Optogenetic Fine-Tuning of Sus scrofa Basic Fibroblast Growth Factor Expression in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Media and Culture Conditions
2.3. Construction of Expression Strains
2.4. Blue Light-Induced Expression System
2.5. Protein Expression, Purification and Analysis
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Genetic Optimization of Engineered bFGF-Producing Strain
2.8. Optimization of Blue Light-Induced bFGF Fermentation
3. Results and Discussion
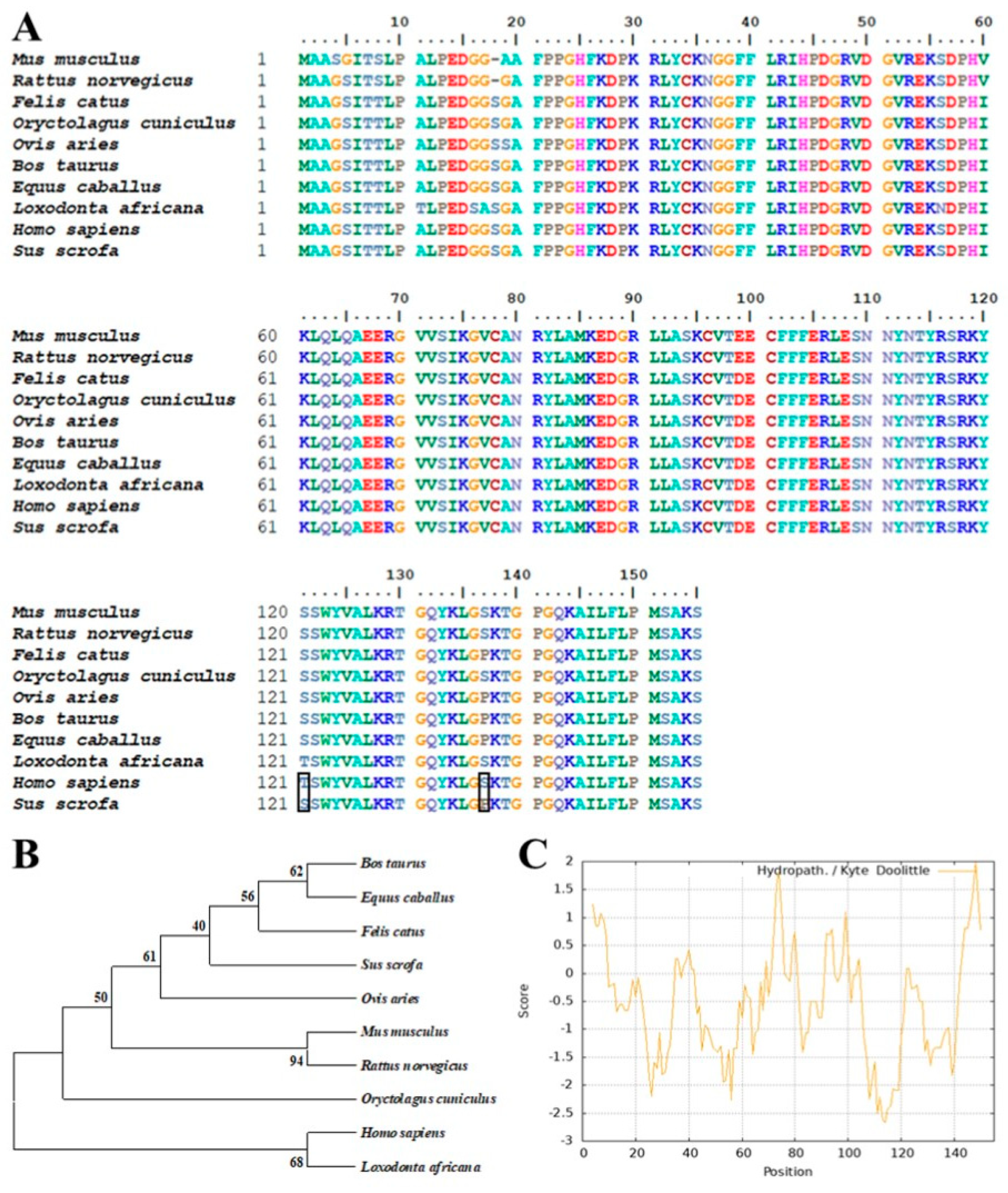
3.1. Bioinformatics Analysis of Porcine bFGF
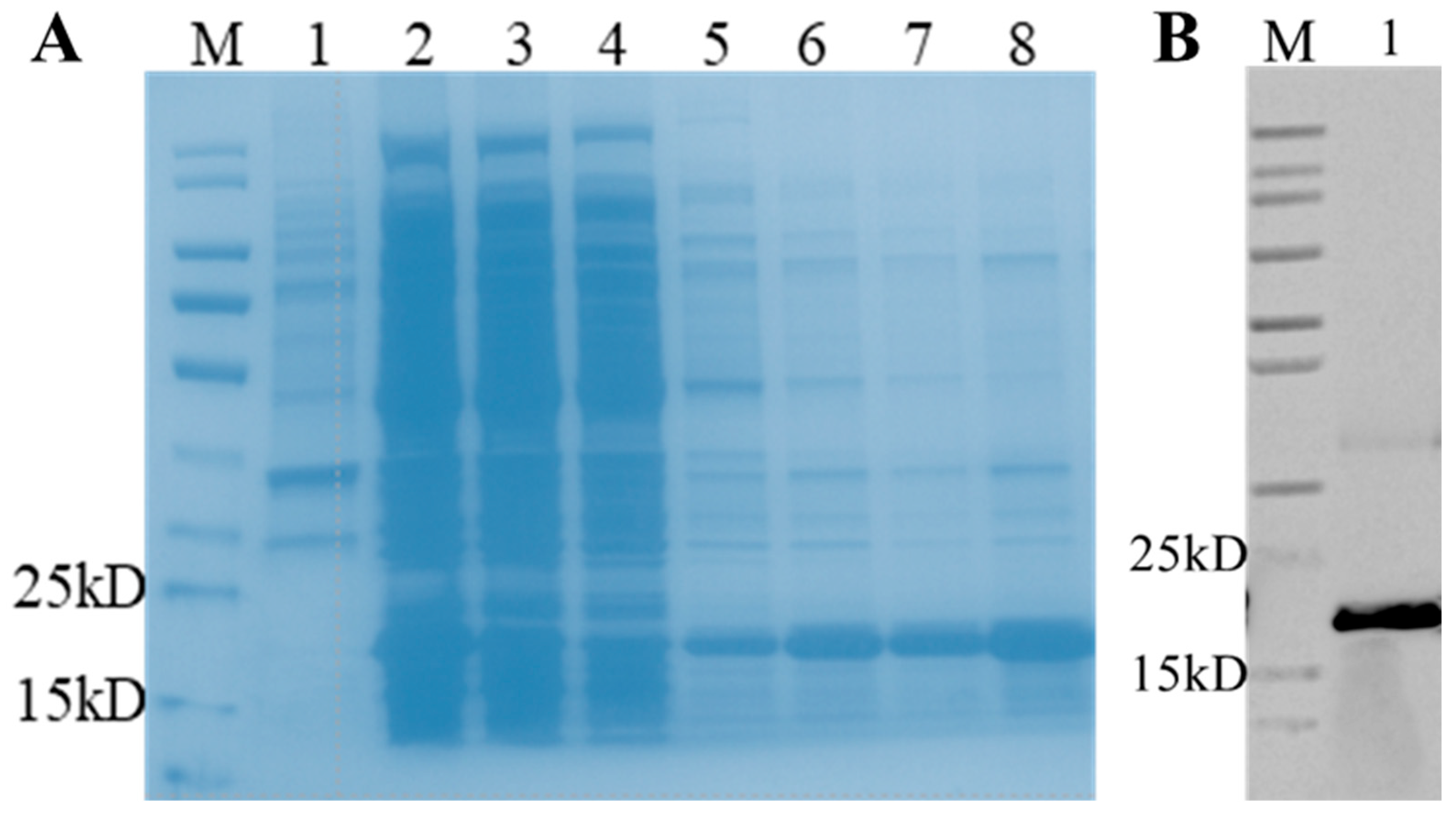
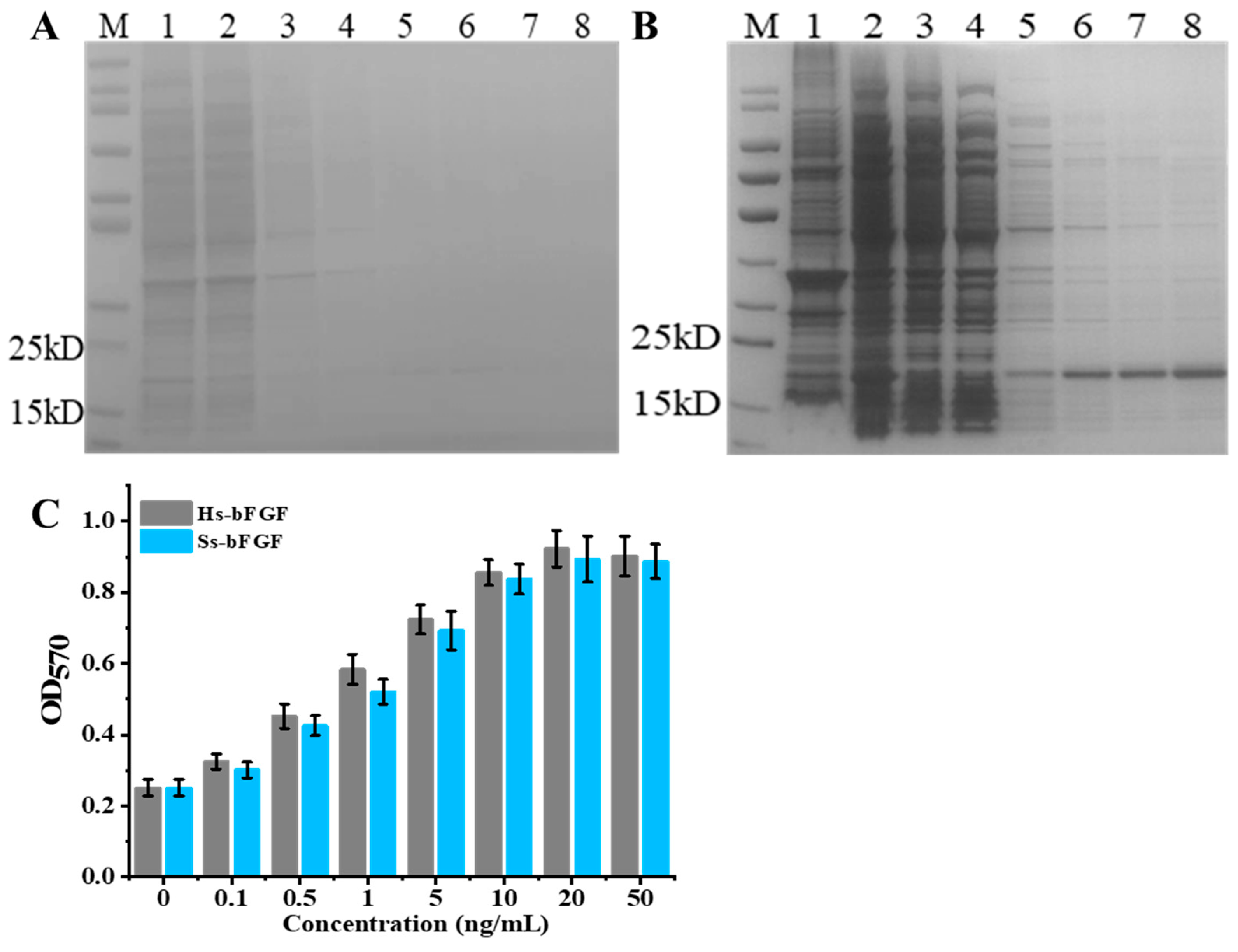
3.2. Expression and Purification of Recombinant Porcine bFGF
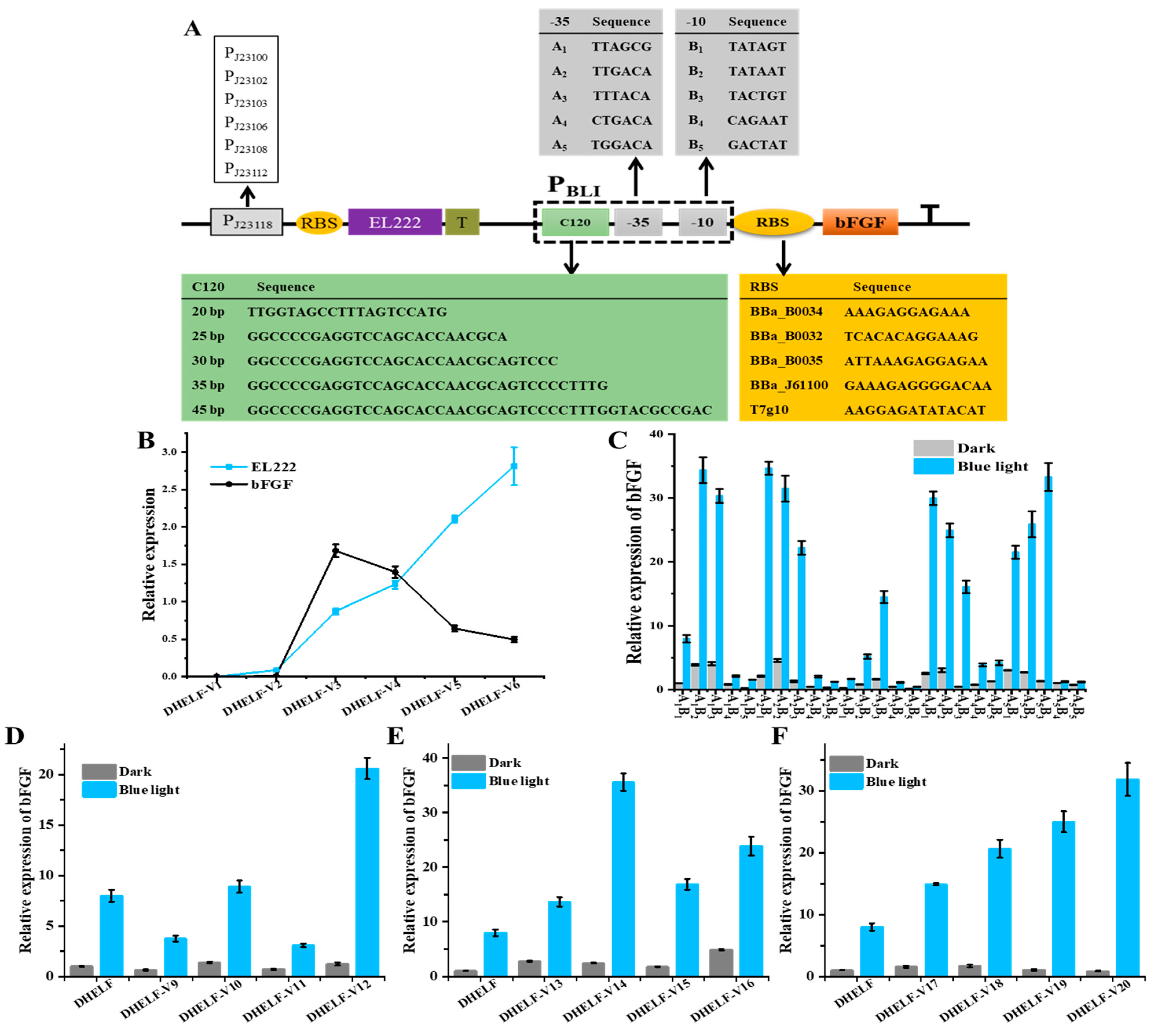
3.3. Optimization of EL222-Based Engineered Strain
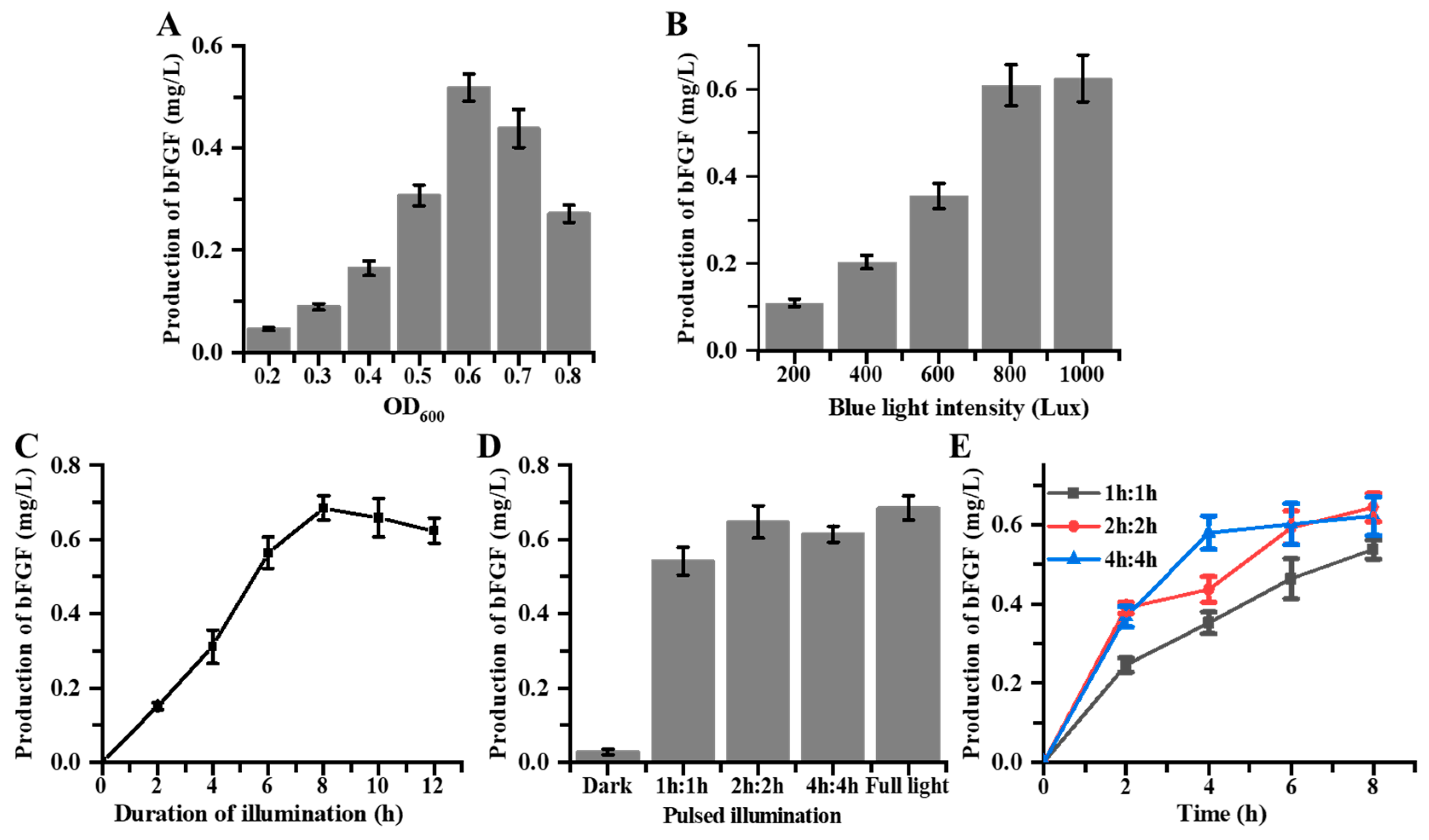
3.4. Fermentation of Blue Light-Induced bFGF Expression in E. coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Haraguchi, Y.; Asahi, T.; Kato, Y.; Kondo, A.; Hasunuma, T.; Shimizu, T. A serum-free culture medium production system by co-culture combining growth factor-secreting cells and L-lactate-assimilating cyanobacteria for sustainable cultured meat production. Sci. Rep. 2024, 14, 19578. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, S.; Post, M.J.; Moutsatsou, P. Towards resource-efficient and cost-efficient cultured meat. Curr. Opin. Food Sci. 2022, 47, 100885. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhao, X.Y.; Li, Y.; Ye, Q.H.; Wu, Y.W.; Niu, Q.Y.; Zhang, Y.; Fan, G.H.; Chen, T.X.; Xia, J.R.; et al. Advances in serum-free media for CHO cells: From traditional serum substitutes to microbial-derived substances. Biotechnol. J. 2024, 19, e2400251. [Google Scholar] [CrossRef]
- Geng, S.L.; Zou, Y.; Bai, Z.Y.; Zhang, M.; Wang, C.; Wang, T.Y. Serum-free medium for recombinant protein expression in insect cells. Biotechnol. Appl. Biochem. 2024, 1–15. [Google Scholar] [CrossRef]
- Romero, S.G.; Boyle, N. Systems biology and metabolic modeling for cultivated meat: A promising approach for cell culture media optimization and cost reduction. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3422–3443. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.J.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhao, X.R.; Li, X.L.; Du, G.C.; Zhou, J.W.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lin, J.X.; Li, J.; Mi, Y.L.; Zeng, W.D.; Zhang, C.Q. Basic fibroblast growth factor suppresses meiosis and promotes mitosis of ovarian germ cells in embryonic chickens. Gen. Comp. Endocrinol. 2012, 176, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhang, G.W.; Gu, T.X.; Li-Ling, J.; Wen, T.; Zhao, Y.; Wang, C.; Fang, Q.; Yu, L.; Liu, B. Exogenous basic fibroblast growth factor promotes cardiac stem cell-mediated myocardial regeneration after miniswine acute myocardial infarction. Coron. Artery Dis. 2011, 22, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nowwarote, N.; Sawangmake, C.; Pavasant, P.; Osathanon, T. Review of the role of basic fibroblast growth factor in dental tissue-derived mesenchymal stem cells. Asian Biomed. 2015, 9, 271–283. [Google Scholar]
- Ramasamy, R.; Tong, C.K.; Yip, W.K.; Vellasamy, S.; Tan, B.C.; Seow, H.F. Basic fibroblast growth factor modulates cell cycle of human umbilical cord-derived mesenchymal stem cells. Cell Prolif. 2012, 45, 132–139. [Google Scholar] [CrossRef]
- Garor, R.; Abir, R.; Erman, A.; Felz, C.; Nitke, S.; Fisch, B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil. Steril. 2009, 91, 1967–1975. [Google Scholar] [CrossRef]
- de Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.J.; Han, M.H.; Yoon, N.K.; Kim, Y.C.; Ahn, J. Effective production of human growth factors in Escherichia coli by fusing with small protein 6HFh8. Microb. Cell Fact. 2021, 20, 9. [Google Scholar] [CrossRef]
- Gasparian, M.E.; Elistratov, P.A.; Drize, N.I.; Nifontova, I.N.; Dolgikh, D.A.; Kirpichnikov, M.P. Overexpression in Escherichia coli and purification of human fibroblast growth factor (FGF-2). Biochemistry 2009, 74, 221–225. [Google Scholar] [CrossRef]
- Mu, X.P.; Kong, N.; Chen, W.L.; Zhang, T.; Shen, M.; Yan, W.Q. High-level expression, purification, and characterization of recombinant human basic fibroblast growth factor in Pichia pastoris. Protein Expr. Purif. 2008, 59, 282–288. [Google Scholar] [CrossRef]
- Imsoonthornruksa, S.; Pruksananonda, K.; Parnpai, R.; Rungsiwiwut, R.; Ketudat-Cairns, M. Expression and purification of recombinant human basic fibroblast growth factor fusion proteins and their uses in human stem cell culture. J. Mol. Microbiol. Biotechnol. 2015, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Soleyman, M.R.; Khalili, M.; Khansarinejad, B.; Baazm, M. High-level expression and purification of active human FGF-2 in Escherichia coli by codon and culture condition optimization. Iran. Red Crescent Med. J. 2016, 18, e21615. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Mirzahoseini, H.; Abad, M.A.K.; Movahed, M.A. High level expression of human basic fibroblast growth factor in Escherichia coli: Evaluating the effect of the GC content and rare codons within the first 13 codons. Afr. J. Biotechnol. 2010, 9, 2456–2462. [Google Scholar]
- Masuda, A.; Xu, J.; Minamihata, K.; Kagawa, G.; Hamada, Y.; Morifuji, Y.; Yano, T.; Hino, M.; Morokuma, D.; Karasaki, N.; et al. Production of a biologically active human basic fibroblast growth factor using silkworm-baculovirus expression vector system. J. Asia-Pacif. Entomol. 2018, 21, 716–720. [Google Scholar] [CrossRef]
- Kurokawa, T.; Sasada, R.; Iwane, M.; Igarashi, K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987, 213, 189–194. [Google Scholar] [CrossRef]
- Cheng, T.; Cao, W.; Wen, R.; Steinberg, R.H.; LaVail, M.M. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Müller cells. Investig. Ophthalmol. Vis. Sci. 1998, 39, 581–591. [Google Scholar]
- Tripathi, N.K.; Shrivastava, A. Recent developments in bioprocessing of recombinant proteins: Expression hosts and process development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Chia, N.; Lee, S.Y.; Tong, Y. Optogenetic tools for microbial synthetic biology. Biotechnol. Adv. 2022, 59, 107953. [Google Scholar] [CrossRef]
- Muller, K.; Naumann, S.; Weber, W.; Zurbriggen, M.D. Optogenetics for gene expression in mammalian cells. Biol. Chem. 2015, 396, 145–152. [Google Scholar] [CrossRef]
- Omelina, E.S.; Yushkova, A.A.; Motorina, D.M.; Volegov, G.A.; Kozhevnikova, E.N.; Pindyurin, A.V. Optogenetic and chemical induction systems for regulation of transgene expression in plants: Use in basic and applied research. Int. J. Mol. Sci. 2022, 23, 1737. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, M.A.; Ip, S.S.; Carrasco-Lopez, C.; Day, C.; Zhao, E.M.; Kawabe, H.; Avalos, J.L. Optogenetic control of the lac operon for bacterial chemical and protein production. Nat. Chem. Biol. 2021, 17, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pouzet, S.; Banderas, A.; Le Bec, M.; Lautier, T.; Truan, G.; Hersen, P. The promise of optogenetics for bioproduction: Dynamic control strategies and scale-up instruments. Bioengineering 2020, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Motta-Mena, L.B.; Reade, A.; Mallory, M.J.; Glantz, S.; Weiner, O.D.; Lynch, K.W.; Gardner, K.H. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 2014, 10, 196–202. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, J.; Moser, F.; Song, M.; Voigt, C.A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 2017, 13, 706–708. [Google Scholar] [CrossRef]
- Hennemann, J.; Iwasaki, R.S.; Grund, T.N.; Diensthuber, R.P.; Richter, F.; Moglich, A. Optogenetic control by pulsed illumination. ChemBioChem 2018, 19, 1296–1304. [Google Scholar] [CrossRef]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Luscher, C.; Mahn, M.; Pan, Z.H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Primers 2022, 2, 55. [Google Scholar] [CrossRef]
- Soffer, G.; Perry, J.M.; Shih, S.C.C. Real-time optogenetics system for controlling gene expression using a model-based design. Anal. Chem. 2021, 93, 3181–3188. [Google Scholar] [CrossRef]
- Nash, A.I.; McNulty, R.; Shillito, M.E.; Swartz, T.E.; Bogomolni, R.A.; Luecke, H.; Gardner, K.H. Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc. Natl. Acad. Sci. USA 2011, 108, 9449–9454. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Motta-Mena, L.B.; Gardner, K.H. Blue light-induced dimerization of a bacterial LOV-HTH DNA-binding protein. Biochemistry 2013, 52, 6653–6661. [Google Scholar] [CrossRef]
- Jayaraman, P.; Devarajan, K.; Chua, T.K.; Zhang, H.; Gunawan, E.; Poh, C.L. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, R.; Möglich, A. Light-regulated gene expression in bacteria: Fundamentals, advances, and perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1029403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Geng, F.; Shen, J.; Zhu, P.; Lu, Z.; Lu, F.; Zhou, L. Blue light-mediated gene expression as a promising strategy to reduce antibiotic resistance in Escherichia coli. Biotechnol. J. 2024, 19, 2400023. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz, A.; Savatier, P.; Cortay, V.; Kennedy, H.; Dehay, C. Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J. Neurosci. 2002, 22, 6610–6622. [Google Scholar] [CrossRef]
- Hung, J.H.; Weng, Z. Sequence alignment and homology search with BLAST and ClustalW. Cold Spring Harb. Protoc. 2016, 2016, 1016–1021. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022, bioRxiv:2022.04.08.487609. [Google Scholar]
- Nielsen, H.; Teufel, F.; Brunak, S.; von Heijne, G. SignalP: The Evolution of a Web Server. Methods Mol. Biol. 2024, 2836, 331–367. [Google Scholar]
- Ding, Q.; Ma, D.; Liu, G.Q.; Li, Y.; Guo, L.; Gao, C.; Hu, G.; Ye, C.; Liu, J.; Liu, L.; et al. Light-powered Escherichia coli cell division for chemical production. Nat. Commun. 2020, 11, 2262. [Google Scholar] [CrossRef]
- Stohr, A.M.; Ma, D.R.; Chen, W.L.; Blenner, M. Engineering conditional protein-protein interactions for dynamic cellular control. Biotechnol. Adv. 2024, 77, 108457. [Google Scholar] [CrossRef]
- Li, M.; Chen, H.; Liu, C.; Guo, J.; Xu, X.; Zhang, H.; Nian, R.; Xian, M. Improvement of isoprene production in Escherichia coli by rational optimization of RBSs and key enzymes screening. Microb. Cell Fact. 2019, 18, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Xu, Z.; Fan, X.; Wang, Z.; Zhou, L. Optogenetic Fine-Tuning of Sus scrofa Basic Fibroblast Growth Factor Expression in Escherichia coli. Fermentation 2024, 10, 612. https://doi.org/10.3390/fermentation10120612
Meng F, Xu Z, Fan X, Wang Z, Zhou L. Optogenetic Fine-Tuning of Sus scrofa Basic Fibroblast Growth Factor Expression in Escherichia coli. Fermentation. 2024; 10(12):612. https://doi.org/10.3390/fermentation10120612
Chicago/Turabian StyleMeng, Fanqiang, Zhimin Xu, Xia Fan, Zhisheng Wang, and Libang Zhou. 2024. "Optogenetic Fine-Tuning of Sus scrofa Basic Fibroblast Growth Factor Expression in Escherichia coli" Fermentation 10, no. 12: 612. https://doi.org/10.3390/fermentation10120612
APA StyleMeng, F., Xu, Z., Fan, X., Wang, Z., & Zhou, L. (2024). Optogenetic Fine-Tuning of Sus scrofa Basic Fibroblast Growth Factor Expression in Escherichia coli. Fermentation, 10(12), 612. https://doi.org/10.3390/fermentation10120612




