial Cellulose Composite Membranes Modified with Chitosan or Chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk Sericin-Functionalized Bacterial Cellulose as a Potential Wound-Healing Biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a Biomaterial. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–113. [Google Scholar]
- Wiegand, C.; Elsner, P.; Hipler, U.-C.; Klemm, D. Protease and ROS Activities Influenced by a Composite of Bacterial Cellulose and Collagen Type I in Vitro. Cellulose 2006, 13, 689–696. [Google Scholar] [CrossRef]
- Oliveira, M.H.; Pinto, F.C.M.; Ferraz-Carvalho, R.S.; Albuquerque, A.V.; Aguiar, J.L. BIO-NAIL: A Bacterial Cellulose Dressing as a New Alternative to Preserve the Nail Bed after Avulsion. J. Mater. Sci. Mater. Med. 2020, 31, 121. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Kawecki, M.; Wysota, K.; Sakiel, S.; Wróblewski, P.; Glik, J.; Nowak, M.; Bielecki, S. Biomedical Applications of Microbial Cellulose in Burn Wound Recovery. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 307–321. [Google Scholar]
- Jankau, J.; Błażyńska-Spychalska, A.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Pankiewicz, T.; Ludwicka, K.; Dettlaff, A.; Pęksa, R. Bacterial Cellulose Properties Fulfilling Requirements for a Biomaterial of Choice in Reconstructive Surgery and Wound Healing. Front. Bioeng. Biotechnol. 2022, 9, 805053. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A Review of Bacterial Cellulose-Based Drug Delivery Systems: Their Biochemistry, Current Approaches and Future Prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical Caffeine Delivery Using Biocellulose Membranes: A Potential Innovative System for Cellulite Treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef]
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial Cellulose Membranes Applied in Topical and Transdermal Delivery of Lidocaine Hydrochloride and Ibuprofen: In Vitro Diffusion Studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef]
- Weyell, P.; Beekmann, U.; Küpper, C.; Dederichs, M.; Thamm, J.; Fischer, D.; Kralisch, D. Tailor-Made Material Characteristics of Bacterial Cellulose for Drug Delivery Applications in Dentistry. Carbohydr. Polym. 2019, 207, 1–10. [Google Scholar] [CrossRef]
- Inoue, B.S.; Streit, S.; dos Santos Schneider, A.L.; Meier, M.M. Bioactive Bacterial Cellulose Membrane with Prolonged Release of Chlorhexidine for Dental Medical Application. Int. J. Biol. Macromol. 2020, 148, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Badshah, M.; Mäkilä, E.; Salonen, J.; Shahbazi, M.-A.; Santos, H.A.; Khan, T. Fabrication, Characterization and Evaluation of Bacterial Cellulose-Based Capsule Shells for Oral Drug Delivery. Cellulose 2017, 24, 1445–1454. [Google Scholar] [CrossRef]
- Meneguin, A.B.; da Silva Barud, H.; Sábio, R.M.; de Sousa, P.Z.; Manieri, K.F.; de Freitas, L.A.P.; Pacheco, G.; Alonso, J.D.; Chorilli, M. Spray-Dried Bacterial Cellulose Nanofibers: A New Generation of Pharmaceutical Excipient Intended for Intestinal Drug Delivery. Carbohydr. Polym. 2020, 249, 116838. [Google Scholar] [CrossRef]
- Badshah, M.; Ullah, H.; Khan, S.A.; Park, J.K.; Khan, T. Preparation, Characterization and in-Vitro Evaluation of Bacterial Cellulose Matrices for Oral Drug Delivery. Cellulose 2017, 24, 5041–5052. [Google Scholar] [CrossRef]
- Badshah, M.; Ullah, H.; Khan, A.R.; Khan, S.; Park, J.K.; Khan, T. Surface Modification and Evaluation of Bacterial Cellulose for Drug Delivery. Int. J. Biol. Macromol. 2018, 113, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Liang, S. Advances in drug delivery applications of modified bacterial cellulose-based materials. Front. Bioeng. Biotechnol. 2023, 11, 1252706. [Google Scholar] [CrossRef]
- Lin, Z.; Guan, Z.; Huang, Z. New Bacterial Cellulose/Polyaniline Nanocomposite Film with One Conductive Side through Constrained Interfacial Polymerization. Ind. Eng. Chem. Res. 2013, 52, 2869–2874. [Google Scholar] [CrossRef]
- Chung, C.K.; Beekmann, U.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Bacterial Cellulose as Drug Delivery System for Optimizing Release of Immune Checkpoint Blocking Antibodies. Pharmaceutics 2022, 14, 1351. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Ho Kim, J.; Hossain, M.d.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.-W. 3D Network of Cellulose-Based Energy Storage Devices and Related Emerging Applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial Cellulose-Based Scaffold Materials for Bone Tissue Engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Dorati, R.; DeTrizio, A.; Modena, T.; Conti, B.; Benazzo, F.; Gastaldi, G.; Genta, I. Biodegradable Scaffolds for Bone Regeneration Combined with Drug-Delivery Systems in Osteomyelitis Therapy. Pharmaceuticals 2017, 10, 96. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Hong, L.; Jia, S.R.; Huang, Y.; Zhu, Y.; Wang, Y.L.; Jiang, H.J. Synthesis and Characterization of Hydroxyapatite-Bacterial Cellulose Nanocomposites. Compos. Sci. Technol. 2006, 66, 1825–1832. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D Printed Polycaprolactone/Gelatin/Bacterial Cellulose/Hydroxyapatite Composite Scaffold for Bone Tissue Engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.T.; Luz, É.P.C.G.; Andrade, F.K.; Rosa, M.d.F.; Borges, M.d.F.; Arcanjo, M.R.A.; Vieira, R.S. Advances in Bacterial Cellulose/Strontium Apatite Composites for Bone Applications. Polym. Rev. 2021, 61, 736–764. [Google Scholar] [CrossRef]
- Mori, R.; Nakai, T.; Enomoto, K.; Uchio, Y.; Yoshino, K. Increased Antibiotic Release from a Bone Cement Containing Bacterial Cellulose. Clin. Orthop. Relat. Res. 2011, 469, 600–606. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, R.; Chen, H.; Lu, Y.; Zhou, J.; Chang, X.; Qiu, G.; Wu, Z.; Yang, G. Investigation on Artificial Blood Vessels Prepared from Bacterial Cellulose. Mater. Sci. Eng. C 2015, 46, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, Y.S. The Role of Bacterial Cellulose in Artificial Blood Vessels. Mol. Cell Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, W.; Cui, L.; Cao, Y. Tissue Engineering of Blood Vessel. J. Cell Mol. Med. 2007, 11, 945–957. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Bouhlouli, M.; Pourhadi, M.; Karami, F.; Talebi, Z.; Ranjbari, J.; Khojasteh, A. Applications of Bacterial Cellulose as a Natural Polymer in Tissue Engineering. ASAIO J. 2021, 67, 709–720. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial Synthesized Cellulose—Artificial Blood Vessels for Microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of Cultivation Conditions on Mechanical and Morphological Properties of Bacterial Cellulose Tubes. Biotechnol. Bioeng. 2007, 97, 425–434. [Google Scholar] [CrossRef]
- Zamani, M.; Khafaji, M.; Naji, M.; Vossoughi, M.; Alemzadeh, I.; Haghighipour, N. A Biomimetic Heparinized Composite Silk-Based Vascular Scaffold with Sustained Antithrombogenicity. Sci. Rep. 2017, 7, 4455. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD Modified Polymers: Biomaterials for Stimulated Cell Adhesion and Beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface Modification and Endothelialization of Biomaterials as Potential Scaffolds for Vascular Tissue Engineering Applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Gao, C.; Han, M.; Liang, H.; Ren, K.; Wang, Y.; Luo, H. Preparation and Characterization of Bacterial Cellulose/Heparin Hybrid Nanofiber for Potential Vascular Tissue Engineering Scaffolds. Polym. Adv. Technol. 2011, 22, 2643–2648. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Chen, S.; Li, Z.; Yao, J.; Peng, X.; Feng, C.; Xu, Y.; Wang, H. Use of Heparinized Bacterial Cellulose Based Scaffold for Improving Angiogenesis in Tissue Regeneration. Carbohydr. Polym. 2018, 181, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.d.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Passuello, A.C.B.; Oliveira, A.F.; Costa, E.B.; Kirchheim, A.P. Application of Life Cycle Assessment in the Evaluation of the Environmental Impacts of Innovative Construction Materials: Case Study of the Carbon Footprint of Alternative Clinkers. Ambiente Construído 2014, 14, 7–20. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Life Cycle Assessment (LCA)–Principles and Guidelines. In Waste Management for the Food Industries; Academic Press: Cambridge, MA, USA, 2008; Volume 14040. [Google Scholar]
- Kamal, T.; Ul-Islam, M.; Fatima, A.; Ullah, M.W.; Manan, S. Cost-Effective Synthesis of Bacterial Cellulose and Its Applications in the Food and Environmental Sectors. Gels 2022, 8, 552. [Google Scholar] [CrossRef]
- Basu, A.; Vadanan, S.V.; Lim, S. A Novel Platform for Evaluating the Environmental Impacts on Bacterial Cellulose Production. Sci. Rep. 2018, 8, 5780. [Google Scholar] [CrossRef]
- Mehrotra, R.; Sharma, S.; Shree, N.; Kaur, K. Bacterial Cellulose: An Ecological Alternative as A Biotextile. Biosci. Biotechnol. Res. Asia 2023, 20, 449–463. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayah, I.; Gervasi, C.; Achour, S.; Gervasi, T. Fermentation Techniques and Biotechnological Applications of Modified Bacterial Cellulose: An Up-to-Date Overview. Fermentation 2024, 10, 100. https://doi.org/10.3390/fermentation10020100
Sayah I, Gervasi C, Achour S, Gervasi T. Fermentation Techniques and Biotechnological Applications of Modified Bacterial Cellulose: An Up-to-Date Overview. Fermentation. 2024; 10(2):100. https://doi.org/10.3390/fermentation10020100
Chicago/Turabian StyleSayah, Islam, Claudio Gervasi, Sami Achour, and Teresa Gervasi. 2024. "Fermentation Techniques and Biotechnological Applications of Modified Bacterial Cellulose: An Up-to-Date Overview" Fermentation 10, no. 2: 100. https://doi.org/10.3390/fermentation10020100
APA StyleSayah, I., Gervasi, C., Achour, S., & Gervasi, T. (2024). Fermentation Techniques and Biotechnological Applications of Modified Bacterial Cellulose: An Up-to-Date Overview. Fermentation, 10(2), 100. https://doi.org/10.3390/fermentation10020100







